Abstract
A highly efficient and sustainable one-pot method for the synthesis of 2,3-diphenylquinoxaline (DPQ) is presented. The reaction employs sodium hypochlorite as an inexpensive and eco-friendly oxidant for the conversion of benzoin, followed by condensation with o-phenylenediamine in an ethanol/water system. This green approach demonstrates remarkable versatility, affording excellent yields under photochemical (96%), reflux (92%), and electrochemical (83%) conditions. To provide mechanistic insight, computational studies were conducted, revealing the reaction pathway and identifying key energetic barriers. This work offers a practical and environmentally benign alternative for synthesizing important quinoxaline derivatives.
1. Introduction
Quinoxaline derivatives represent a class of organic compounds with diverse applications, including their utilization as pharmacophores, organic semiconductors, and molecular recognition agents [1,2]. Among these derivatives, 2,3-diphenylquinoxaline (DPQ) stands out as a molecule of particular interest due to its unique structural features and potential for various biological and material science applications [3,4]. While the synthesis of DPQ has been previously reported using different methodologies, the existing approaches often suffer from drawbacks such as the use of volatile and environmentally harmful solvents, low product yields, and harsh reaction conditions [5,6,7,8,9,10,11]. The development of a sustainable and efficient synthetic strategy for the preparation of DPQ is therefore of significant interest.
In recent years, the increasing emphasis on sustainable and environmentally friendly chemical processes has spurred the exploration of alternative synthetic methodologies that minimize the use of toxic reagents and solvents, reduce energy consumption, and generate minimal waste [12]. The integration of green chemistry principles in the Synthesis of complex organic molecules has emerged as a crucial area of research, aiming to address the challenges posed by conventional synthetic protocols [13].
In this context, our study focuses on the development of an innovative and eco-friendly approach for the Synthesis of DPQ, employing Sodium Hypochlorite (NaOCl·5H2O) as a key reagent. The proposed method involves a one-pot synthesis, starting with the oxidation of benzoin, an α-hydroxyketone, to benzyl, followed by its condensation with o-phenylenediamine in a water/ethanol solvent system. This synthetic pathway not only minimizes the use of hazardous organic solvents but also ensures an efficient conversion of the starting materials to the desired product. The utilization of NaOCl·5H2O offers several advantages, including its low cost, ready availability, and its eco-friendly nature compared to conventional catalysts. Furthermore, using a water/ethanol solvent system adds to the sustainable aspects of the process, as it reduces the generation of harmful waste and offers the potential for recycling and reuse.
The green synthesis of DPQ described in this study presents a significant advancement in the field of sustainable organic synthesis. By utilizing a benign catalytic system and a sustainable solvent, the approach not only offers a more environmentally friendly alternative to conventional methods but also demonstrates a high yield of the target compound, ranging from 83% to 96%. The mild reaction conditions provided by this methodology contribute to the preservation of the structural integrity of the desired product, ensuring its suitability for further functionalization and application in various fields. Notably, the versatility of the reaction conditions allows for its execution using reflux, photochemical, or electrochemical methods, thereby providing flexibility and adaptability to different laboratory setups and preferences. The ability to perform synthesis using multiple methodologies further enhances the practicality and accessibility of this green synthetic approach.
2. Results and Discussion
The successful Synthesis of 2,3-diphenylquinoxaline 3 using 2-hydroxy-1,2-diphenylethan-1-one 1 and benzene-1,2-diamine 2 under various conditions underscores the adaptability and efficiency of the green synthesis method. In this study, a systematic exploration of various parameters on the yield of the targeted reaction was conducted, as elucidated in Table 1. We investigated different combinations of factors, including the amount of NaOCl·5H2O, choice of solvent, and reaction conditions. Initially, the optimization of the photochemical reaction commenced with the use of 0.5 equivalent NaOCl·5H2O (Entry 1). Subsequent increments in the amount of NaOCl·5H2O to 1.0, 1.5, 2.0, and 2.5 equivalents (Entries 2 to 5) revealed that 1.5 equivalents and above consistently provided the same yield. Further exploration involved different solvents (Entries 6 to 9), unveiling varied yields in the photochemical setting. Notably, DMF and DMSO yielded relatively higher percentages, whereas Ethanol/Water emerged as the optimal solvent. Reflux conditions in Ethanol/Water resulted in a notable yield of 92% (Entry 10). Lastly, grinding and sonication yielded 75% and 83%, respectively (Entries 11 and 12), suggesting the influential role of mechanical processes on the reaction outcome (Scheme 1).

