Abstract
Macrocycles represent an important class of compounds that are widespread in nature. Of particular interest to researchers are aromatic macrocyclic compounds, which, due to their rigid structure and unique physicochemical properties, can find application in many areas of science, industry and medicine. Previously, we synthesized polyether aromatic macrodiolides, which showed intriguing antitumor properties. In the work, Peyrottes S. and co-authors showed that the introduction of biphenyl or naphthyl rings, as well as triple bonds, into the structure of the compounds they synthesized, not only helps to reduce the molecular flexibility of the molecule, but also increases the bioavailability after oral administration of the corresponding neutral prodrugs. Studies in mice have shown that the presence of two aromatic groups is well tolerated and has resulted in compounds with valuable properties in vitro and in vivo. Based on these results, in continuation of our research on the synthesis of biologically active macrodiolides, in the framework of this work, new aromatic macrocycles were synthesized, the structure of which, along with the 1Z,5Z-diene fragment, contains phenyl or naphthyl rings. The target polyester macrodiolides were obtained by Hf-catalyzed intermolecular cyclocondensation of 1,14-tetradeca-5Z,9Z-dienedioic acid with diols synthesized from dihydroxybenzenes and naphthalenediols.
1. Introduction
Most aromatic macrocycles contain a phenyl fragment with various substituents; macrocycles with biphenyl or naphthalene functional groups are less common. At the same time, the naphthalene framework, due to its diverse biological activity, is a promising building block in the development of drugs. In particular, new derivatives of naphthalene, and hybrid molecules based on it, are known, which exhibit antiviral, antibacterial, fungicidal, and antitumor properties [1,2,3,4,5,6].
Currently, a large number of naphthalene-based drugs, such as naphyrone, tolnaftate, naftifine, nafcillin, terbinafine, propranolol, nabumetone, nafimidone, naproxen, etc., are approved by the FDA and are sold as therapeutic agents [7,8,9,10,11,12].
Over the past few years, our research group under the direction of prof. V. A. D’yakonov has been conducting research in the field of synthesis of unsaturated macrocyclic compounds that demonstrate good antitumor activity [13,14,15,16,17,18,19]. Recently, we have obtained polyether aromatic macrodiolides that are effective inducers of apoptosis in tumor cells [19]. In connection with the interesting properties of compounds with a naphthalene skeleton in the structure, in the development of our research within the framework of this work, the idea of synthesizing new polyether aromatic macrodiolides containing a 1Z,5Z-diene fragment, including together with a naphthalene framework, arose.
2. Results and Discussion
Previously, we showed that direct cyclocondensation between α,ω-alka-nZ,(n+4)Z-dienedioic acids and dihydroxybenzenes or naphthalenediols does not occur; however, if the hydroxyl group is located at a distance from the aromatic ring, the occurrence of these reactions becomes possible [18,19]. In connection with the above, in order to obtain new synthetic aromatic macrocycles, we synthesized diols 5a–e, obtained in two stages from dihydroxybenzenes (pyrocatechol, resorcinol, hydroquinone) and naphthalenediols (naphthalene-2,6-diol, naphthalene-2,7-diol) with ethyl bromoacetate [20]. The synthesis of the target macrodiolides was accomplished by cyclocondensation of 1,14-tetradeca-5Z,9Z-dienedioic acid 4 with aromatic diols 5a–e (Scheme 1).
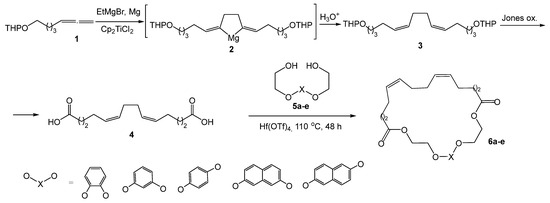
Scheme 1.
Synthesis of aromatic polyether macrodiolides.
Based on our previous studies [18,19], macrocyclization using carbodiimides (DCC, EDCI) catalyzed by 4-dimethylaminopyridine (DMAP) was studied, but in these reactions it was not possible to achieve acceptable yields of the target products. At the same time, intermolecular cyclocondensation catalyzed by Hf(OTf)4 allows the synthesis of target macrocycles with good yields (64–75%).
