Abstract
In this work, we synthesized methylxanthine and azobenzene derivatives, linked to secondary amines via a seven-carbon chain, to evaluate their acetylcholinesterase (AChE) inhibitory activity. Among the azobenzene compounds, 3a exhibited the highest activity with an IC50 of 1.1 µM. Meanwhile, the theobromine derivative 2a was the most potent inhibitor among the methylxanthines, with an IC50 of 0.19 µM. These results highlight the importance of structure–activity relationship analysis to optimize AChE inhibition by modifying pharmacophore fragments and secondary amines.
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative condition that predominantly affects older adults, leading to cognitive impairment and memory loss. A key feature of AD pathology is the degeneration of neurons and brain atrophy, leading to impairments in neurotransmission, particularly involving acetylcholine (ACh) [1]. Acetylcholinesterase (AChE) is an enzyme that regulates ACh levels, and its inhibition has been widely studied as a strategy to alleviate AD symptoms by enhancing cholinergic transmission.
Currently available AChE inhibitors (AChEIs) such as donepezil, rivastigmine, and galantamine temporarily improve cognitive symptoms in AD patients but are not capable of halting disease progression. This has prompted ongoing research to develop new compounds that target multiple biological pathways simultaneously [2]. Previous studies from our group demonstrated that hybrids of caffeine and pyrrolidine were effective AChE inhibitors and could also activate nicotinic acetylcholine receptors (nAChRs), suggesting the potential for multifunctional therapeutic agents [3,4,5].
In this study, we expanded on this concept by synthesizing new derivatives based on methylxanthines, such as theobromine and theophylline, linked to secondary amines via a seven-carbon chain. Additionally, inspired by the resveratrol scaffold—due to its interesting biological properties and previous findings from our group—we synthesized new azobenzene derivatives, replacing the methylxanthine fragment with azobenzene (Ph-N=N-Ph), known for its photo-modulable properties [6,7]. The primary aim was to perform a structure–activity relationship analysis to evaluate the impact of the pharmacophore (azobenzene or methylxanthine) and the type of amino group (diethylamine or N-benzylmethylamine) on the inhibitory activity against AChE. This comparison allowed us to assess which combination of these structural features results in more potent AChE inhibitors.
2. Materials and Methods
All solvents employed in the reactions were previously distilled and dried using standard procedures; for instance, drying agents were thermally activated prior to use. Dimethylformamide (DMF) was distilled and stored over 4 Å molecular sieves under an inert nitrogen atmosphere. Chromatographic separations were performed on Merck silica gel 60 (particle size 0.2–0.63 mm, 240–400 mesh). Reaction monitoring was conducted via thin-layer chromatography (TLC), using silica gel 60 F254 chromatofoils (Merck, Darmstadt, Germany), visualized under UV light at 254 nm. In the purification of azobenzene derivatives, neutral alumina obtained from Merck (Darmstadt, Germany) was utilized. TLC spots were also developed using p-anisaldehyde/acetic acid staining reagent (Mallinckrodt, NY, USA), with UV visualization at 254 and 366 nm.
Microwave-assisted syntheses were conducted using a CEM Discover Benchmate reactor (CEM Corp., Matthews, NC, USA). The structural characterization of all final compounds was carried out by nuclear magnetic resonance (NMR) spectroscopy. Spectra for 1H and 13C nuclei, along with two-dimensional experiments such as COSY, HSQC, and HMBC, were acquired on a Bruker Avance ARX-300 spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany), operating at room temperature and using CDCl3 as a solvent. Chemical shifts (δ) are given in parts per million (ppm) relative to tetramethylsilane (TMS, δ = 0.00 ppm) as an internal standard.
2.1. Preparation of Alkylbrominated Intermediates 1, 2, and 3
Alkylbrominated intermediates were obtained using theophylline or 4-(phenyldiazenyl)phenol as the starting reagent, as previously reported [4,7]. The same methodology was used to synthesize intermediate 2 by reacting or using theobromine with 1,7-dibromoheptane (Scheme 1). Synthesis of 1-(7-bromoheptyl)-3,7-dimethyl-1H-purine-2,6(3H,7H)-dione (2): 7-dibromoheptane was added (2 mmol) to a solution of theobromine (1.0 mmol) and NaH (1 mmol) in dry DMF (10 mL). The solution was placed in a 25 mL round-bottom flask equipped with a magnetic stirrer and heated conventionally at 80 °C for 2.5 h with continuous stirring. The solvent was subsequently removed by the addition of distilled H2O (3 mL) and extraction with dichlomethane (3 × 2 mL). The organic phase was dried over anh. Na2SO4 and filtered, and the solvent was evaporated to obtain the desired product. The residue was purified by column chromatography on silica gel 60 (70–230 mesh) with dichlomethane–methanol (95:5) to obtain the desired ether (yields of 33%).