Table 1.
Optimization of the reaction.
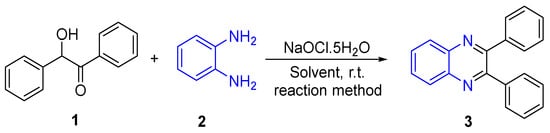
Scheme 1.
Optimization of the reaction.
2.1. Reaction Methods
2.1.1. Refluxing at 65 °C
The synthesis involved refluxing a mixture of alpha-hydroxy ketone (Benzoin) 1, o-phenylenediamine 2, and Sodium Hypochlorite in Ethanol/Water at 65 °C for 2 h. The reaction progress was monitored by TLC. After completion, the mixture was processed, yielding 92% pure quinoxaline 3 (Scheme 2). Identity confirmation included melting point comparison, Carbonyl functional group test, and a negative primary amine test. Additionally, the compound was identified by 1H NMR Spectroscopy.
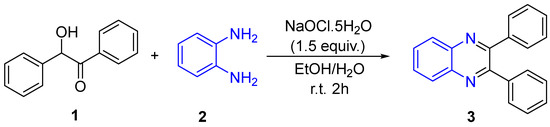
Scheme 2.
Reaction strategies with three different methods.
2.1.2. Photochemical Reactor
In the photochemical synthesis, a pilot photocatalytic reactor was used for reaction [Capacity (1 KL), Voltage 230 V, Frequency 50 Hz, Size 600 mm (H) × 480 mm (W) × 480 mm (D), UV lamp type Medium Pressure Mercury vapour lamp] immersion mixture of Benzoin 1, o-phenylenediamine 2, and Sodium Hypochlorite in Ethanol/Water was stirred in a UV Tube for 2 h. Progress was monitored by TLC, and the resulting yield improved to 96% (Scheme 2).
2.1.3. Electrochemical Cell
Utilizing an electrochemical cell, a mixture of Benzoin 1, o-phenylenediamine 2, and Sodium Hypochlorite in Ethanol/Water was subjected to deep well graphite electrodes for 2 h. The reaction progress was monitored by TLC, and the yield of the product was 83% (Scheme 1). Identity confirmation included various tests, and the electrochemical cell parameters included a cell potential of 30 V.
2.2. Characterization
2,3-Diphenylquinoxaline (3): White Crystalline Powder; m.p.: 127 °C; FT-IR (νmax, cm−1): 3045.0 (C-H, st), 1566.5 (C=N, st), 1330.0 (C-N, st) 771.53 (C-H, b); 1H NMR (400 MHz, CDCl3) δ ppm: 8.21 (2H, dd), 7.81 (2H, dd) 7.55 (4H, dd) 7.38 (6H, m) (Figure S1); 13C NMR (400 MHz, CDCl3) δ ppm: 153.51, 141.24, 139.08, 130.00, 129.85, 129.22, 128.83, 128.30 (Figure S2).
2.3. Computational Calculations
In order to shed light into the reaction mechanism for the formation of quinoxaline, we performed theoretical calculations with Gaussian 09, Rev. D.01 [14]. Gas-phase geometries were optimized without constraints or symmetry restrictions using ωB97X-D [15] and the def2-SVPP basis set [16]. Harmonic frequency analyses confirmed the nature of each stationary point (0 imaginary frequencies for minima; 1 for transition states). Thermal and entropic corrections (298 K, 1 atm) were taken from the standard thermochemistry output. Solvation was modeled by single-point PCM/SMD calculations [17,18,19,20,21] on the gas-phase geometries with ethanol (ε = 24.852) as solvent. Electronic energies were refined by single-point calculations with def2-TZVPP [22]. Final reported energies correspond to SMD(ethanol):ωB97X-D/def2-TZVPP//ωB97X-D/def2-SVPP.