3. Materials and Methods
Chemistry
NMR spectra were recorded in CDCl3 on Bruker Ascend 500 ((500 MHz (1H), 126 MHz (13C)) instruments. The mass spectra were obtained on an UltraFlex III TOF/TOF (Bruker Daltonik GmbH, Bremen, Germany) operating in linear (TOF) and reflection (TOF/TOF) positive and negative ion modes. Macrocyclic compounds were synthesized similarly according to the procedure described in the literature [14].
(9Z,13Z)-2,3,6,7,8,11,12,15,16,17,20,21-dodecahydrobenzo[e][1,4,7,10]tetraoxacyclotetracosine-5,18-dione (6a). White waxy solid; yield 72%. 1H NMR (500 MHz, CDCl3): δ = 6.94–6.86 (m, 4H), 5.48–5.31 (m, 4H), 4.56–4.48 (m, 4H), 4.36–4.28 (m, 4H), 2.34–2.23 (m, 4H), 2.11–1.95 (m, 8H), 1.71–1.64 (m, 4H). 13C NMR (126 MHz, CDCl3): δ = 173.6, 149.7, 130.1, 129.2, 122.3, 116.8, 66.7, 62.5, 33.3, 27.6, 26.7, 24.7. ESI-MS: calcd. for C24H32O6 + H+ [M + H]+ 417.2272; found 417.2281
(10Z,14Z)-2,5,20,23-tetraoxa-1(1,3)-benzenacyclotricosaphane-10,14-diene-6,19-dione (6b). White waxy solid; yield 75%. 1H NMR (500 MHz, CDCl3): δ = 7.21 (t, J = 7,8 Hz, 1H), 6.57–6.50 (m, 3H), 5.46–5.32 (m, 4H), 4.52–4.46 (m, 4H), 4.36–4.24 (m, 4H), 2.33–2.19 (m, 4H), 2.11–1.94 (m, 8H), 1.70–1.62 (m, 4H). 13C NMR (126 MHz, CDCl3): δ = 173.5, 159.6, 130.1, 130.0, 129.2, 107.2, 101.9, 68.2, 64.1, 33.4, 27.7, 26.6, 24.8. ESI-MS: calcd. for C24H32O6 + Na+ [M + Na]+ 439.2091; found 439.2082
(10Z,14Z)-2,5,20,23-tetraoxa-1(1,4)-benzenacyclotricosaphane-10,14-diene-6,19-dione (6c). White waxy solid; yield 71%. 1H NMR (500 MHz, CDCl3): δ = 6.86 (s, 4H), 5.45–5.32 (m, 4H), 4.51–4.40 (m, 4H), 4.37–4.27 (m, 4H), 2.35–2.22 (m, 4H), 2.12–1.91 (m, 8H), 1.74–1.68 (m, 4H). 13C NMR (126 MHz, CDCl3): δ = 173.5, 152.5, 130.2, 129.1, 115.4, 66.5, 62.4, 33.4, 27.5, 26.8, 24.8. ESI-MS: calcd. for C24H32O6 + H+ [M + H]+ 417.2272; found 417.2279
(10Z,14Z)-2,5,20,23-tetraoxa-1(2,7)-naphthalenacyclotricosaphane-10,14-diene-6,19-dione (6d). White waxy solid; yield 67%. 1H NMR (500 MHz, CDCl3): δ = 7.69 (d, J = 8.3 Hz, 2H), 7.13–7.04 (m, 4H), 5.29–5.17 (m, 4H), 4.60–4.48 (m, 4H), 4.36–4.24 (m, 4H), 2.44–2.30 (m, 4H), 2.14–1.87 (m, 8H), 1.70–1.62 (m, 4H). 13C NMR (126 MHz, CDCl3): δ = 173.6, 157.2, 135.7, 131.9, 130.4, 129.2, 124.8, 116.2, 107.4, 66.4, 62.8, 33.5, 27.2, 26.9, 24.7. ESI-MS: calcd. for C28H34O6 + H+ [M + H]+ 467.2428; found 467.2439
(10Z,14Z)-2,5,20,23-tetraoxa-1(2,6)-naphthalenacyclotricosaphane-10,14-diene-6,19-dione (6e). White waxy solid; yield 64%. 1H NMR (500 MHz, CDCl3): δ = 7.63 (d, J = 5.8 Hz, 2H), 7.17–7.07 (m, 4H), 5.35–5.16 (m, 4H), 4.55–4.46 (m, 4H), 4.36–4.25 (m, 4H), 2.39–2.21 (m, 4H), 2.06–1.92 (m, 8H), 1.76–1.65 (m, 4H). 13C NMR (126 MHz, CDCl3): δ = 173.6, 155.5, 131.2, 130.2, 129.9, 128.2, 125.0, 119.3, 66.5, 63.2, 33.6, 27.2, 26.4, 24.7. ESI-MS: calcd. for C28H34O6 + H+ [M + H]+ 467.2428; found 467.2431
4. Conclusions
As a result of the conducted research, stereoselective synthesis of polyether aromatic macrodiolides containing pharmacophoric 1Z,5Z-diene, phenyl, and naphthyl fragments was carried out for the first time with yields of 64–75%.