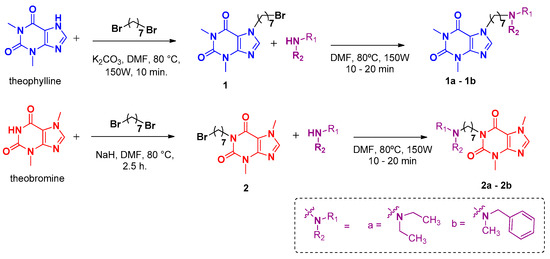
Scheme 1.
Synthesis of methylxanthine derivatives 1a–1b and 2a–2b.
2.2. Preparation of Compounds 1a–1b and 2a–2b
In a microwave reaction vessel, a solution of compounds 1–2 (0.1 mmol) in dry DMF (1 mL) was prepared, followed by the addition of diethylamine or benzylmethylamine (0.3 mmol). A glass vial was placed in a microwave reactor at 150 W and 100 °C for 10–20 min until complete conversion was observed by TLC. The mixture was then partitioned between water and CH2Cl2. The separated organic layer was dried over anhydrous MgSO4 and filtered. The solvent was evaporated under reduced pressure, and the crude reaction product was purified by column chromatography using silica gel 60 (0.2–0.63 mm, 240–400 mesh) as the stationary phase and mixtures of CH2Cl2–methanol (90:10 and 80:20).
2.3. Preparation of Compounds 3a–3b
A total of 0.3 mmol of diethylamine or N-methylbenzylamine was added to a solution containing 0.1 mmol of 3 in 1 mL of dry DMF placed in a microwave reaction glass tube containing a magnetic stirrer. The glass tube was placed in a microwave reactor at 150 W, 80 °C, for 10 to 40 min until observing total conversion by TLC. The solvent was subsequently removed by the addition of distilled H2O (3 mL) and extraction with ethyl acetate (3 × 2 mL). The separated organic layer was dried over anhydrous NaSO4 and filtered. The solvent was evaporated under reduced pressure, and the crude reaction product was purified by column chromatography on neutral aluminum oxide (Fluka AG, Buchs SG, Switzerland), using hexane–ethyl acetate (70:30) as an eluent.
2.4. Cholinesterase Inhibition Assay
Acetylcholinesterase (AChE) from Electrophorus electricus (500 U; Sigma, Buenos Aires, Argentina) was employed as the enzymatic source. Inhibition assays were conducted in vitro following a modified version of Ellman’s colorimetric protocol [8,9]. Absorbance at 405 nm was measured over a 120-s interval at 25 °C. The percentage of enzymatic inhibition was calculated by comparing the reaction rate of each sample with that of a blank (no inhibitor). IC50 values were determined by fitting the inhibition curves to a nonlinear regression model (log[inhibitor] vs. response) using GraphPad Prism 5. Tacrine served as the positive control.
3. Results and Discussion
Drawing on our prior investigations, we designed a new series of caffeine-based hybrids in which the pyrrolidine moiety was substituted with alternative secondary amines. This modification strategy has shown promise across various chemical scaffolds, as reported in multiple studies [5,10,11]. Furthermore, to evaluate the impact of substitution positions in the methylxanthine core (N1 or N7) on biological activity, the natural alkaloids theobromine and theophylline were used as starting materials. The synthetic approach employed for the generation of these derivatives is outlined in Scheme 1. The synthetic route involved the initial alkylation of the methylxanthine scaffold using 1,7-dibromoheptane, followed by nucleophilic substitution with either diethylamine or benzylmethylamine. The seven-carbon linker was selected in accordance with previous findings that indicated that this chain length provides optimal inhibitory activity in caffeine-based AChE inhibitors [4]. All reactions were carried out under microwave irradiation, which enabled short reaction times and satisfactory to high product yields. Enzymatic inhibition against AChE was evaluated for compounds 1a–b and 2a–b and compared with the activity observed for caffeine and the previously reported caffeine-C7-pyrrolidine hybrid (Table 1). The results show that the potency of the new compounds was higher than that of caffeine, with derivative 2a (IC50 = 0.188 µM) displaying a significantly higher inhibition potency than the previously reported caffeine–pyrrolidine hybrids. Moreover, the results highlight the importance of the methylxanthine fragment for inhibition potency, as hybrids synthesized from theobromine were more active than their theophylline counterparts.

Table 1.
Inhibition of cholinesterase activity by theophylline derivatives 1a–1b, theobromine derivatives 2a–2b, and azobenzene derivatives 3a–3b.
