Abstract
This study explores the Diels–Alder reaction using spilanthol, a natural diene isolated from Heliopsis longipes roots, to synthesize potentially bioactive compounds. Spilanthol was purified through silica gel column chromatography, yielding 16 g/kg of dried roots, and characterized by 1H NMR spectroscopy. Among the dienophiles tested, only p-anisaldehyde reacted efficiently in the presence of BF3·OEt2 as a Lewis acid catalyst. A cyclic adduct was obtained with yields of 9.72% (endo) and 24.32% (exo). 1H NMR analysis confirmed the formation of a pyran ring, demonstrating the viability of this synthetic pathway for producing functionalized cyclic compounds with potential biological activity.
1. Introduction
Spilanthol 1 (Scheme 1), N-isobutyl-(2E,6Z,8E)-deca-2,6,8-trienamide, is an alkylamide isolated from the roots of Heliopsis longipes, a medicinal plant endemic to regions of Mexico such as the Sierra Álvarez and Sierra Gorda [1]. Its architecture—an unsaturated amide appended to a conjugated diene—renders spilanthol 1 a competent diene for Diels–Alder cycloadditions [2]. Beyond its synthetic utility, spilanthol 1 displays analgesic, anti-inflammatory, antimicrobial, and antioxidant activities, underscoring its pharmacological promise [3]. Leveraging these features, spilanthol 1 can serve as a renewable scaffold for the sustainable construction of bioactive heterocycles.
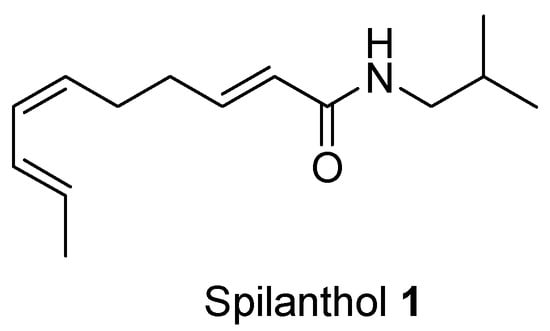
Scheme 1.
Chemical structure of spilanthol 1 (2E,6Z,8E)-N-isobutyl-2,6,8-decatrienamide.
In this study, we assess the feasibility and selectivity of the Diels–Alder reaction between spilanthol 1 and p-anisaldehyde. We further examine the influence of different catalysts on yield and selectivity, aiming to optimize conditions and advance greener, more efficient synthetic methodologies.
2. Materials and Methods
2.1. Extraction and Identification of Spilanthol 1
Ten grams of ground root of H. longipes were macerated in absolute ethanol (1:10 w/v) for one week, and this was repeated three times with fresh solvent. The extract was filtered, evaporated, and analyzed via thin-layer chromatography using hexane/ethyl acetate (3:2), identifying the target compound with an Rf = 0.3. Structural identification was confirmed by proton nuclear magnetic resonance spectroscopy (1H-NMR, 400 MHz, CDCl3), comparing chemical shifts with the reported literature data [4,5].
Spilanthol 1. IR (film) νmax: 3276, 2958, 2928, 2871, 1669, 1629, 1552, 1247, 1159, 980, 947, 820 cm−1.1HNMR (CDCl3, 400 MHz) δH: 6.85 (dt, 1H, J1 15.0 Hz, J2 6.6 Hz, H-3), 6.30–6.26 (m, 1H, H-8), 5.97 (dd, 1H, J1 = J2 = 10.8 Hz, H-7), 5.80 (dt, 1H, J1 15.6 Hz, J2 1.8 Hz, H-2), 5.70 (dq, 1H, J1 12.0, J2 6.0, H-9), 5.52 (br, s, 1H, NH), 5.26 (dt, 1H, J1 10.8 Hz, J2 7.2 Hz, H-6), 3.15 (dd, 2H, J1 = J2 6.3 Hz, H-1′), 2.36–2.22 (m, 4H, H-4 and H-5), 1.82–1.77 (m, 2H, H-2′ and H-10), 0.92 (d, 1H, J 6.0 Hz, H-3′ and H-4′) ppm; 13CNMR (CDCl3, 100 MHz) δC: 165.9 (C=O), 143.4 (C-3), 129.9 (C-9), 129.4 (C-7), 127.8 (C-6), 126.6 (C-8), 124.1 (C-2), 46.8 (C-1′), 32.1 (C-4), 28.6 (C-2′), 26.4 (C-5), 20.1 (C-3′ andC-4′), 18.3 (C-10) ppm; EIMS m/z (%) 221 (M+, 6), 141 (61), 126 (17), 98 (18), 81 (100), 79 (19), 41 (31); HREIMS m/z 221.1801 (calcd for C14H23NO 221.1779).
2.2. Diels-Alder Reaction
A solution of spilanthol 1 (1 mmol), the corresponding dienophile (1 mmol), and 5 mol% of catalyst in dry tetrahydrofuran (THF, 0.2 M) was stirred for 24 h under two conditions—at room temperature and under reflux —while monitoring progress by TLC and visualized with potassium permanganate; upon completion, distilled water was added, and the mixture was extracted with dichloromethane (3 × 25 mL), after which the combined organic layers were dried, filtered, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using a hexane/ethyl acetate gradient (100:0 to 80:20), affording a purified adduct that was analyzed by 1H-NMR to determine chemical structure.
