Abstract
Bis-heterocyclic compounds containing imidazo[1,2-a]pyridines (IMPs) are privileged heterocyclic drug scaffolds due to their potential applications. The Groebke–Blackburn–Bienaymé reaction (GBBR) is a greener alternative to synthesizing IMPs. On the other hand, 1,2,3-triazole scaffolds are biososteres of the trans amide, and their incorporation in bioactive molecules provides advantages such as resistance to cleavage mediated by proteases and improved stability. In this context, the CuAAC reaction is the most efficient approach to synthesizing 1,4-disustituted-1,2,3-triazoles. Herein, we describe a novel one-pot synthesis of IMPs by the GBBR-CuAAC strategy assisted by microwave irradiation.
1. Introduction
Imidazo[1,2-a]pyridines (IMPs) have been identified as highly versatile building blocks in synthetic organic chemistry due to their diverse biological activities such as antifungal, antiviral, anticancer, antibacterial, anti-inflammatory, anthelmintic, analgesic, antituberculosis, antipyretic and antiepileptic activities. Additionally, several IMPs have been studied for their optical properties for their possible applications in cell imaging, metal sensing and OLEDs [1]. Bis-heterocyclic compounds combine two heterocyclic cores with different connectivities such as linked, bound, spaced or fused [2]. In particular bis-heterocycles containing IMPs are privileged heterocyclic drug scaffolds [3].
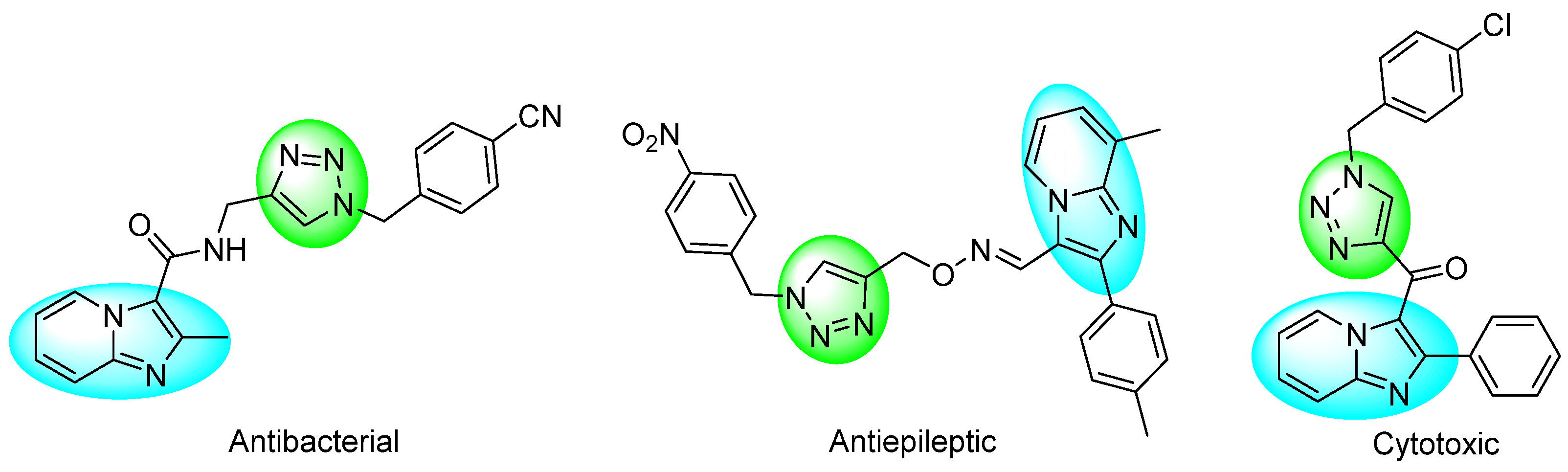
On the other hand, the 1,2,3-triazole scaffolds are biososteres of the trans-amide bond [4], and they are of great interest in the design of novel heterocycles compounds with potential applications in medicinal chemistry. Their incorporation provides advantages such as resistance to proteases and improved stability under hydrolytic, oxidative, and reducing conditions [5]. IMPs connected to 1,4-disustituted-1,2,3-Ts (1,4-DS-1,2,3-Ts) have been reported for their biological properties (Figure 1) [6,7,8].