Author Contributions
Conceptualization, I.I.; methodology, validation, and execution of chemistry experiments, I.G. and I.I.; manuscript preparation I.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded within approved plans for research projects at the IPC RAS State Registration No. FMRS-2022-0075.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Acknowledgments
The structural studies of the synthesized compounds were performed with the use of Collective Usage Centre “Agidel” at the Institute of Petrochemistry and Catalysis of RAS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Caldarelli, S.; Fangour, S.E.; Wein, S.; Tran Van Ba, C.; Périgaud, C.; Pellet, A.; Vial, H.; Peyrottes, S. New Bis-thiazolium Analogues as Potential Antimalarial Agents: Design, Synthesis, and Biological Evaluation. J. Med. Chem. 2013, 56, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Peyrottes, S.; Caldarelli, S.; Wein, S.; Périgaud, C.; Pellet, A.; Vial, H. Choline analogues in malaria chemotherapy. Curr. Pharm. Des. 2012, 18, 3454–3466. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abozeid, M.A.; El-Sawi, A.A.; Abdelmoteleb, M.; Awad, H.; Abdel-Aziz, M.M.; Abdel-Rahman, A.R.H.; El-Desoky, E.S.I. Synthesis of novel naphthalene-heterocycle hybrids with potent antitumor, anti-inflammatory and antituberculosis activities. RSC Adv. 2020, 10, 42998–43009. [Google Scholar] [CrossRef] [PubMed]
- Mahesha, P.; Shetty, N.S. Naphthyl-Based Chalcone Derivatives: A Multifaceted Player in Medicinal Chemistry. ChemistrySelect 2024, 9, e202400522. [Google Scholar] [CrossRef]
- Wang, G.; Liu, W.; Peng, Z.; Huang, Y.; Gong, Z.; Li, Y. Design, synthesis, molecular modeling, and biological evaluation of pyrazole-naphthalene derivatives as potential anticancer agents on MCF-7 breast cancer cells by inhibiting tubulin polymerization. Bioorg. Chem. 2020, 103, 104141. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Jia, J.J.; Zhong, Q.; Zhong, X.; Zheng, S.; Wang, G.; He, L. Synthesis and anticancer activity evaluation of naphthalene-substituted triazole spirodienones. Eur. J. Med. Chem. 2021, 213, 113039. [Google Scholar] [CrossRef] [PubMed]
- Makar, S.; Saha, T.; Singh, S.K. Naphthalene, a versatile platform in medicinal chemistry: Sky-high perspective. Eur. J. Med. Chem. 2019, 161, 252–276. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.E.; Aroulanda, C.; Celebre, G.; Merlet, D.; De Luca, G. The conformational behaviour of naproxen and flurbiprofen in solution by NMR spectroscopy. New J. Chem. 2015, 39, 9086–9097. [Google Scholar] [CrossRef]
- Wang, X.; Xing, Y.; Su, J.; Wang, C.; Wang, Z.; Yu, Y.; Xu, H.; Ma, D. Synthesis of two new naphthalene-containing compounds and their bindings to human serum albumin. J. Biomol. Struct. Dyn. 2021, 39, 3435–3448. [Google Scholar] [CrossRef] [PubMed]
