Photoelectroactive Corrole Monomer Functionalized with a Triphenylamine–Chalcone Derivative: Synthesis, Electropolymerization, and Electrochromic Applications †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
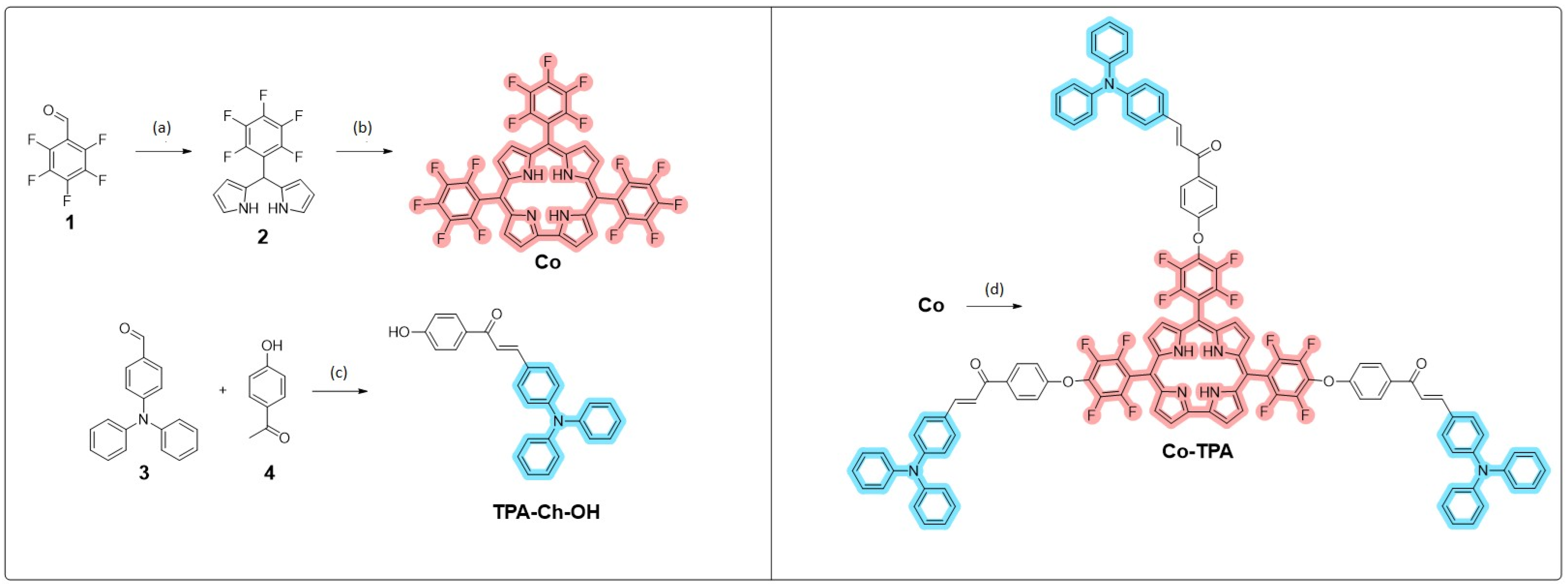
2.2. Synthesis
2.3. Spectroscopic Studies
2.4. Electrochemistry and Electrodeposition
2.5. Spectroelectrochemistry
3. Results and Discussion
3.1. Design, Synthesis, and Characterization of Co-TPAmonomer
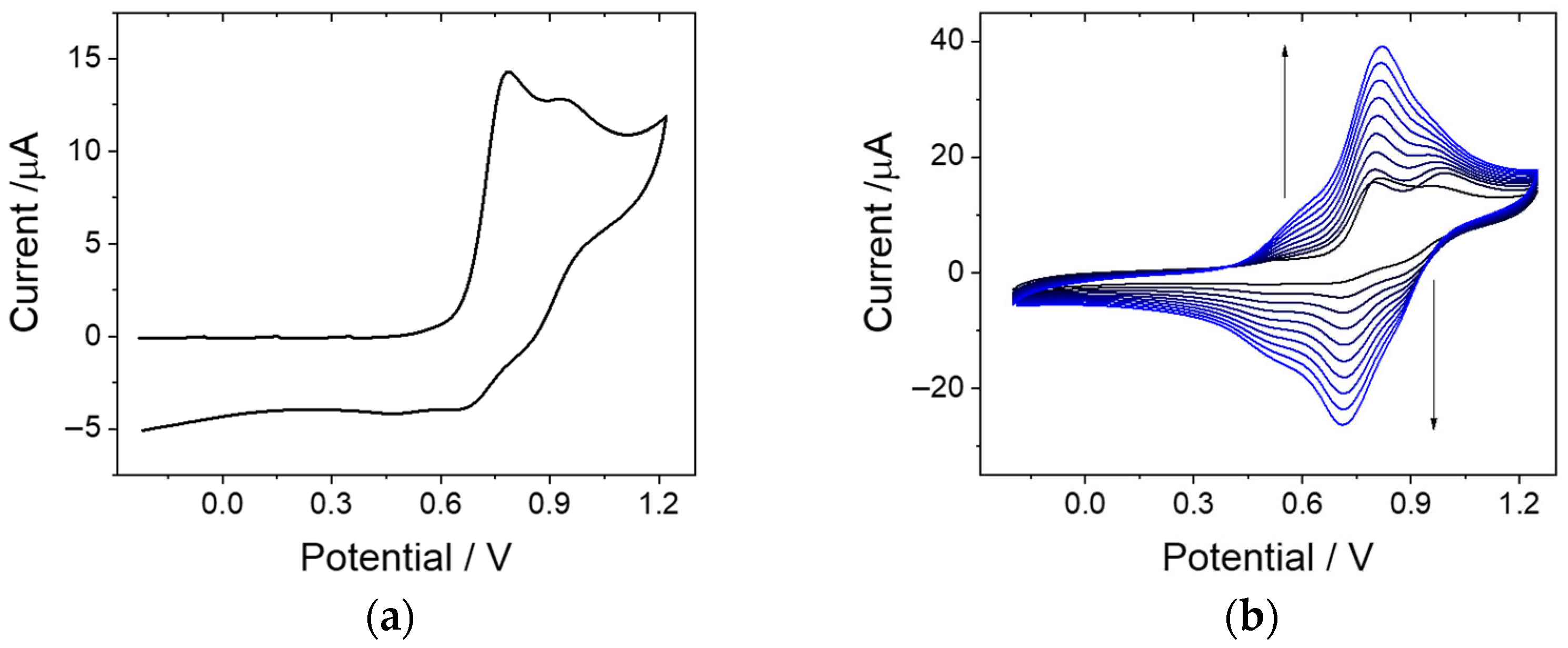
3.2. Electrochemical Characterization of Co-TPA and Electrochemical Synthesis and Deposition of P-Co-TPA Polymeric Film
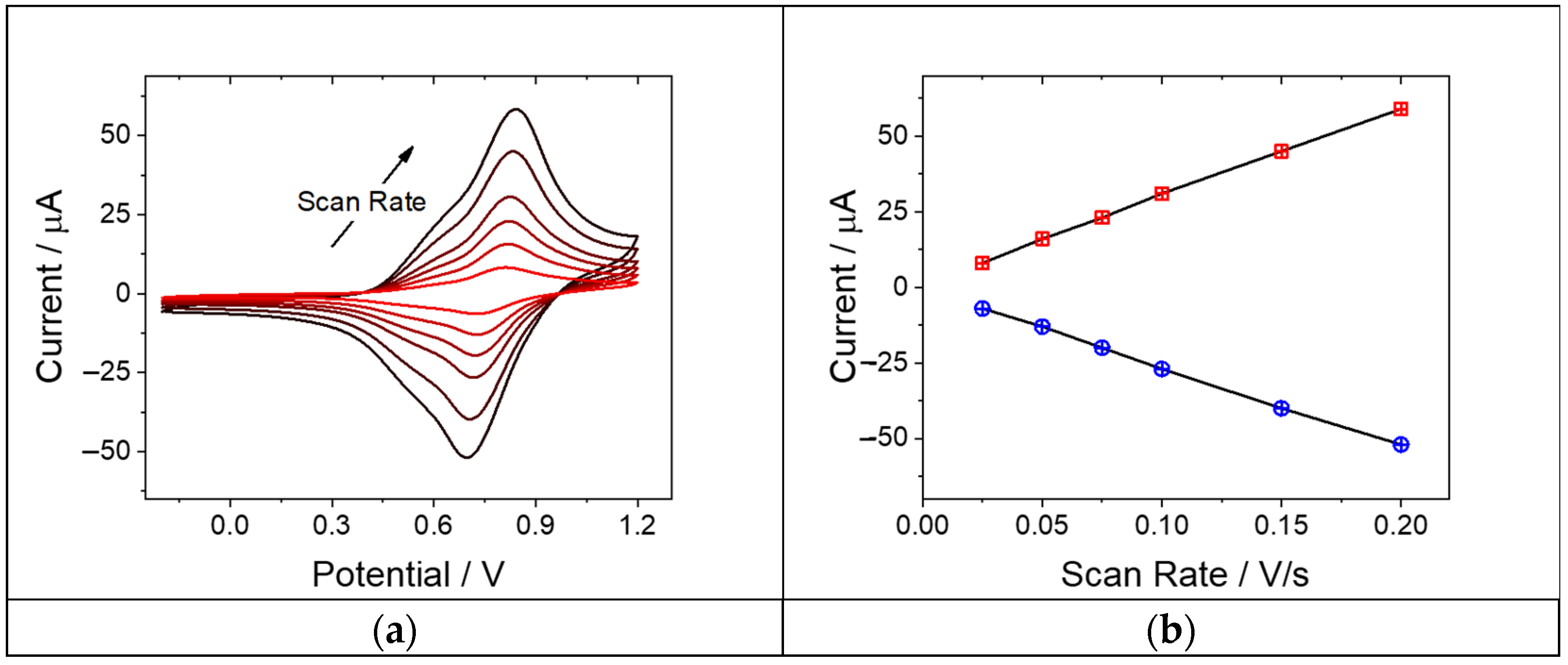
3.3. Electrochemical Characterization of P-Co-TPA
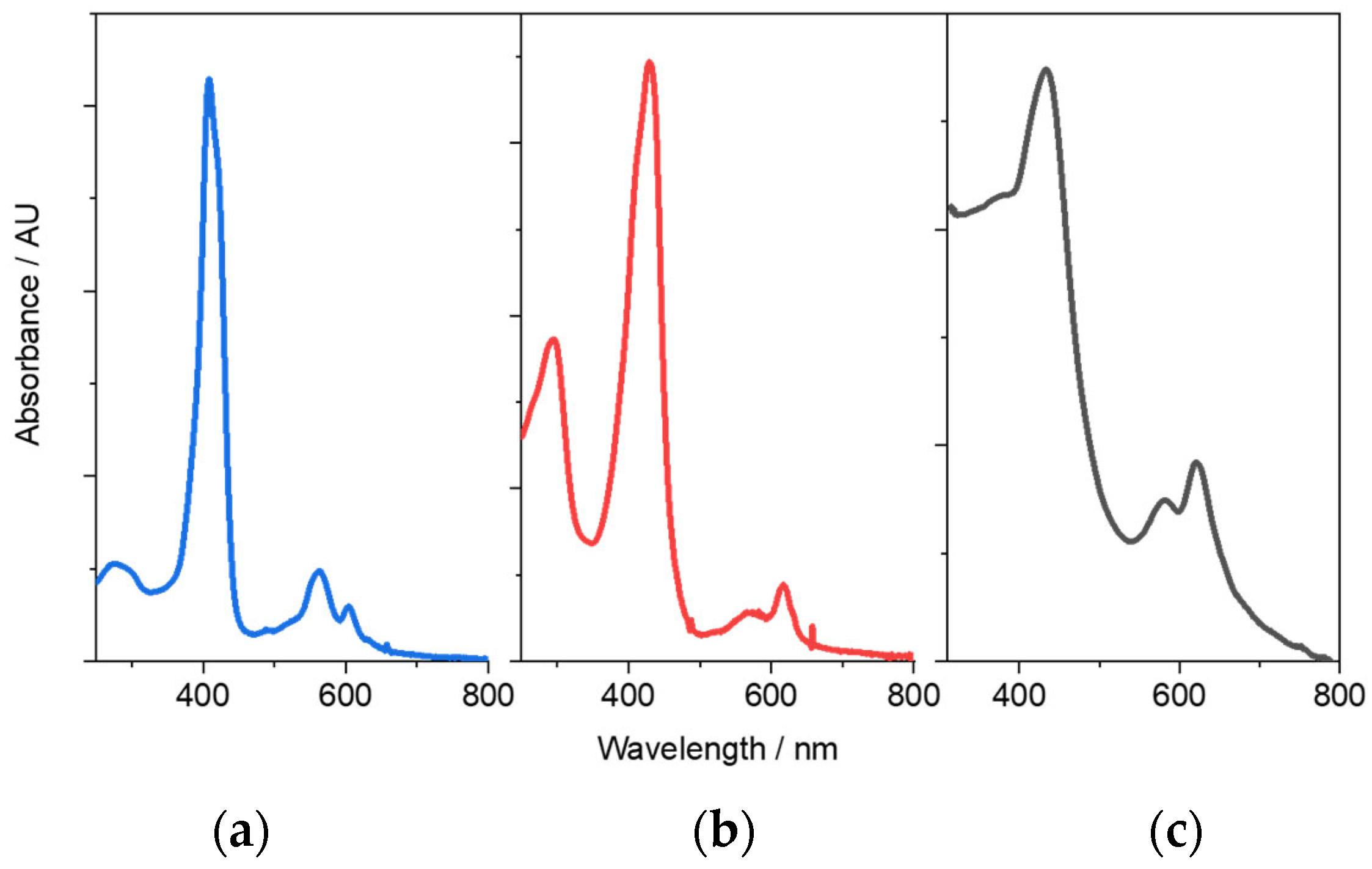
3.4. Spectroscopic Characterization of Co, Co-TPA, and P-Co-TPA
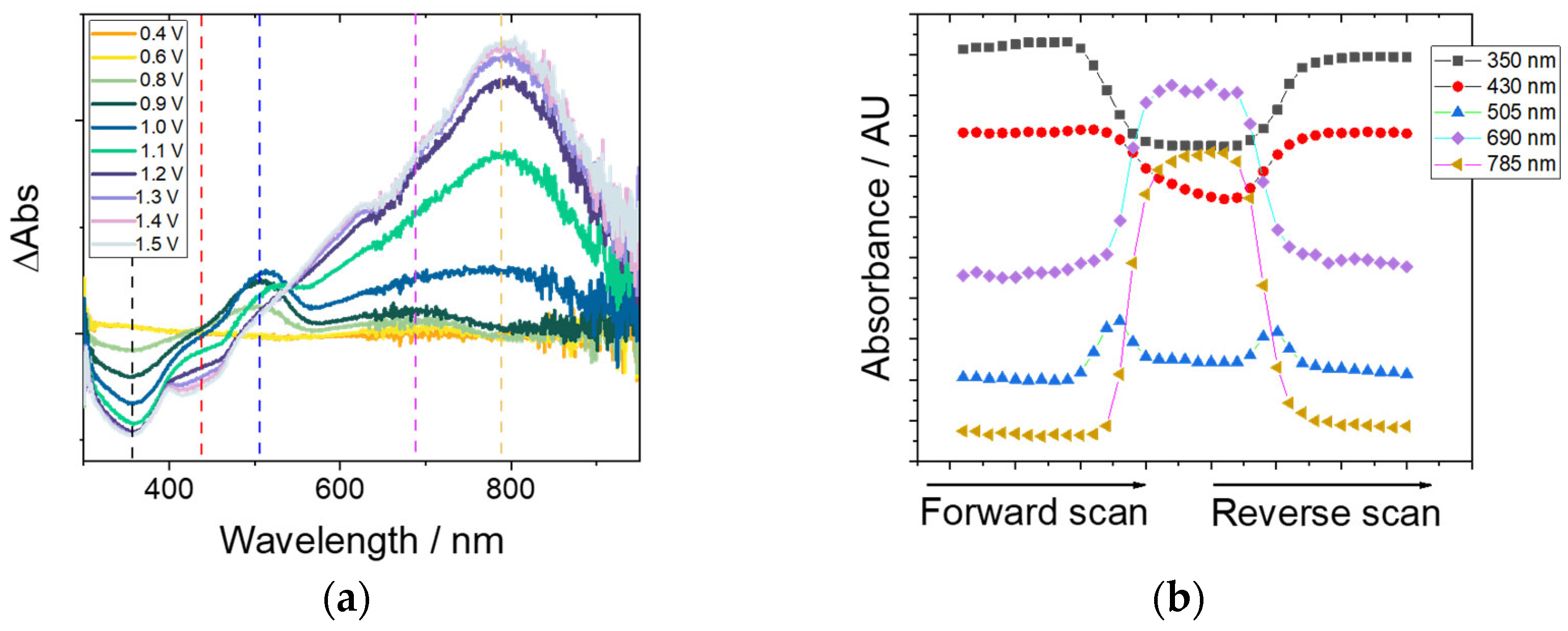