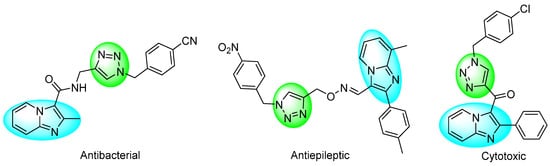
Figure 1.
Biological activities of IMPs connected to 1,4-DS-1,2,3-Ts.
The most common methods to synthetize IMP include transition-metal catalysis, condensation reactions, cyclization reactions, heteroannulation and photocatalytic reactions. Nevertheless, in the majority of instances, they are subjected to harsh conditions, such as high temperatures, non-ecofriendly solvents, side products, long reaction times, expensive operations, low yields and limited scope [9].
In this context, multicomponent reactions (MCRs) represent the most efficient synthetic tools for organic synthesis, exhibiting high overall yields, convergence, atomic economy and broad scope [10,11,12]. For example, the isocyanide multicomponent reaction (IMCR) Groebke–Blackburn–Bienaymé reaction (GBBR) has attracted the interest of the scientific community; since its discovery, it remains the best methodology to synthesize imidazo[1,2-a] pyridine-3-amines [13,14,15]. The use of alternative energy sources (AESs) in GBBR allows for chemical activation as well as accelerated and cleaner reactions; for example, the use of MW irradiation allows for homogeneous and efficient temperature increases through the effect of dielectric heating [16].
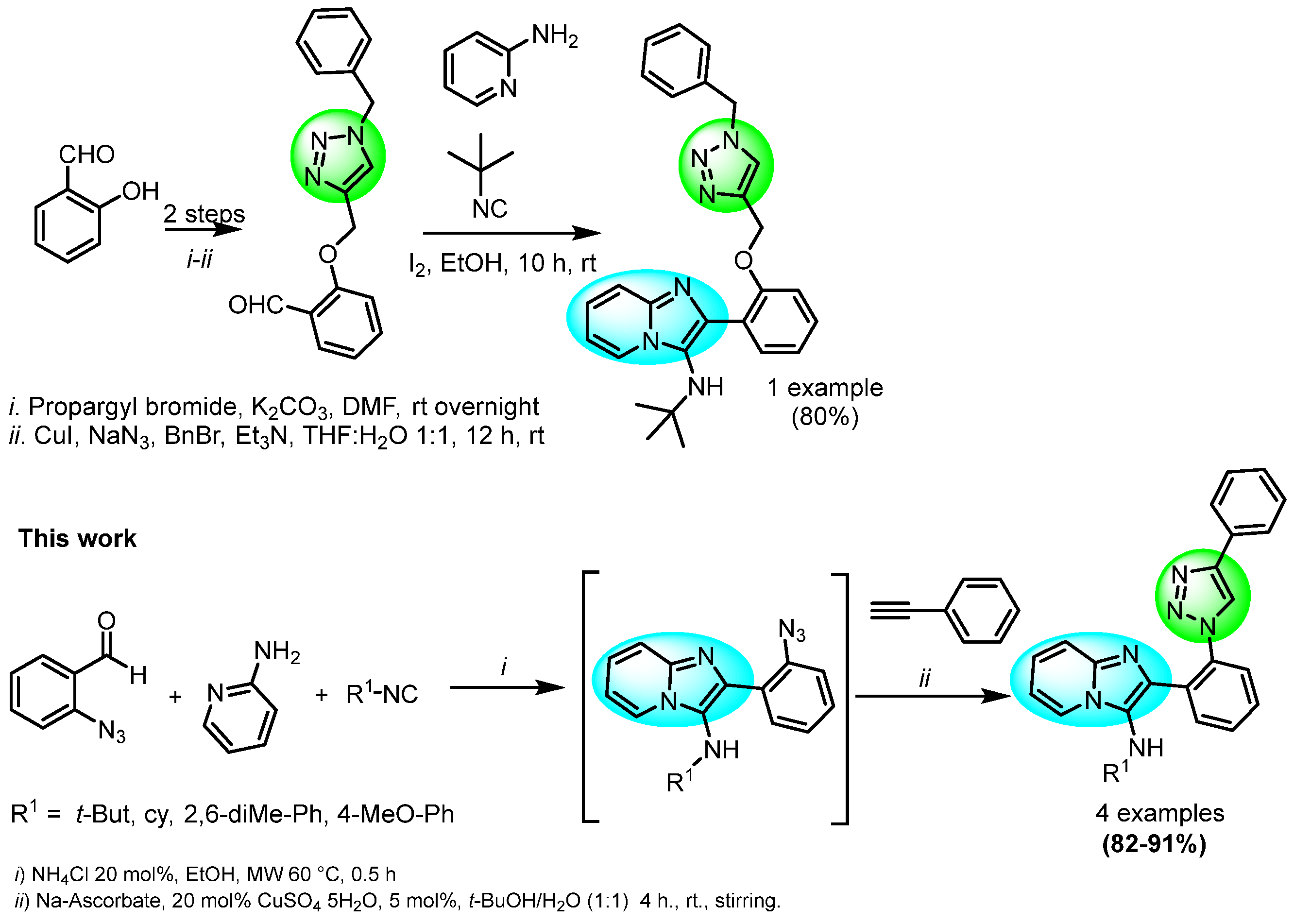
