Abstract
Hybrid molecules, integrating multiple pharmacophores within a single scaffold, represent a modern strategy in drug discovery, offering improved selectivity and safety. Anthranilic acid is a versatile building block with diverse biological activities. In this work, we designed and synthesized novel anthranilic acid-based hybrids with enhanced pharmacokinetic potential. The methods used include cheminformatics- guided library design, followed by amide bond formation between anthranilic acid derivatives and substituted 2-phenylethylamines. Purification and structural characterization were achieved via NMR, IR, and HRMS. The compounds exhibited favorable, predicted ADME/Tox profiles and synthetic accessibility. These results provide a foundation for further biological evaluation toward therapies for smooth muscle dysfunction and inflammation.
1. Introduction
Modern drug discovery increasingly relies on molecular hybridization, a strategy in which two or more pharmacophoric elements are combined into a single scaffold (Figure 1). This approach enables modulation of multiple biological targets simultaneously, potentially yielding compounds with improved selectivity, synergistic efficacy, and reduced side effects, compared to single-target agents. Recent reviews have highlighted that hybrid molecules are especially promising in tackling complex, multifactorial diseases such as chronic inflammation, cancer, metabolic syndrome, and neurodegeneration [1,2].
Figure 1.
Advantages of hybrid molecules in novel drug design (visualized with Napkin AI).
Anthranilic acid is a valuable scaffold in medicinal chemistry, on account of its dual reactive groups (–NH2 and –COOH) which allow for diverse derivatization. Various recent studies report anthranilic acid derivatives with potent biological activities, including anti-inflammatory, antimicrobial, anticonvulsant, enzyme inhibition, and receptor modulatory effects [3,4]. For example, novel anthranilic acid hydrazones have shown potent inhibition of cholinesterases and α-glycosidase with favorable ADME/Tox profiles [5]. A very recent work describes the hybridization of quinoline with anthranilic acid to produce compounds with strong in vitro and in vivo anti-inflammatory effects and good drug-likeness [6].
In this context, our study aims to design and synthesize novel anthranilic acid-based hybrid molecules that not only retain the broad spectrum of biological activities associated with anthranilic analogs, but also exhibit improved pharmacokinetic and pharmacodynamic properties through rational hybrid design and in silico filtering.
2. Materials and Methods
A combination of cheminformatics tools was used to guide the design of a focused library of target compounds. Publicly available software products (SwissADME [7], PASS Online [8], ProTox 3.0 [9]) were used for the in silico screening to identify the most favorable candidates for synthesis. The main criteria in this process included properties such as synthetic availability, Lipinski’s rule of 5, gastrointestinal absorption, blood–brain barrier (BBB) permeability, antispasmodic activity, and toxicity.
The synthetic procedure relied on an efficient ring-opening reaction of isatoic anhydride with substituted 2-phenylethylamines at room temperature to limit side products formation (Scheme 1) [10,11,12,13,14]. Obtaining the desired hybrid products (3–5) was monitored chromatographically (TLC).
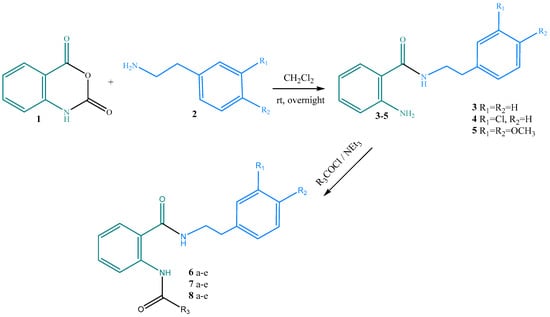
Scheme 1.
Synthetic procedure for obtaining the desired hybrid diamides 6–8 a–e with anthranilic acid (green) and phenylethylamine (blue) structural units.
The hybrids were acylated with various alkyl- and aryl-substituted acyl chlorides (R3 = CH3, C6H5, CH2-C6H5, 2-Cl-C6H4, CH(Cl)C6H5), yielding the desired diamides 6–8 a–e with high purity.