In addition to these derivatives, two analogs were synthesized by replacing the methylxanthine scaffold with azobenzene, aiming to obtain resveratrol-like derivatives with photo-modulable properties. The synthesis and evaluation of these azobenzene derivatives are illustrated in Scheme 2. The enzymatic inhibition assays revealed that all compounds demonstrated AChE inhibitory capacity. Among the azobenzene derivatives, 3a ((E)-N,N-diethyl-7-(4-(phenyldiazenyl)phenoxy)heptan-1-amine) was the most active, with an IC50 of 1.1 µM.

Scheme 2.
Synthesis of azobenzene derivatives 3a–3b.
4. Conclusions
In summary, this study on the structure–activity relationship revealed that among the synthesized derivatives, the theobromine–diethylamine hybrid, 2a, (7-(7-(benzyl(methyl)amino)heptyl)-1,3-dimethyl-1H-purine-2,6(3H,7H)-dione) emerged as the most potent AChE inhibitor, with an IC50 of 0.19 µM. This study underscores the superiority of the theobromine pharmacophore over theophylline and azobenzene, as well as the diethylamine group over benzylamine, in the development of potent AChE inhibitors. The findings demonstrate that both the scaffold and the nature of the amino substituents play a crucial role in enhancing inhibitory potency, making theobromine derivatives promising candidates for further optimization in the search for new cholinesterase inhibitors.
Author Contributions
Conceptualization, B.B. and A.P.M.; methodology, B.B., S.G. and M.D.; validation, B.B., S.G. and A.P.M.; formal analysis, B.B.; investigation, B.B. and A.P.M.; resources, A.P.M.; writing—original draft preparation, B.B.; writing—review and editing, A.P.M.; visualization, B.B.; supervision, A.P.M.; project administration, A.P.M.; funding acquisition, A.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Scientific and Technical Research Council (CONICET grant PIP2021 GI 11220200100834CO), the National Agency for Promotion of Science and Technology (ANPCyT grant PICT2020-01187), and Universidad Nacional del Sur (grant PGI-UNS 24/Q141), from Argentina.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Nasb, M.; Tao, W.; Chen, N. Alzheimer’s Disease Puzzle: Delving into Pathogenesis Hypotheses. Aging Dis. 2024, 15, 43–73. [Google Scholar] [CrossRef]
- Fabiani, C.; Murray, A.P.; Corradi, J.; Antollini, S.S. A novel pharmacological activity of caffeine in the cholinergic system. Neuropharmacology 2018, 135, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, C.; Biscussi, B.; Munafó, J.P.; Murray, A.P.; Corradi, J.; Antollini, S.S. New Synthetic Caffeine Analogs as Modulators of the Cholinergic System. Mol. Pharmacol. 2022, 101, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Munafó, J.P.; Biscussi, B.; Obiol, D.; Costabel, M.; Bouzat, C.; Murray, A.P.; Antollini, S. New Multitarget Molecules Derived from Caffeine as Potentiators of the Cholinergic System. ACS Chem. Neurosci. 2024, 15, 994–1009. [Google Scholar] [CrossRef] [PubMed]
- Broichhagen, J.; Jurastow, I.; Iwan, K.; Kummer, W.; Trauner, D. Optical Control of Acetylcholinesterase with a Tacrine Switch. Angew. Chem. Int. Ed. 2014, 53, 7657–7660. [Google Scholar] [CrossRef]
- Biscussi, B.; Sequeira, M.A.; Richmond, V.; Mañez, P.A.; Murray, A.P. New photochromic azoderivatives with potent acetylcholinesterase inhibition. J. Photochem. Photobiol. A Chem. 2021, 418, 113375. [Google Scholar] [CrossRef]
- Ellman, G.; Courtney, K.; Andres, V.; Featherstone, R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Alza, N.P.; Richmond, V.; Baier, C.J.; Freire, E.; Baggio, R.; Murray, A.P. Synthesis and cholinesterase inhibition of cativic acid derivatives. Org. Med. Chem. 2014, 22, 3838–3849. [Google Scholar] [CrossRef] [PubMed]
- Reshetnikov, D.V.; Ivanov, I.D.; Baev, D.S.; Rybalova, T.V.; Mozhaitsev, E.S.; Patrushev, S.S.; Vavilin, V.A.; Tolstikova, T.G.; Shults, E.E. Design, Synthesis and Assay of Novel Methylxanthine–Alkynylmethylamine Derivatives as Acetylcholinesterase Inhibitors. Molecules 2022, 27, 8787. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sharma, A.; Nuthakki, V.K.; Bhatt, S.; Nandi, U.; Bharate, S.B. Design, synthesis, and structure–activity relationship of caffeine-based triazoles as dual AChE and BACE-1 inhibitors. Drug Dev. Res. 2022, 83, 1803–1821. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).