On the other hand, 1,3-dipolar Copper-catalyzed Alkyne-Azide Cycloaddition (CuAAC) is the most efficient approach to synthesize 1,4-DS-1,2,3-Ts [17,18]. The IMCR coupled with post-transformations strategy has emerged as a research field for the synthesis of novel molecules, with potential application in several fields [2,19]. As far as our survey of the literature is concerned, few reports of the synthesis of IMPs connected to 1,4-DS-1,2,3-Ts by IMCR processes have been reported (Scheme 1) [20].
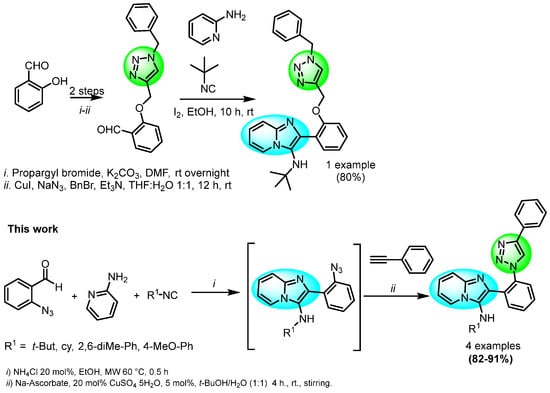
Scheme 1.
Previous reports of synthesis of IMPs connected to 1,4-DS-1,2,3−Ts (Previous work by Thennarasu at al. [20]).
Herein, we report the one-pot synthesis of bis-heterocyclic IMPs-1,4-disustituted-1,2,3-triazoles using a GBBR-CuAAC strategy assisted by AES.
2. Results and Discussion
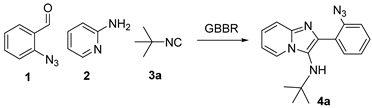
The optimization of the reactions was conducted by use of orthogonal 2-azidobenzaldehyde (1), 2-aminopyridine (2) and tert-butyl isocyanide (3a), employing NH4Cl [21,22] as a green catalyst under conventional conditions (Table 1, Entry 1), giving the desired product at a 82% yield. To decrease the reaction time, the procedure was conducted at 60 °C, resulting in 8 h with a comparable yield (Entry 2). The reaction was performed with the assistance of a MW energy source, which yielded the desired product in a more efficient manner, with a yield of 89% and a reduction in the reaction time to 30 min (Entry 3).

Table 1.
Screening conditions for the synthesis of 4a.
