Abstract
Phenylazocarboxylate is described as a novel non-organometallic aryl nucleophile in palladium-catalyzed arylation.
1. Introduction
The arylation of aromatic electrophiles (halogenoaromatics, triflates, diazoniums) catalyzed by palladium is known [1,2,3] to occur with numerous organometallic aryl nucleophiles of boron (Suzuki-Miyaura), silicon (Hiyama), tin (Stille), zinc (Negishi) and mercury (Heck) [4], which obviously leads to sub-products containing metals (Figure 1).
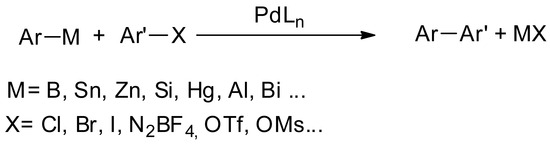
Figure 1.
Arylation of aromatic electrophiles with aryl organometallics as nucleophiles.
If boron and silicon are only slightly or not toxic, a large majority of metals present a toxicity, which can be troublesome during the synthesis of molecules for therapeutic purposes. This is particularly the case for tin in the Stille reaction.
2. Results and Discussion
We investigated the opportunity to use a non-organometallic phenyl anion derived from an elimination reaction as a nucleophilic arylation agent. Several tests using a carboxylate or a sulfinate were not successful, but, on the other hand, using phenylazocarboxylate led to interesting results.
Phenylazacarboxylate was first introduced by Nesmeyanov and Reutov in the synthesis of arylmercury and arylantimony componds [5,6].
To the best our knowledge, this compound was not reused and was never tested in catalyzed palladium couplings. During phenylation, one molecule of nitrogen and one molecule of carbon dioxide are removed (Figure 2).

Figure 2.
Arylation of palladium by phenylazocarboxylate.
The precursor of phenylazocarboxylate is the commercially available 1-phenylsemicarbazide, which can be oxidized to produce 1-phenyldiazocarboxamide (Figure 3). The latter can be converted into potassium phenylazocarboxylate by saponification with potassium hydroxide, under ultrasound irradiation, without heating, with a yield of 81% (Figure 3).

Figure 3.
Formation of phenylazocarboxamide and phenylazocarboxylate.
Several coupling tests of this phenylazocarboxylate were carried out with palladium catalysts with potassium hydroxide as a base with MSTPP as a ligand or with cesium carbonate with a carbene ligand formed with H-Imes, Cl (Figure 4).

Figure 4.
Couplings of phenylazocarboxylate.
A mixture of products, benzene, diphenyl and coupling product, were formed during the reaction. An excess of potassium phenylazocarboxylate (two equivalents relative to the electrophile) led to better coupling product yields. The reaction with potassium phenylazocarboxylate gave a better yield of coupling product at 60 °C than at 35 °C under microwave irradiation. The results obtained are reported in Table 1. The ligands used in the couplings are reported in Figure 5.