- Elrayess, R.A.; Elshihawy, H. Naphthalene: An overview. Rec. Pharm. Biomed. Sci. 2023, 7, 145–153. [Google Scholar] [CrossRef]
- Frolov, N.A.; Seferyan, M.A.; Detusheva, E.V.; Son, E.; Kolmakov, I.G.; Kartseva, A.S.; Firstova, V.V.; Vereshchagin, A.N.; Elinson, M.N. Development of Naphthalene-Derivative Bis-QACs as Potent Antimicrobials: Unraveling Structure–Activity Relationship and Microbiological Properties. Molecules 2024, 29, 5526. [Google Scholar] [CrossRef]
- Mishra, P.; Sethi, P.; Kumar, S.; Rathi, P.; Umar, A.; Kumar, R.; Baskoutas, S. Synthesis and Biomedical Applications of Macrocyclic Complexes. J. Mol. Struct. 2024, 1317, 139098. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Dzhemilev, U.M. Natural compounds with bis-methylene-interrupted Z-double bonds: Plant sources, strategies of total synthesis, biological activity, and perspectives. Phytochem. Rev. 2021, 20, 325–342. [Google Scholar] [CrossRef]
- Dzhemileva, L.U.; D’yakonov, V.A.; Islamov, I.I.; Yunusbaeva, M.M.; Dzhemilev, U.M. New 1Z,5Z-diene macrodiolides: Catalytic synthesis, anticancer activity, induction of mitochondrial apoptosis, and effect on the cell cycle. Bioorg. Chem. 2020, 99, 103832. [Google Scholar] [CrossRef] [PubMed]
- Islamov, I.I.; Yusupova, A.V.; D’yakonov, V.A.; Dzhemilev, U.M. Synthesis of polyether macrodiolides based on acetylenic derivatives of (5Z, 9Z)-tetradeca-5, 9-diene-1, 14-dioic acid. Russ. Chem. Bull. 2023, 72, 2473–2483. [Google Scholar] [CrossRef]
- Islamov, I.I.; Makarov, A.A.; Makarova, E.K.; Yusupova, A.V.; D’yakonov, V.A.; Dzhemilev, U.M. Synthesis of macrocyclic and linear compounds with 1 Z, 5 Z-diene and alkynylcarbinol fragments based on (5 Z, 9 Z)-tetradeca-5, 9-diene-1, 14-diol. Russ. Chem. Bull. 2023, 72, 925–931. [Google Scholar] [CrossRef]
- Islamov, I.I.; Gaisin, I.V.; Dzhemilev, U.M.; D’yakonov, V.A. Synthesis of macrocyclic mono-and diolides based on new ω-hydroxyalkadienoic acids with (Z, Z)-1, 5-diene moiety. Russ. Chem. Bull. 2024, 73, 1623–1630. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Islamov, I.I.; Dzhemileva, L.U.; Makarova, E.K.; Dzhemilev, U.M. Direct synthesis of polyaromatic cyclophanes containing bis-methylene-interrupted Z-double bonds and study of their antitumor activity in vitro. Int. J. Mol. Sci. 2021, 22, 8787. [Google Scholar] [CrossRef] [PubMed]
- Islamov, I.I.; Dzhemileva, L.U.; Gaisin, I.V.; Dzhemilev, U.M.; D′yakonov, V.A. New Polyether Macrocycles as Promising Antitumor Agents─ Targeted Synthesis and Induction of Mitochondrial Apoptosis. ACS Omega 2024, 9, 19923–19931. [Google Scholar] [CrossRef]
- Matsumoto, C.; Yasutake, K.J.; Nishino, H. Synthesis of naphthalenophane-type macrocyclic compounds using Mn (III)-based dihydrofuran-clipping reaction. Tetrahedron 2016, 72, 6963–6971. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).