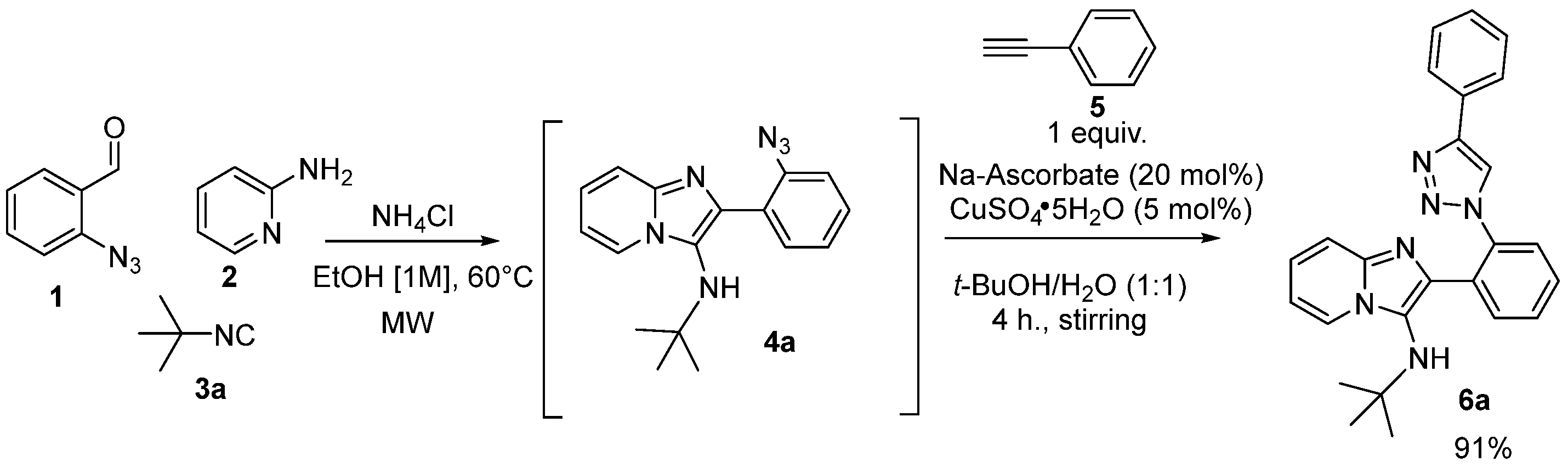
After optimizing the conditions, we explored the one-pot reaction to synthetize 6a via the GBBR/CuAAC strategy, according to the Sharpless conditions for the click reaction (Scheme 2) [17,18].
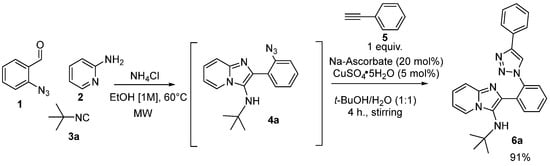
Scheme 2.
One-pot optimization for the synthesis of via GBBR/CuAAC strategy.
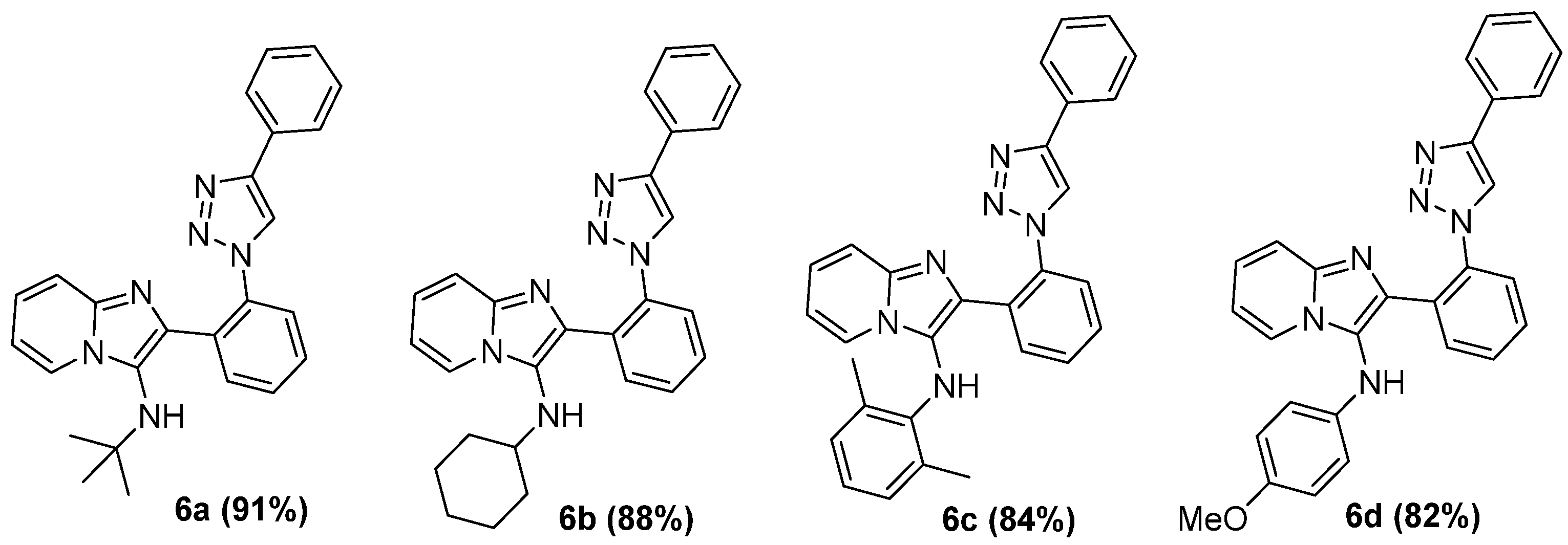
Additionally, we explored the versatility of the methodology through variations in isocyanide reagents. The respective imidazo[1,2-a]pyridine-1,2,3-triazoles (6a–d, Figure 2) synthesized under AES were obtained in good yields (82–91%).
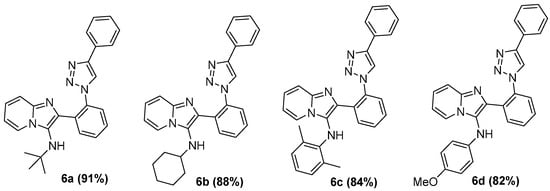
Figure 2.
Substrate scope of imidazo[1,2-a]pyridine-1,2,3-triazoles.
3. Experimental Section
3.1. General Information, Instrumentation and Chemicals
The 1H and 13C NMR spectra were acquired on Bruker Avance III spectrometers (500 MHz). Deuterated chloroform (CDCl3) was used. Chemical shifts are reported in parts per million (δ/ppm). The internal reference for NMR spectra was tetramethylsilane (TMS) at 0.0 ppm. Coupling constants are reported in Hertz (J/Hz). Multiplicities of signals are reported using the standard abbreviations: singlet (s), doublet (d), triplet (t), quartet (q) and multiplet (m). NMR spectra were analyzed using MestreNova software version 10.0.1-14719. IR spectra were acquired on a Perkin Elmer 100 spectrometer. High-resolution mass spectrometry (HRMS) samples were ionized in Electrospray ionization (ESI) mode and recorded via the time-of-flight (TOF) method. Microwave-assisted reactions were performed in closed-vessel mode using a monomodal CEM Discover unit. Reaction progress was monitored by thin-layer chromatography (TLC) on precoated silica gel Kieselgel 60 F254 plates, and spots were visualized under UV light at 254 or 365 nm. Mixtures of hexanes with ethyl acetate (EtOAc) were used as eluents for TLC and for measuring retention factors (Rfs). Flash column chromatography was performed using silica gel (230–400 mesh) and mixtures of hexanes with EtOAc in different proportions (v/v) as the mobile phase. Melting points were determined on an electrothermal apparatus and were uncorrected. All starting materials were purchased from Sigma-Aldrich and were used without further purification. Chemical names and drawings were obtained using the ChemBioDraw Ultra 13.0.2.3020 software package.