Table 1.
Coupling of phenylazocarboxylate in the presence of palladium catalysts.
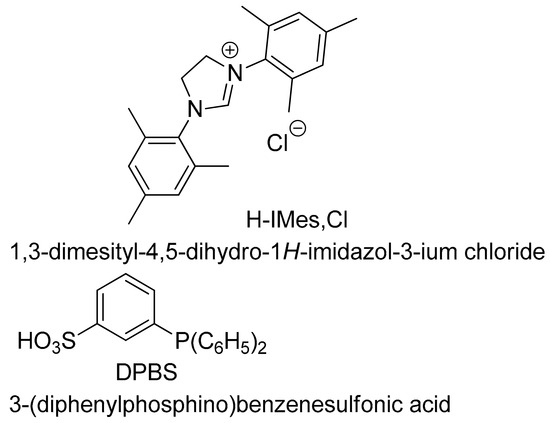
Figure 5.
Ligands used in aryl couplings.
Therefore, it appears that arylazocarboxylates can be non-organometallic arylnucleophiles in aromatic arylations.
3. Experimental
3.1. General Information
The 1H NMR and 13C NMR spectra were recorded on a Bruker DPX 250 and DPX 400 spectrometer at 250 and 400 MHz. (Bruker corporation, Billerica, MA, USA) The samples were recorded in CDCl3 solutions using TMS as an internal standard. The chemical shifts are expressed in δ units (ppm) and quoted downfield from TMS.
The various analyses were carried out by GC/MS to identify the products, which were then measured by GC (Varian CP 3800(GC) and Saturn 2000 (MS/MS) and column CPSIL8CB (30 m × 0.25 nm) 220 °C, gaz He.) (Waters, Milford, MA, USA).
Microwave irradiations were performed at 2450 MHz with a Prolabo Synthewave 402 under argon.
3.2. Starting Products
1-phenylazocarboxamide, RN:4203-28-5.
A total of 7.55 g of 1-phenylsemicarbazide (0.05 mol) is dissolved in 150 mL of glacial acetic acid. A total of 5 g of chromic anhydride in 50 mL of water is then added slowly while stirring. The mixture is stirred for 5 days at room temperature and then poured into 500 mL of water. The aqueous phase is discarded and the organic phase is evaporated. In total, 7.17 g of a brown solid are then obtained (yield = 96%).
mp:115 °C (litt. = 112–114 °C , Chern S. F. Jr., J. Org. Chem. 1977, 42, 178–179).
1H NMR (CDCl3, 400 MHz): 6.35–6.49 (m wide, 2H, NH2); 7.50–7.60 (m, 3H, Ar-H); 7.95 (d, 7,0 Hz, 2H, Ar-H). UV-Vis (ethanol): λmax = 432 nm.
Potassium phenylazocarboxylate, RN:13444-03-6.
A total of 1 g of phenylazacarboxamide (6.7 mmol) is dissolved in a minimum amount of water. A total of 2 mL of 50% potassium hydroxide is added with stirring. The mixture was irradiated by ultrasounds with an ultrasonic laboratory bath for 4 h under a nitrogen atmosphere at 20 °C. The mixture is then cooled to 0 °C and the precipitated product is recovered by filtration on a Büchner funnel. The flask is rinsed with diethyl ether. In total, 1.02 g of a brown solid is obtained (yield = 81%). This solid decomposes quite violently at around 160 °C.
Potassium phenylazocarboxylate is stable in basic aqueous media before filtration and can be stored without decomposition in aqueous frozen form. It is sufficient to melt the medium and recover the salt by filtration just before use. In neutral or acidic media, the salt decomposes at room temperature quickly with a release of gas.
3.3. Couplings
Example of coupling with 4-iodotoluene.
Condition a: Potassium phenylazocarboxylate/R-C6H4-I/Pd2(dba)3/DPBS/KOH = 2/1/0.01/0.04/2; dioxane/water = 10/1, microwave at 60 °C for 6 min.
In a typical experiment, potassium phenylazocarboxylate (2 mmol.), 4-iodotoluene (1 mmol,), potassium hydroxide (2.2 mmol), Pd2(dba)3 (0.01 mmol), DPBS (0.04 mmeq.) are dissolved in dioxane/water (10 mL:1 mL) under argon. The mixture is stirred and irradiated under microwaves at 60 °C for 6 min. The reaction mixture is analyzed by GC/MS. The ether extraction of the reaction mixture provides 4-methylbiphenyle.
Condition b: Potassium phenylazocarboxylate/R-C6H4-I/Pd2(dba)3/HIMes,Cl/Cs2CO3 = 2/1/0.01/0.04/1; dioxane/acetonitrile = 1/1, microwave at 60 °C for 6 min.
4. Conclusions
We have shown that arylazocarboxylates can be efficient non-organometallic arylnucleophiles in aromatic arylations. Moreover, arylazocarboxylates are stable as solids and can be easily prepared.
The coupling reactions take place in green conditions, and the only by-products are nitrogen, carbon dioxide and a potassium salt.
Author Contributions
Conceptualization, D.V.; investigation, D.V. and A.J.; writing—review and editing, D.V. and N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Acknowledgments
The authors thank Karine Jarsalé for the mass spectrometry spectra.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Magano, J.; Dunetz, J.R. Transition Metal-Catalyzed Couplings in Process Chemistry; Wiley: Hoboken, NJ, USA, 2003; ISBN 9783527332793. [Google Scholar] [CrossRef]
- Foubelo, F.; Nájera, C.; Yus, M. The Hiyama Cross-Coupling Reaction: New Discoveries. Chem. Rec. 2016, 16, 2521–2533. [Google Scholar] [CrossRef] [PubMed]
- KKostasloannis, D.; Kostas, D. Suzuki-Miyaura Cross_Coupling Reaction and Potential Applications; MDPI: Basel, Switzerland, 2022. [Google Scholar] [CrossRef]
- Heck, R.F.; Nolley, J.P., Jr. Acylation, methylation, and carboxyalkylation of olefins by Group VIII metal derivatives. J. Am. Chem. Soc. 1968, 90, 5518–5526. [Google Scholar] [CrossRef]
- Nesmeyanov, A.N.; Reutov, O.A. Chem. Abstr. 1948, 43, 171b.
- Reutov, O.A.; Bundel, Y.G. Synthesis of aromatic arsenic-organic compounds through arylazocarboxylic salts. Russ. Chem. Bull. 1952, 1, 911–916. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).