On Novel Non-Organometallic Aryl Nucleophile in Palladium-Catalyzed Arylation †
Abstract
1. Introduction
2. Results and Discussion
3. Experimental
3.1. General Information
3.2. Starting Products
3.3. Couplings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magano, J.; Dunetz, J.R. Transition Metal-Catalyzed Couplings in Process Chemistry; Wiley: Hoboken, NJ, USA, 2003; ISBN 9783527332793. [Google Scholar] [CrossRef]
- Foubelo, F.; Nájera, C.; Yus, M. The Hiyama Cross-Coupling Reaction: New Discoveries. Chem. Rec. 2016, 16, 2521–2533. [Google Scholar] [CrossRef] [PubMed]
- KKostasloannis, D.; Kostas, D. Suzuki-Miyaura Cross_Coupling Reaction and Potential Applications; MDPI: Basel, Switzerland, 2022. [Google Scholar] [CrossRef]
- Heck, R.F.; Nolley, J.P., Jr. Acylation, methylation, and carboxyalkylation of olefins by Group VIII metal derivatives. J. Am. Chem. Soc. 1968, 90, 5518–5526. [Google Scholar] [CrossRef]
- Nesmeyanov, A.N.; Reutov, O.A. Chem. Abstr. 1948, 43, 171b.
- Reutov, O.A.; Bundel, Y.G. Synthesis of aromatic arsenic-organic compounds through arylazocarboxylic salts. Russ. Chem. Bull. 1952, 1, 911–916. [Google Scholar] [CrossRef]
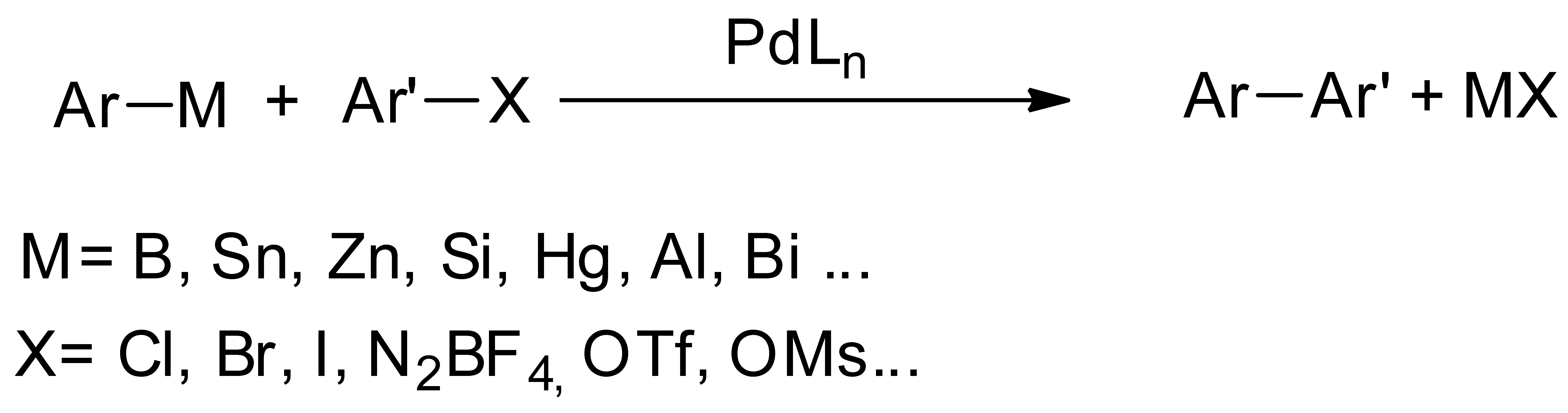

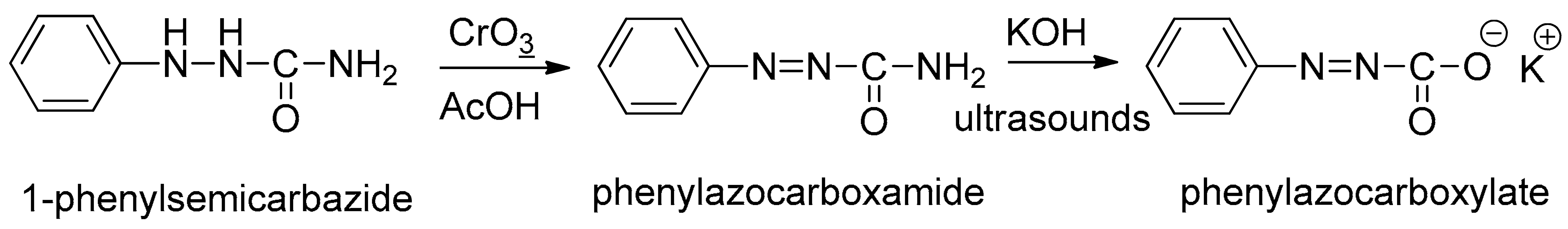


| Entry | R | Conditions | Yield (%) of C6H5Ar |
|---|---|---|---|
| 1 | H | a MW 60 °C, 6 min | 68 |
| 2 | CH3 | a MW 60 °C, 6 min | 62 |
| 3 | OCH3 | a MW 60 °C, 6 min | 58 |
| 4 | COOCH3 | a MW 60 °C, 6 min | 75 |
| 5 | NO2 | a MW 60 °C, 6 min | 80 |
| 6 | H | b MW 60 °C, 6 min | 75 |
| 7 | OCH3 | b MW 60 °C, 6 min | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villemin, D.; Jullien, A.; Bar, N. On Novel Non-Organometallic Aryl Nucleophile in Palladium-Catalyzed Arylation. Chem. Proc. 2024, 16, 97. https://doi.org/10.3390/ecsoc-28-20147
Villemin D, Jullien A, Bar N. On Novel Non-Organometallic Aryl Nucleophile in Palladium-Catalyzed Arylation. Chemistry Proceedings. 2024; 16(1):97. https://doi.org/10.3390/ecsoc-28-20147
Chicago/Turabian StyleVillemin, Didier, Arnaud Jullien, and Nathalie Bar. 2024. "On Novel Non-Organometallic Aryl Nucleophile in Palladium-Catalyzed Arylation" Chemistry Proceedings 16, no. 1: 97. https://doi.org/10.3390/ecsoc-28-20147
APA StyleVillemin, D., Jullien, A., & Bar, N. (2024). On Novel Non-Organometallic Aryl Nucleophile in Palladium-Catalyzed Arylation. Chemistry Proceedings, 16(1), 97. https://doi.org/10.3390/ecsoc-28-20147