3.2. General Procedure (GP)
In a 10 mL MW-sealed tube equipped with a magnetic stirring bar, azidobenzaldehyde (1, 1.0 equiv.), 2-aminopyridine (2, 1 equiv.), the corresponding isocyanide (3a–d, 1 equiv.) and NH4Cl (20% mol) were added and dissolved in EtOH (1M), the reaction mixture was MW heated (150 W, 60 °C) for 30 min. The reactions were monitored by TLC, and once the starting materials disappeared, the solvent was removed to dryness, the residue was diluted in tert-BuOH/H2O (1:1 v/v) [0.3 M] and the phenylacetylene (5, 1.0 equiv.) was added. Sodium ascorbate (0.20 equiv.) and CuSO4*5H2O (0.05 equiv.) were added sequentially. Then, the vial was closed, and the reaction mixture was stirred at room temperature and monitored by TLC. Once the starting material disappeared, the reaction mixture was diluted in water (5.0 mL) and extracted with ethyl acetate (2 × 10 mL). The organic layer was washed with water (2 × 10 mL) and brine (2 × 10 mL). The new organic layer was dried over anhydrous Na2SO4 and concentrated under vacuum to afford the crude product. The residue was purified by flash chromatography to afford the corresponding imidazo[1,2-a]pyridines-1,2,3-triazole 6a–d.
3.3. Spectral Data
3.3.1. Characterization of the N-(tert-butyl)-2-(2-(4-phenyl-1H-1,2,3-triazol-1-yl)phenyl)imidazo[1,2-a]pyridin-3-amine (6a)
Brown oil, 91%; Rf = 0.32 (Hexanes/EtOAc, 3:7); 1H NMR (500 MHz, CDCl3): δ 8.27–8.21(m, 1H), 7.93–7.88 (m, 1H), 7.83 (s, 1H), 7.78–7.70(m, 3H), 7.70–7.60 (m, 3H), 7.40–7.28 (m, 4H), 6.95–6.87 (m, 1H), 2.70 (s, 1H), 0.92 (s, 9H).; 13C NMR (126 MHz, CDCl3, 25 °C): δ 147.8, 140.8, 135.9, 132.8, 130.2, 130.0(2), 128.8, 128.6, 128.4, 127.2, 126.5, 126.1, 125.8, 124.1, 122.1, 116.0, 113.2, 55.9, 29.9.; HRMS (ESI+): m/z calcd. for C25H25N6+ [M + H]+ 409.2135, found 409.2133.
3.3.2. Characterization of the N-cyclohexyl-2-(2-(4-phenyl-1H-1,2,3-triazol-1-yl)phenyl)imidazo [1,2-a]pyridin-3-amine (6b)
Brown oil, 88%; Rf = 0.33(hexanes/EtOAc, 3:7); 1H NMR (500 MHz, CDCl3, 25 °C): δ 8.04–8.00 (m,1H), 7.85 (s, 1H), 7.82–7.78 (m, 1H), 7.72–7.67 (m, 3H), 7.64–7.58 (m, 3H), 7.38–7.33 (m, 2H), 7.30–7.27 (m, 1H), 7.22–7.18 (m, 1H), 6.84–6.68 (m, 1H), 2.67 (s, 1H), 2.63–2.55 (m, 1H), 1.59–1.49 (m, 4H), 1.48–1.43 (m, 1H), 1.06–1.02 (m, 2H), 0.98–0.93 (m, 2H), 0.89–0.84 (m, 1H); 13C NMR (126 MHz, CDCl3): δ 147.6, 141.0, 136.0, 132.5, 130.1, 129.9, 129.6, 129.0, 128.8, 128.2, 127.0, 126.3, 125.8, 125.5, 123.3, 122.0, 116.7, 112.6, 56.3, 33.6, 25.5, 24.4; HRMS (ESI+): m/z calcd. for C27H27N6+ [M + H]+ 435.2292, found 435.2306.
3.3.3. Characterization of the N-(2,6-Dimethylphenyl)-2-(2-(4-phenyl-1H-1,2,3-triazol-1-yl)phenyl)imidazo[1,2-a]pyridin-3-amine (6c)
Brown oil, 84%; Rf = 0.30(hexanes/EtOAc, 3:7); 1H NMR (500 MHz, CDCl3): δ 7.93 (s, 1H), 7.76–7.71 (m, 2H), 7.61–7.54 (m, 2H),7.53–7.44 (m, 4H),7.40–7.35 (m, 2H), 7.32–7.26 (m, 1H), 7.14–7.07 (m, 1H), 6.86–6.79 (m, 2H), 6.70–6.74 (m, 1H), 6.67–6.62 (m, 1H), 5.15 (s, 1H), 1.79 (s, 6H); 13C NMR (126 MHz, CDCl3, 25 °C): δ 147.5, 141.2, 140.0, 136.4, 133.9, 132.1, 130.2, 129.9, 129.5, 129.3(2), 128.9, 128.3, 126.9, 125.8, 125.6, 124.1, 123.9, 122.5, 122.9, 122.1, 121.6, 117.7, 112.4, 18.2; HRMS (ESI+): m/z calcd. for C24H25N6+ [M + H]+ 457.2135, found 457.2142.