The calculated reaction mechanism (Scheme 3), for the Synthesis of 2,3-dyphenylquinoxaline 3, starts with the dehydration reaction between benzoin 1 and NaOCl·5H2O to produce benzoil and NaCl·6H2O as subproduct. Then, o-phenylenediamine is added to form A1 adduct and all this process was calculated to be highly exergonic (ΔGR1 = −60.1 kcal/mol). Next, the first aminol group is formed through proton transfer assisted by ethanol (TSA1->4-S) with an energy barrier of ΔG‡1 = 26.3 kcal/mol. Generation of intermediate 4-S is endergonic by ΔGR2 = 10.3 kcal/mol. From here, anti-diaminol 5-S (ΔGR3 = −5.8 kcal/mol) can be obtained via proton transfer (TS4-S->5-S) with ΔG‡2 = 12.6 kcal/mol. We also found a pathway to syn-diaminol (5SC, Figure S9) with ΔG‡2’ = 14.3 kcal/mol. The anti-diaminol is favored both kinetically and thermodynamically over the syn-isomer, a preference that originates from reduced steric repulsion due to the larger spatial separation of the substituents. Later, we found that an intermolecular proton transfer between two diaminol molecules (from 5-S to A2 adduct, ΔGR4 = −5.3 kcal/mol) presents an accessible energy barrier (via TSA2->A3 with ΔG‡3 = 26.7 kcal/mol) to produce intermediate A3 and water (ΔGR5 = −5.4 kcal/mol). This last step was also explored by ethanol-assistance, but the energy barrier was higher (ΔG‡3’ = 33.3 kcal/mol, TS5-S->6, Figure S6). This reactivity discrepancy can be rationalized by the greater acidity of the syn-diaminol proton relative to ethanol, resulting from efficient delocalization of the transient excess electron density across the diaminol scaffold. Finally, a second intermolecular proton transfer via TSA3->3 (ΔG‡4 = 18.7 kcal/mol) leads to 2,3-diphenylquinoxaline (3) and a second water molecule (ΔGR6 = −25.5 kcal/mol). We also examined the reaction route from 4-S to the first condensation via TS4-S->5 (Figure S7) but this resulted in a higher energy barrier of 31.8 kcal/mol (see Figures S3–S11 for other explored pathways).
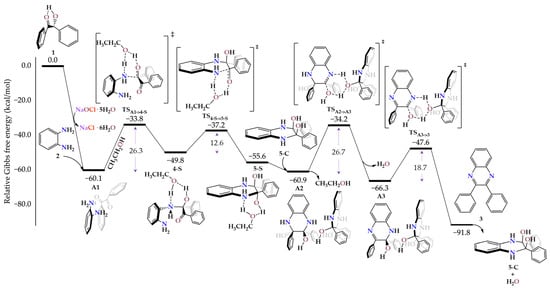
Scheme 3.
Energy profile of the proposed reaction mechanism to get quinoxaline 3. Relative Gibbs free energy values are expressed in kcal/mol. All calculations were done at (SMD:ethanol)ω-B97X-D/def2-TZVPP//ω-B97X-D/def2-SVPP level.
The diverse synthetic approaches explored in this study showcase the adaptability of the green synthesis method for 2,3-diphenylquinoxaline under various reaction conditions. Refluxing at 65 °C, employing a photochemical reactor, and utilizing an electrochemical cell all demonstrated high yields of the target compound. The environmentally friendly nature of the Sodium Hypochlorite catalyst, combined with the use of Ethanol/Water as a solvent system, aligns with the principles of green chemistry. The versatility of the reaction conditions provides practical options for different laboratory setups, ensuring accessibility and applicability. This study contributes valuable insights into the realm of sustainable organic synthesis, offering a promising avenue for further advancements and applications in the synthesis of quinoxaline derivatives.