Compound 2: 1H NMR (400 MHz, CDCl3) δ 1H NMR (400 MHz, CDCl3) δ 7.40 (m), 6.80 (m), 5.90 (d), 5.75 (m), 5.58 (Br s), 5.35 (m), 4.20 (m), 2.40 (m, 1H), 1.95 (m, 2H), 1.90 (s, 3H), 1.01 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 166.0, 145.9, 138.6, 132.9, 129.0, 128.9, 128.4, 128.2, 126.1, 124.1, 122.4, 82.9, 77.0, 47.1, 36.4, 35.7, 28.7, 26.9, 20.4, 20.3, 15.2; EIMS m/z (%) 327 (M+, 16), 328 (23), 133 (17), 98 (18), 81 (100); HREIMS m/z 327.2215 (calcd for C21H29NO2 327.2201).
3. Results and Discussion
3.1. Isolation and Characterization of Spilanthol 1
The isolated compound, purified from the ethanolic extract of Heliopsis longipes, was then subjected to 1H NMR analysis. The spectrum exhibited characteristic chemical shift signals corresponding to specific proton environments. Three methyl group signals (–CH3) were observed at δH 0.93 and 1.74 ppm, along with a methine proton (–CH–) at δH 1.76 ppm. Three methylene group signals (–CH2–) were detected in the δH 2.35–2.40 ppm region. Additionally, five olefinic protons (C=CH) were identified in the δH 5.59–6.00 ppm range. The proton signals were consistent with those previously reported in the literature [6], supporting the structural identification of the compound. The extraction yielded approximately 16 g of Spilanthol 1 per kilogram of dried root material.
3.2. Diels-Alder Reaction Between Spilanthol 1 and p-Anisaldehyde
The Diels-Alder reaction between spilanthol 1 and p-anisaldehyde was examined to probe diene–dienophile reactivity under thermal conditions and in the presence of Lewis acids (Scheme 2). Reactions were first run without a catalyst in dry THF, exploring two temperature regimes to distinguish kinetic control (favouring the endo adduct) from thermodynamic control (favouring the more stable exo isomer) [7].
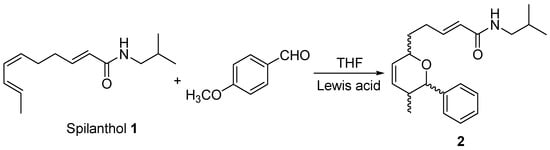
Scheme 2.
Reaction of spilanthol with p-anisaldehyde.
Under catalyst-free conditions, no appreciable cycloaddition was detected, indicating that activation of the dienophile is required. This lack of reactivity is consistent with insufficient orbital overlap between the HOMO of spilanthol 1 and the LUMO of p-anisaldehyde and with an unfavourable HOMO–LUMO gap that hampers formation of the cycloaddition transition state [8].
To promote activation, several Lewis acids were screened—AuCl3, AlCl3, AgSbF6, and BF3·OEt2 (Table 1). Of these, only BF3·OEt2 effectively delivered the Diels–Alder adducts, attributable to coordination at the aldehyde carbonyl, which stabilizes the dienophile LUMO and narrows the HOMO–LUMO gap, thereby facilitating the transition state [9].

Table 1.
Catalysts used in the synthesis.
The purified compound obtained from the Diels–Alder reaction was characterized by 1H-NMR to determine its structural identity, as well as the spectral purity and integrity of the isolated cycloadduct. Figure 1 shows the 1H-NMR spectrum of the compound recorded in chloroform at 400 MHz.
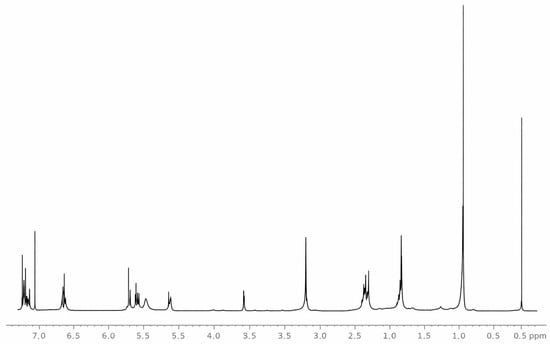
Figure 1.
1H NMR spectrum of the Diels-Alder Adduct recorded in CDCl3 at 400 MHz.
In the aliphatic region, signals were observed at δH 1.01 and 1.90 ppm, corresponding to methyl protons (–CH3). An additional signal at δH 1.95 ppm was attributed to methylene protons (–CH2–), while the resonance at δH 2.40 ppm was assigned to a methine proton (–CH–). Signals at δH 5.58, 5.75, 5.90, and 6.80 ppm were characteristic of olefinic protons (C=C). Finally, the signal at δH 7.40 ppm indicated the presence of aromatic protons. Collectively, these resonances support the proposed structure of the compound 2 as a pyran, a six-membered heterocyclic system composed of five carbon atoms and one oxygen atom, bearing an unsaturation (double bond) [10].