3.3.4. Characterization of the N-(4-Methoxyphenyl)-2-(2-(4-phenyl-1H-1,2,3-triazol-1-yl)phenyl)imidazo[1,2-a]pyridin-3-amine (6d)
Brown oil, 82%; Rf = 0.29(hexanes/EtOAc, 3:7); 1H NMR (500 MHz, CDCl3, 25 °C): δ 7.89 (s, 1H), 7.77–7.72 (m, 3H), 7.67 (d, J = 6.8 Hz, 1H), 7.58–7.49 (m, 4H), 7.40–7.35 (m, 2H),.7.33–7.28 (m, 1H),.7.18 (t, J = 6.8 Hz, 1H), 6.72 (t, J = 6.8 Hz, 1H), 6.62 (d, J = 8.8 Hz, 2H), 6.26 (d, J = 8.8 Hz, 2H), 5.34 (s, 1H); 3.65 (s, 3H); 13C NMR (126 MHz, CDCl3, 25 °C): δ 153.3, 147.5, 142.5, 137.9, 135.8, 135.4, 132.3, 130.2, 129.9, 129.8, 129.3, 128.8, 128.2, 126.2, 125.7, 124.9, 123.4, 122.0, 121.5, 117.8, 114.9, 114.6, 112.3, 55.6.HRMS (ESI+): m/z calcd. for C28H23N6O+ [M + H]+ 459.1927, found 459.1934.
4. Conclusions
This is a contribution to the class of multicomponent one-pot processes in the design of novel drug scaffolds like analogs of IMPs incorporating conformationally restricted bioisosteres of amide bonds. The bis-heterocycles containing privileged IMP and triazole cores add aggregate value, increasing their possible applications in medicinal chemistry. The developed strategy contributes to the multicomponent and click chemistry fields.
Author Contributions
Writing—original draft preparation, investigation, M.A.R.-G.; investigation, D.C.-R.; resources, formal analysis, F.R.-L.; resources, formal analysis, A.C.-D.; methodology, writing—original draft preparation, R.G.-M. All authors have read and agreed to the published version of the manuscript.
Funding
R.G.-M. is thankful for the financial support from DAIP-UG (132/2023 and 066/2024) and CONAHCYT (CB-2016-285622) project. M.A.R.-G. (707974/585367), D.C.-R. (666925/2782364), F.R.-L. (892154/764724) and A.C.-D. (490344/2907767) thank CONAHCYT for the scholarships.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to the article.