3. Conclusions
In conclusion, the eco-friendly synthesis of 2,3-diphenylquinoxaline, using Sodium Hypochlorite, demonstrates exceptional adaptability and sustainability across a range of methodologies. The successful outcomes observed through refluxing at 65 °C, employing a photochemical reactor, and utilizing an electrochemical cell highlight the versatility of this environmentally conscious approach. The adoption of Ethanol/Water as a solvent aligns seamlessly with green chemistry principles, emphasizing the environmentally benign nature of the process. The robustness of the synthetic route is confirmed through rigorous characterization methods. This study contributes to the sustainable synthesis of quinoxaline and sets the stage for broader applications in green organic synthesis, underscoring the significance of environmentally conscious chemical processes.
2,3-Diphenylquinoxaline: Purification with n-Hexane: EtOAc (8:2) as eluent; yellow solid, mp = 125–128 °C). 1H NMR (400 MHz, CDCl3) δ 8.22 (dd, J = 6.4 Hz, 3.2 Hz, 2H), 7.88 (dd, J = 6.4 Hz, 3.2 Hz, 2H), 7.52 (d, J = 7.6 Hz, 4H), 7.36–7.34 (m, 6H); 13C NMR (100 MHz, CDCl3) δ (ppm): 153.51, 141.24, 139.08, 130.00, 129.85, 129.22, 128.83, 128.30 HRMS (ESI-TOF) m/z: [M + H+] Calcd for C20H15N2 283.1230, Found 283.1233.
Computational details
All DFT calculations were performed using Gaussian09 rev. E.01 software optimizations were carried out in gas-phase with ωB97X-D [S2], a long-range hybrid functional, in combination with Ahlrichs’ def2-sv(p) basis set (written in this paper as def2-SVPP) [S3]. Subsequently, harmonic frequency calculation was done for each optimized geometry to corroborate the kind of each critical point in the Potential Energy Surface (PES), where reactants, intermediates, and products must present all the frequencies as positive values; the transition states must have one and just one negative frequency.
In order to improve our energy calculations, solvent effects were included through the implicit PCM model using the SMD parameters according to the Truhlar’s framework with ethanol (ε = 24.852) as solvent [S4,S5]. These calculations were done as single points of the optimized geometry at level of theory mentioned above. Also, we performed single points calculations of each optimized geometry at higher level of theory ωB97X-D/def2-TZVPP to get better accuracy [S6]. Finally, these energy corrections were added to gas-phase values and were reported as our definitive results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ecsoc-29-26872/s1, Figure S1: 1H NMR of 2,3-Diphenylquinoxaline; Figure S2: 13C NMR of 2,3-Diphenylquinoxaline; Figure S3: Proposed favorable reaction mechanism for obtain Quinoxaline (3) through diaminol intermediate (5-S); Figure S4. Relatives Gibbs free energies for possibles bimolecular transition states using diaminol molecules. code: blue (nitrogen), red (oxygen), gray (carbon), and white (hydrogen); Figure S5 Energy profile to obtain aminol intermediate (4) without solvent assistance; Figure S6. Energy profile for obtain Quinoxaline (3) through aminol-imine pathway with water assistance; Figure S7. Energy profile for obtain Quinoxaline (3) through aminol-imine pathway with ethanol assistance; Figure S8. Energy profile to form imine-aminol intermediate (6) with ethanol assistance; Figure S9. Energy profile to form imine-aminol intermediate (6C) with ethanol assistance. Figure S10. Energy profile to form imine-aminol intermediate (6) through syn-diaminol intermediate (5-C); Figure S11. Energy profile to form imine-aminol intermediate (6) through anti-diaminol intermediate (5-T); Table S1. Cartesian coordinates (xyz format) of all the optimized structures involved in the reaction mechanisms and energy profiles, calculated at ωB97X-D/def2-SVPP level of theory.
Author Contributions
N.R.D.: Conceptualization, methodology, validation, writing—original draft, resources. V.P.S.: validation, formal analysis. J.O.C.J.-H.: computational resources. P.: writing, design. A.G.D.: data curation. D.I.M.-V.: computing resources, visualization. T.J.P.: conceptualization, resources, writing—original draft. S.V.P.: conceptualization, methodology, writing—original draft, review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data created is within the manuscript and Supplementary Materials.