3.5. Spectroelectrochemical Characterization of P-Co-TPA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Natale, C.; Gros, C.P.; Paolesse, R. Corroles at Work: A Small Macrocycle for Great Applications. Chem. Soc. Rev. 2022, 51, 1277–1335. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Lopez, E.J.; Martínez, S.R.; Aiassa, V.; Santamarina, S.C.; Domínguez, R.E.; Durantini, E.N.; Heredia, D.A. Tuning the Molecular Structure of Corroles to Enhance the Antibacterial Photosensitizing Activity. Pharmaceutics 2023, 15, 392. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Chen, C.-L.; Chung, M.-W.; Chen, Y.-J.; Chou, P.-T. Effects of Multibranching on 3-Hydroxyflavone-Based Chromophores and the Excited-State Intramolecular Proton Transfer Dynamics. J. Phys. Chem. A 2010, 114, 10412–10420. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Tian, M.; Zhang, Z.; Lu, Q.; Liu, Z.; Niu, G.; Yu, X. Simultaneous Two-Color Visualization of Lipid Droplets and Endoplasmic Reticulum and Their Interplay by Single Fluorescent Probes in Lambda Mode. J. Am. Chem. Soc. 2021, 143, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Rohand, T.; Dolusic, E.; Ngo, T.H.; Maes, W.; Dehaen, W. Efficient Synthesis of Aryldipyrromethanes in Water and their Application in the Synthesis of Corroles and Dipyrromethenes. Arkivoc 2007, 10, 307–324. [Google Scholar] [CrossRef]
- Yurchenko, O.; Freytag, D.; zur Borg, L.; Zentel, R.; Heinze, J.; Ludwigs, S. Electrochemically Induced Reversible and Irreversible Coupling of Triarylamines. J. Phys. Chem. B 2012, 116, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Renfige, M.; Gonzalez Lopez, E.J.; Macor, L.; Solis, C.; Durantini, J.E.; Morales, G.; Otero, L.; Durantini, E.N.; Heredia, D.A.; Gervaldo, M. Electrochemical Synthesis of Donor–Acceptor Triazine-Based Polymers with Halochromic and Electrochromic Properties. Electrochim. Acta 2024, 486, 141428. [Google Scholar] [CrossRef]
