Abstract
2,5-diketopiperazines (2,5-DKPs) are the smallest cyclic peptides in nature and display a variety of antibacterial, antifungal, and anticancer properties. Very little has been reported on the one-pot synthesis of 2,5-DKPs. Herein, we report a novel one-pot synthesis of 2,5-DKPs via the Ugi-4CR/SN2-cyclization strategy, under mild conditions, and ethanol was used as a solvent for both procedures.
1. Introduction
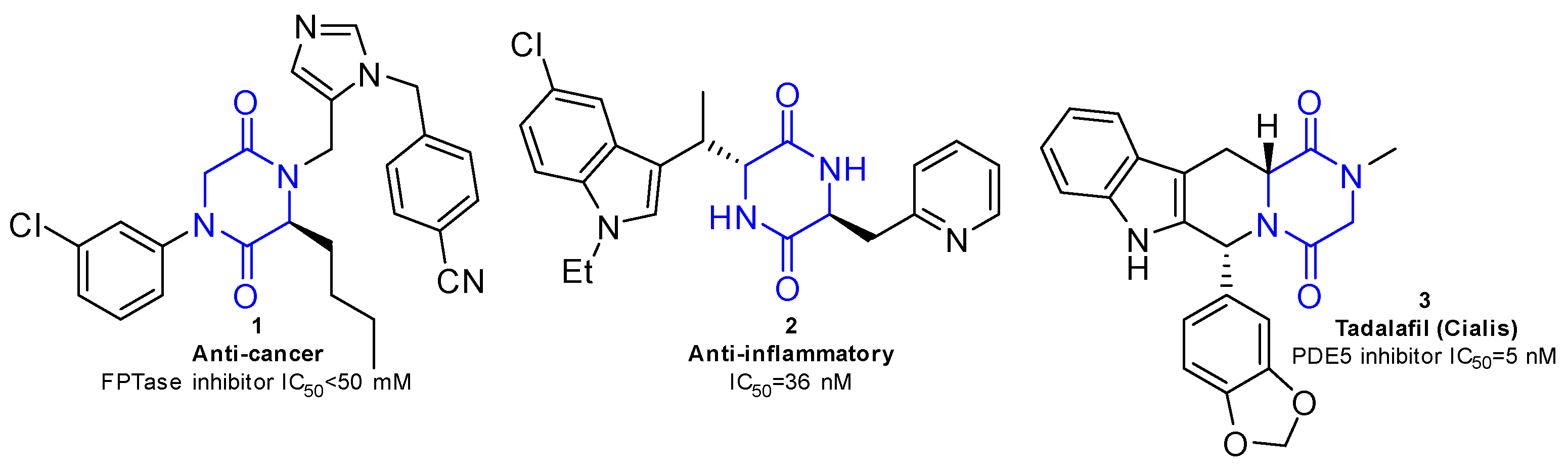
2,5-diketopiperazines (2,5-DKPs) are the only cyclic peptides found in nature, composed of two α-amino acids cyclized by peptide bonds [1]. The 2,5-DKP cores exhibit several pharmacological properties (Figure 1). Therefore, 2,5-DKPs have attracted attention in the field of drug discovery as biologically validated platforms because of their rigid conformation, structural stability, high resistance to enzymatic degradation, and cell permeability [2,3].
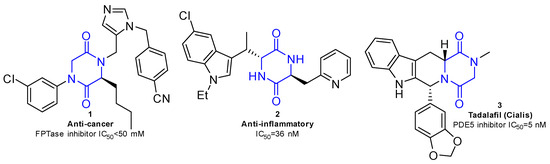
Figure 1.
Bioactive molecules containing 2,5-DKP scaffold.
Conventional synthesis of 2,5-DKPs involve cyclization of a dipeptide ester, cleavage-induced cyclization, or intermolecular cyclization of α-haloacyl derivates of amino acids. These have several disadvantages, including the use of drastic reaction conditions that include high temperatures, strong bases and/or toxic reagents (for deprotection-based cyclization), and low global yields [4]. In this context, the isocyanide-based multicomponent reaction (IMCR) approach is advantageous in terms of facile reaction conditions, scope of the starting materials, broadness of synthetic variations, and synthetic efficacy, which contributes significantly to the development of environmentally friendly strategies [5,6,7,8].