Acknowledgments
Laboratorio Nacional de Caracterización de Propiedades Fisicoquímicas y Estructura Molecular (CONACYT-México, Project: 123732).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chaudhran, P.A.; Sharma, A. Progress in the Development of Imidazopyridine-Based Fluorescent Probes for Diverse Applications. Crit. Rev. Anal. Chem. 2022, 54, 2148–2165. [Google Scholar] [CrossRef] [PubMed]
- Ishwar Bhat, S. One-Pot Construction of Bis-Heterocycles through Isocyanide Based Multicomponent Reactions. ChemistrySelect 2020, 5, 8040–8061. [Google Scholar] [CrossRef]
- Devi, N.; Singh, D.; Rawal, R.K.; Bariwal, J. Virender Singh Medicinal Attributes of Imidazo[1,2-a]Pyridine Derivatives: An Update. Curr. Top. Med. Chem. 2016, 16, 2963–2994. [Google Scholar] [CrossRef] [PubMed]
- Agouram, N.; El Hadrami, E.M.; Bentama, A. 1,2,3-Triazoles as Biomimetics in Peptide Science. Molecules 2021, 26, 2937. [Google Scholar] [CrossRef]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Ulloora, S.; Shabaraya, R.; Adhikari, A.V. New dihydropyridine derivatives: Anti-inflammatory, analgesic and docking studies. Bioorg. Med. Chem. Lett. 2013, 23, 3368–3372. [Google Scholar] [CrossRef]
- Reddyrajulaa, R.; Dalimba, U.K. Structural modification of zolpidem led to potent antimicrobial activity in imidazo[1,2-a]pyridine/pyrimidine-1,2,3-triazoles. New J. Chem. 2019, 43, 16281–16299. [Google Scholar] [CrossRef]
- Sayeed, I.B.; Vishnuvardhan MV, P.S.; Nagarajan, A.; Kantevari, S.; Kamal, A. Imidazopyridine linked triazoles as tubulin inhibitors, effectively triggering apoptosis in lung cancer cell line. Bioorganic Chem. 2018, 80, 714–720. [Google Scholar] [CrossRef]
- Panda, J.; Raiguru, B.P.; Mishra, M.; Mohapatra, S.; Nayak, S. Recent Advances in the Synthesis of Imidazo[1,2-a]Pyridines: A Brief Review. ChemistrySelect 2022, 7, e202103987. [Google Scholar] [CrossRef]
- Tietze, L.F.; Beifuss, U. Sequential Transformations in Organic Chemistry: A Synthetic Strategy with a Future. Angew. Chem. Int. Ed. Engl. 1993, 32, 131–163. [Google Scholar] [CrossRef]
- Tzitzikas, T.Z.; Chandgude, A.L.; Dömling, A. Multicomponent Reactions, Union of MCRs and Beyond. Chem. Rec. 2015, 15, 981–996. [Google Scholar]
- Zhu, J.; Wang, Q.; Wang, M.-X. Multicomponent Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Devi, N.; Rawal, R.K.; Singh, V. Diversity-oriented synthesis of fused-imidazole derivatives via Groebke–Blackburn–Bienayme reaction: A review. Tetrahedron 2015, 71, 183. [Google Scholar] [CrossRef]
- Shaaban, S.; Abdel-Wahab, B.F. Groebke-Blackburn-Bienaymé multicomponent reaction: Emerging chemistry for drug discovery. Mol. Divers. 2016, 20, 233. [Google Scholar] [CrossRef]
- Boltjes, A.; Dömling, A. The Groebke-Blackburn-Bienaymé Reaction. Eur. J. Org. Chem. 2019, 2019, 7007–7049. [Google Scholar]
- Fairoosa, J.; Saranya, S.; Radhika, S.; Anilkumar, G. Recent Advances in Microwave Assisted Multicomponent Reactions. ChemistrySelect 2020, 5, 5180–5197. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Brandner, L.; Müller, T.J.J. Multicomponent synthesis of chromophores—The one-pot approach to functional π-systems. Front. Chem. 2023, 11, 1124209. [Google Scholar]
- Mala, R.; Nandhagopal, M.; Narayanasamy, M.; Thennarasu, S. An Imidazo[1,2-a]pyridine Derivative That Enables Selective and Sequential Sensing of Cu2+ and CN− Ions in Aqueous and Biological Samples. ChemistrySelect 2019, 4, 13131–13137. [Google Scholar] [CrossRef]
- Kurva, M.; Claudio-Catalán, M.Á.; Rentería-Gómez, Á.; Jiménez-Halla, J.O.C.; González-García, G.; Velusamy, J.; Ramos-Ortíz, G.; Castaño-González, K.; Piazza, V.; Gámez-Montaño, R. Multicomponent one-pot synthesis of luminescent imidazo [1,2-a]pyridine-3-amines. Studies of fluorescence, solvatochromism, TD-DFT calculations and bioimaging application. J. Mol. Struct. 2023, 1276, 134797. [Google Scholar] [CrossRef]
- Kurva, M.; Pharande, S.G.; Quezada-Soto, A.; Gámez-Montaño, R. Ultrasound assisted green synthesis of bound type bis-heterocyclic carbazolyl imidazo[1,2-a]pyridines via Groebke-Blackburn-Bienayme reaction. Tetrahedron Lett. 2018, 59, 1596. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).