Acknowledgments
The author is thankful to Sarthi, Government of Maharashtra, G. E. Society’s HPT Arts and RYK Science College, Nashik for providing support, encouragement and laboratory facilities.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Dirlam, J.P.; Presslitz, J.E.; Williams, B.J. Synthesis and Antibacterial Activity of Some 3-[(Alkylthio)Methyl]Quinoxaline 1-Oxide Derivatives. J. Med. Chem. 1983, 26, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Synthesis of New Quinoxaline-2-Carboxylate 1,4-Dioxide Derivatives as Anti- Mycobacterium t Uberculosis Agents. J. Med. Chem. 2005, 48, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, N.; Zeng, Z.; Gu, X.; Fang, B.; Yang, F.; Zhang, B.; Ding, H. Tissue Deposition and Residue Depletion of Cyadox and Its Three Major Metabolites in Pigs after Oral Administration. J. Agric. Food Chem. 2013, 61, 9510–9515. [Google Scholar] [CrossRef] [PubMed]
- Parhi, A.K.; Zhang, Y.; Saionz, K.W.; Pradhan, P.; Kaul, M.; Trivedi, K.; Pilch, D.S.; LaVoie, E.J. Antibacterial Activity of Quinoxalines, Quinazolines, and 1,5-Naphthyridines. Bioorg. Med. Chem. Lett. 2013, 23, 4968–4974. [Google Scholar] [CrossRef]
- Ayaz, M.; Xu, Z.; Hulme, C. Novel Succinct Routes to Quinoxalines and 2-Benzimidazolylquinoxalines via the Ugi Reaction. Tetrahedron Lett. 2014, 55, 3406–3409. [Google Scholar] [CrossRef]
- Srinivas, C.; Kumar, C.N.S.S.P.; Rao, V.J.; Palaniappan, S. Efficient, Convenient and Reusable Polyaniline-Sulfate Salt Catalyst for the Synthesis of Quinoxaline Derivatives. J. Mol. Catal. A Chem. 2007, 265, 227–230. [Google Scholar] [CrossRef]
- More, S.V.; Sastry, M.N.V.; Yao, C.-F. Cerium (Iv) Ammonium Nitrate (CAN) as a Catalyst in Tap Water: A Simple, Proficient and Green Approach for the Synthesis of Quinoxalines. Green Chem. 2006, 8, 91–95. [Google Scholar] [CrossRef]
- Bhosale, R.S.; Sarda, S.R.; Ardhapure, S.S.; Jadhav, W.N.; Bhusare, S.R.; Pawar, R.P. An Efficient Protocol for the Synthesis of Quinoxaline Derivatives at Room Temperature Using Molecular Iodine as the Catalyst. Tetrahedron Lett. 2005, 46, 7183–7186. [Google Scholar] [CrossRef]
- Zhao, Z.; Wisnoski, D.D.; Wolkenberg, S.E.; Leister, W.H.; Wang, Y.; Lindsley, C.W. General Microwave-Assisted Protocols for the Expedient Synthesis of Quinoxalines and Heterocyclic Pyrazines. Tetrahedron Lett. 2004, 45, 4873–4876. [Google Scholar] [CrossRef]
- Nagare, Y.K.; Shah, I.A.; Yadav, J.; Pawar, A.P.; Rangan, K.; Choudhary, R.; Iype, E.; Kumar, I. Electrochemical Oxidative Addition of Nucleophiles on 2-Arylindoles: Synthesis of C2-Heteroquaternary Indolin-3-Ones. J. Org. Chem. 2022, 87, 15771–15782. [Google Scholar] [CrossRef]
- Deore, N.R.; Pawar, T.J.; Nagare, Y.K.; Patil, S.V. Electrochemical Synthesis of Imidazopyridine and Benzylidene Malononitrile. Chem. Proc. 2022, 12, 83. [Google Scholar] [CrossRef]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319. [Google Scholar] [CrossRef] [PubMed]
- Pollok, D.; Waldvogel, S.R. Electro-Organic Synthesis—A 21 St Century Technique. Chem. Sci. 2020, 11, 12386–12400. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Rev. E.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Mennucci, B.; Tomasi, J. Ab Initio Study of Ionic Solutions by a Polarizable Continuum Dielectric Model. Chem. Phys. Lett. 1998, 286, 253–260. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comp. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).