All compounds were purified and characterized using standard spectroscopic techniques, including NMR, IR, and HRMS, and melting point temperature determination.
3. Results and Discussion
The applied in silico approaches, including cheminformatics and ADME/Tox predictions, generated a comprehensive dataset on the physicochemical, pharmacokinetic, and toxicological profiles of the tested structures. Based on the analysis of key parameters, three phenylethylanthranilamide compounds and their diamide derivatives were identified as the most promising candidates.
Lipophilicity, a major determinant of oral bioavailability, was found to be below the critical threshold of logP = 5. This finding is consistent with Lipinski’s Rule of Five, which states that orally active drugs are more likely to succeed when not violating more than one of the following criteria: molecular weight ≤ 500 Da, logP ≤ 5, ≤ 5 hydrogen bond donors, and ≤ 10 hydrogen bond acceptors [15]. Compliance with these pharmacokinetic guidelines is crucial, since a significant proportion of drug candidates fail at early stages of development due to poor ADME properties [16].
Further ADME predictions revealed high gastrointestinal absorption and BBB permeability. While BBB penetration is advantageous in the context of central nervous system drug discovery, particularly for neurodegenerative disorders, it also raises safety concerns due to the risk of neurotoxicity [17].
Preliminary toxicity assessment placed the compounds in toxicity class 4, with calculated LD50 values ranging from 1000 to 2025 mg/kg. The SwissADME tool (Figure 2) confirmed overall organ safety, yet it predicted approximately 70% probability of respiratory and neurotoxicity. In this study, such findings are interpreted as potentially favorable, since BBB penetration is a desired feature for the conceptualized therapeutic application. Comparable or even higher toxicity levels have been reported for clinically used drugs in this therapeutic area, suggesting that the investigated structures remain within an acceptable safety range for further pharmacological exploration [18,19].
Figure 2.
Toxicity calculation results for compound 6b, obtained with SwissADME.
Application of the synthetic methodology allowed for the efficient synthesis of three hybrid molecules and fifteen diamides in total, with practical yields ranging between 78 and 83% [12,13,14].
4. Conclusions
The synthesized hybrid structures integrate multiple pharmacophores and demonstrate favorable predicted ADME properties and toxicity. The synthetic procedures were efficient and reproducible, yielding structurally confirmed compounds ready for further biological exploration.
The generated hybrid structures show promising in silico drug-likeness and synthetic accessibility. This work provides a strong foundation for developing new small molecules aimed at treating complex disorders involving smooth muscle dysfunction and inflammation. The biological effects of all hybrids are to be thoroughly studied as a next step of the novel drug candidates design and development project.
Author Contributions
Conceptualization, I.S. and S.A.N.; methodology, S.A.N.; software, V.G. and M.M.; investigation, M.M., M.S., M.T. and V.G.; resources, M.M.; data curation, M.M.; writing—original draft preparation, M.M. and V.G.; writing—review and editing, S.A.N. and I.S.; visualization, M.M., V.G. and M.T.; supervision, S.A.N. and I.S.; project administration, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This study is supported by the Bulgarian Ministry of Education, National Program “Young Scientists and Postdoctoral Students–2”, Project № MUPD-HF-017.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADME | Absorption, distribution, metabolism, excretion |
| Tox | Toxicity |
| BBB | Blood–brain barrier |
| IR | Infrared spectroscopy |