These findings are relevant for the synthesis of functionalized cyclohexene-type scaffolds, with potential applications in the development of bioactive compounds. Moreover, the fact that the reaction proceeds under mild conditions and employs acceptable solvents positions it as a strategy aligned with the principles of green chemistry, by enabling the formation of new molecular architectures without the need for intermediate steps or protecting groups [11].
4. Conclusions
Spilanthol 1 was successfully isolated and purified from the dried roots of Heliopsis longipes, and 1H-NMR confirmed its structural identity. Its reactivity as a diene in the Diels–Alder reaction was evaluated, and it was found that only p-anisaldehyde, in the presence of the Lewis acid catalyst BF3·OEt2, enabled the formation of the desired cyclic adduct.
The study confirmed that the reaction proceeds through a hetero-Diels–Alder mechanism, as evidenced by the formation of a six-membered oxygen-containing ring, consistent with a pyran structure. Furthermore, both the yield and the stereoselectivity of the reaction were strongly influenced by the reaction conditions and the nature of the catalyst used.
These findings validate the use of Spilanthol 1 as a natural diene in cycloaddition reactions with synthetic potential, under conditions compatible with the principles of green chemistry. The developed methodology provides a solid foundation for synthesising structurally complex derivatives with potential biological activity.
Author Contributions
Methodology, R.B.-V.; conceptualization, M.M.-A. and J.G.M.; writing—review and editing, E.I.J.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI) (Grant No. CBF-2025-I-2852).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the Secretaría de Investigación y Posgrado/Instituto Politécnico Nacional (SIP/IPN) for their valuable support and resources.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cilia-Lopez, V.G.; Aguirre-Rivera, J.R.; Reyes-Agüero, J.A.; Juárez-Flores, B.I. Ethnobotany of Heliopsis longipes (Asteraceae: Heliantheae). Bol. Soc. Bot. Méx. 2008, 83, 81–87. [Google Scholar]
- Skolia, E.; Kokotos, C.G. Direct Diels-Alder Reaction of Biomass-Derived Furfurol with Maleimides in a Bio-Based Green Solvent. EurJOC 2024, 27, e202400105. [Google Scholar] [CrossRef]
- Abdul-Rahim, R.; Ayu-Jayusman, P.; Muhammad, N.; Mohamed, N.; Lim, V.; Hazwani Ahmad, N.; Mohamad, S.; Abdul-Hamid, Z.A.; Ahmad, F.; Mokhtar, N.; et al. Potential Antioxidant and Anti-Inflamatory Effects of Spilanthes acmella and Its Health Beneficial Effects: A Review. Int. J. Environ. Res. Public Health 2021, 18, 3532. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, I.; Takeya, K.; Itokawa, H. The geometric structure of spilanthol. Chem. Pharm. Bull. 1980, 28, 2251–2253. [Google Scholar] [CrossRef]
- Nakatani, N.; Nagashima, M. Pungent Alkamides from Spilanthes acmella L. var. oleracea Clarke. Biosci. Biotechnol. Biochem. 1992, 56, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Alperth, F.; Erhart, S.; Kunert, O.; Bucar, F. Simple Green Purification of Spilanthol from Natural Deep Eutectic Solvent and Ethanolic Acmella oleracea (L.) R.K. Jansen Extracts Using Solid-Phase Extraction. Separations 2024, 11, 251. [Google Scholar] [CrossRef]
- Houk, K.N.; Liu, F.; Yang, Z.; Seeman, J.I. Evolution of the Diels-Alder Reaction Mechanism Since the 1930s: Woodward, Houk with Woodward, and the Influence of Computational Chemistry on Understanding Cycloadditions. Angew. Chem. Int. Ed. 2020, 60, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-Y.; Gu, A.; Li, J.; Liu, Y. Advanced green synthesis: Solvent-free and catalyst-free reaction. Green Synth. Catal. 2025, 6, 36–66. [Google Scholar] [CrossRef]
- Vermeeren, P.; Hamlin, T.A.; Fernéndez, I.; Bickelhaupt, F.M. How Lewis Acids Catalyze Diels-Alder Reactions Angew. Chem. Int. 2020, 59, 6201–6206. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Lone, W.I.; Rashid, A.; Bhat, B.A. Inverse electron demand Diels-Alder reaction in total synthesis of bioactive natural products. Tetrahedron. Chem. 2024, 9, 100066. [Google Scholar] [CrossRef]
- Raiol, A.; Pinheiro, M.; Belo, E.; Da Cunha, R.A.; Marinho, A.M.R.; Silva, S.Y.S.; Silva, S.C.; Andrade-Filho, T.; Gester, R. Experimental and Theoretical Spectroscopic Characterization, NLO Response, and Reactivity of the Pharmacological Agent Spilanthol and Analogues. J. Mol. Struct. 2021, 1227, 129423. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).