- Sravani, R.; Nenavath, S.; Palacharla, S.P.; Giribabu, L. Triphenylamine–Corrole Donor–Acceptor Systems: Synthesis, Spectroscopy, and Photophysical Studies. Chem. Asian J. 2025, e70310. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto, E.B.; López, E.J.G.; Solis, C.; Calosso, A.; Otero, L.; Durantini, E.N.; Macor, L.; Gervaldo, M.; Heredia, D.A. Photoelectroactive Corrole Monomer Functionalized with a Triphenylamine–Chalcone Derivative: Synthesis, Electropolymerization, and Electrochromic Applications. Chem. Proc. 2025, 18, 29. https://doi.org/10.3390/ecsoc-29-26913
Prieto EB, López EJG, Solis C, Calosso A, Otero L, Durantini EN, Macor L, Gervaldo M, Heredia DA. Photoelectroactive Corrole Monomer Functionalized with a Triphenylamine–Chalcone Derivative: Synthesis, Electropolymerization, and Electrochromic Applications. Chemistry Proceedings. 2025; 18(1):29. https://doi.org/10.3390/ecsoc-29-26913
Chicago/Turabian StylePrieto, Elizabeth Bermúdez, Edwin Javier Gónzalez López, Claudia Solis, Andres Calosso, Luis Otero, Edgardo Néstor Durantini, Lorena Macor, Miguel Gervaldo, and Daniel Alejandro Heredia. 2025. "Photoelectroactive Corrole Monomer Functionalized with a Triphenylamine–Chalcone Derivative: Synthesis, Electropolymerization, and Electrochromic Applications" Chemistry Proceedings 18, no. 1: 29. https://doi.org/10.3390/ecsoc-29-26913
APA StylePrieto, E. B., López, E. J. G., Solis, C., Calosso, A., Otero, L., Durantini, E. N., Macor, L., Gervaldo, M., & Heredia, D. A. (2025). Photoelectroactive Corrole Monomer Functionalized with a Triphenylamine–Chalcone Derivative: Synthesis, Electropolymerization, and Electrochromic Applications. Chemistry Proceedings, 18(1), 29. https://doi.org/10.3390/ecsoc-29-26913