| NMR | Nuclear magnetic resonance spectroscopy |
| HRMS | High-resolution mass spectrometry |
| TLC | Thin-layer chromatography |
References
- Morais, T.S. Recent Advances in the Development of Hybrid Drugs. Pharmaceutics 2024, 16, 889. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Sharma, M. Medicinal Chemistry of Anthranilic Acid Derivatives: A Mini Review. Drug Dev. Res. 2021, 82, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekhara Kumar, B.; Bhagwath, A.A.; Chandrashekar, K.S. Pharmaceutical Chemistry of Anthranilic Acid Derivatives: A Brief Review. Int. J. Pharm. Sci. 2024, 2, 2143–2174. [Google Scholar] [CrossRef]
- Nasr, T.M.; Aboshanab, A.M.; Abouzid, K.A.M.; Zaghary, W.A. Hands-On Synthetic Approaches and Biological Activities of Anthranilic Acid Derivatives: A Mini-Review. Egypt. J. Chem. 2023, 66, 329–343. [Google Scholar] [CrossRef]
- Tokalı, F.S.; Taslimi, P.; Taskin-Tok, T.; Karakuş, A.; Sadeghian, N.; Gulçin, İ. Novel Hydrazones Derived from Anthranilic Acid as Potent Cholinesterases and α-Glycosidase Inhibitors: Synthesis, Characterization, and Biological Effects. J. Biochem. Mol. Toxicol. 2024, 38, e23521. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Hussain, K.; Shehzadi, N.; Arshad, M.; Arshad, M.N.; Iftikhar, S.; Saghir, F.; Shaukat, A.; Sarfraz, M.; Ahmed, N. Design, Synthesis, Biological Evaluation and Molecular Docking Studies of Quinoline-Anthranilic Acid Hybrids as Potent Anti-Inflammatory Drugs. Org. Biomol. Chem. 2024, 22, 3708–3724. [Google Scholar] [CrossRef] [PubMed]
- SwissADME. Available online: http://www.swissadme.ch/ (accessed on 26 September 2025).
- PASS Online. Available online: https://way2drug.com/PassOnline/ (accessed on 26 September 2025).
- ProTox 3.0. Available online: https://tox.charite.de/protox3/ (accessed on 26 September 2025).
- Rivero, I.A.; Espinoza, K.; Somanathan, R. Syntheses of Quinazoline-2,4-dione Alkaloids and Analogues from Mexican Zanthoxylum Species †. Molecules 2004, 9, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tan, Z.; Xiong, B.; Jiang, H.F.; Zhang, M. Transition-Metal-Catalyst-Free Synthesis of Anthranilic Acid Derivatives by Transfer Hydrogenative Coupling of 2-Nitroaryl Methanols with Alcohols/Amines. Org. Biomol. Chem. 2018, 16, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Milusheva, M.; Stoyanova, M.; Gledacheva, V.; Stefanova, I.; Todorova, M.; Pencheva, M.; Stojnova, K.; Tsoneva, S.; Nedialkov, P.; Nikolova, S. 2-Amino-N-Phenethylbenzamides for Irritable Bowel Syndrome Treatment. Molecules 2024, 29, 3375. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, M.; Milusheva, M.; Gledacheva, V.; Stefanova, I.; Todorova, M.; Kircheva, N.; Angelova, S.; Pencheva, M.; Stojnova, K.; Tsoneva, S.; et al. Spasmolytic Activity and Anti-Inflammatory Effect of Novel Mebeverine Derivatives. Biomedicines 2024, 12, 2321. [Google Scholar] [CrossRef] [PubMed]
- Milusheva, M.; Todorova, M.; Gledacheva, V.; Stefanova, I.; Feizi-Dehnayebi, M.; Pencheva, M.; Nedialkov, P.; Tumbarski, Y.; Yanakieva, V.; Tsoneva, S.; et al. Novel Anthranilic Acid Hybrids—An Alternative Weapon against Inflammatory Diseases. Pharmaceuticals 2023, 16, 1660. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The Blood–Brain Barrier: Bottleneck in Brain Drug Development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, H.; Zhang, T.; Wang, Z.; Li, H.; Zhang, Y. Nonlinear and Mixed Inhibitory Effect of Matrine on the Cytotoxicity of Oligomeric Amyloid-β Protein. Neurochem. Int. 2020, 137, 104746. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Nilgirwar, P.S.; Bali, S.D. Current pharmacological treatments for neurodegenerative diseases. In The Neurodegeneration Revolution; Sastri, T., Osmani, R.A.M., Singh, E., Dutta, S., Eds.; Elsevier: Amsterdam, NL, USA, 2025; pp. 117–126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).