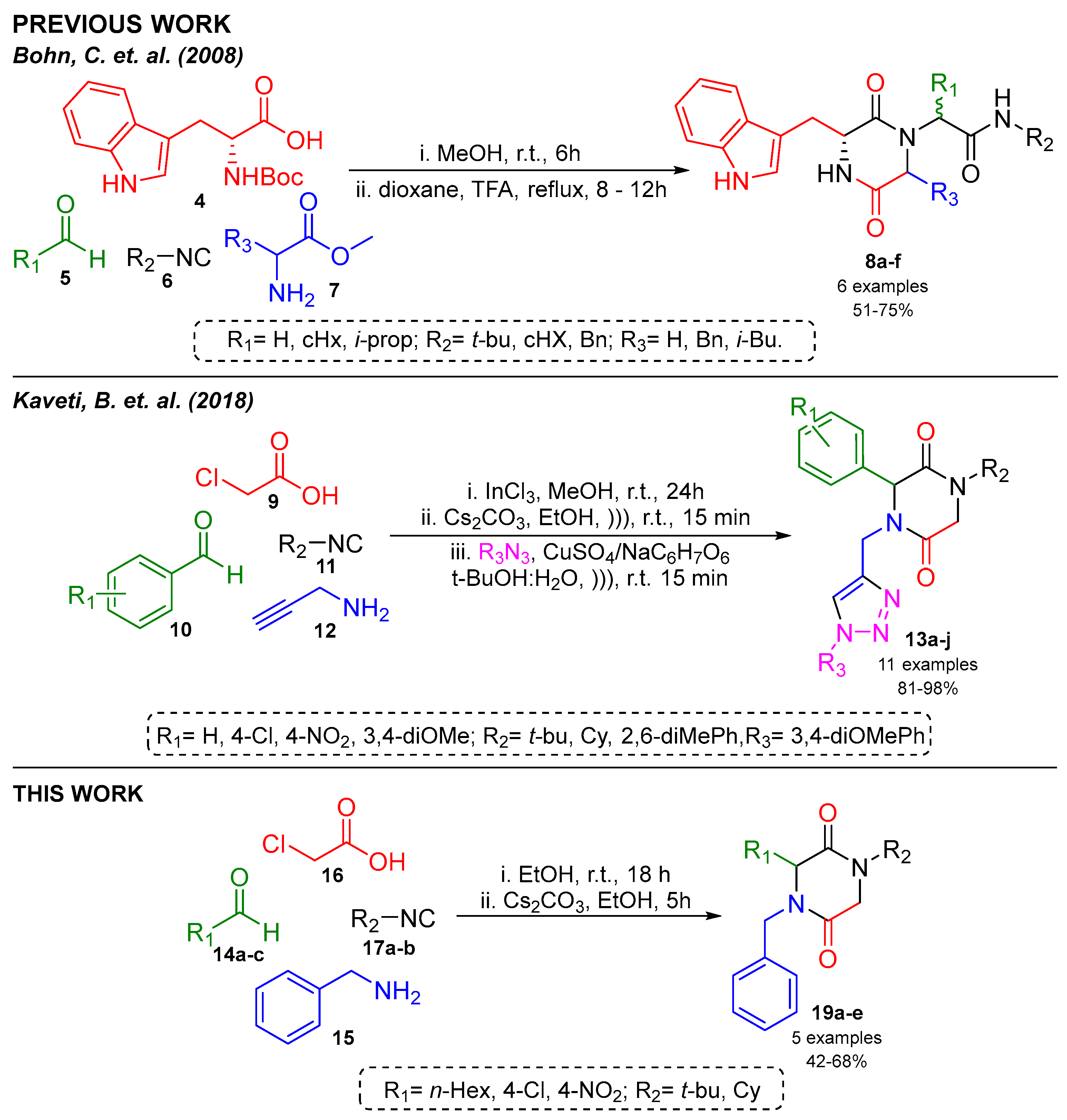
The Ugi reaction (Ugi-4CR) offers an alternative method to form an acyclic precursor which requires post-transformation reactions like deprotection and activation-based cyclization under basic and acid conditions, respectively, to obtain 2,5-DKPs. In 2018, our research group reported a one-pot synthesis of 2,5-DKPs with other heterocyclic peptidomimetic under mild conditions (Scheme 1) [9,10].
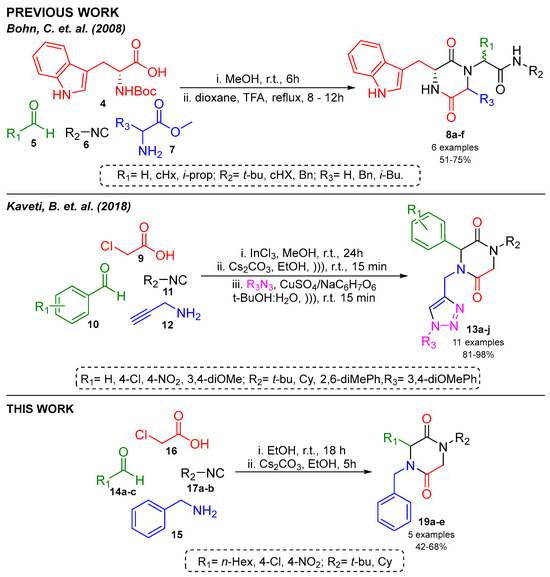
Scheme 1.
Previous reports of synthesis of 2,5-DKPs [9,10].
The one-pot synthesis of 2,5-DKPs via the Ugi-4CR/SN2-cyclization strategy is reported. Herein, we described the one-pot synthesis of 2,5-DKPs under mild conditions using ethanol as a solvent.
2. Results and Discussion
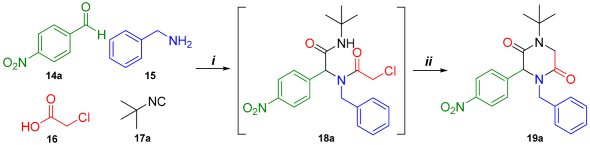
First, the synthesis of Ugi-4CR product (18a) was made via the mixing of 4-nitrobenzaldehyde (14a), benzylamine (15), monochloroacetic acid (16), and tert-butyl isocyanide (17a), using InCl3 as a catalyst. The reaction in MeOH at room temperature resulted in good yields, changing to a greener and less toxic solvent, EtOH, which obtained the desired products with similar yields to MeOH (Table 1, entries 1–2). Therefore, we opted to use EtOH for the synthesized Ugi adduct.

Table 1.
Screening conditions for the synthesis of molecule 19a.
Subsequently, the Ugi adduct was subjected to SN2-cyclization with an inorganic strong base (KOH); the result was the decomposition of the reaction. However, with the use of Cs2CO3, a less strong base, the conversion of the Ugi adduct into the 2,5-DKP (19a) was achieved (Table 1, entries 3–4).
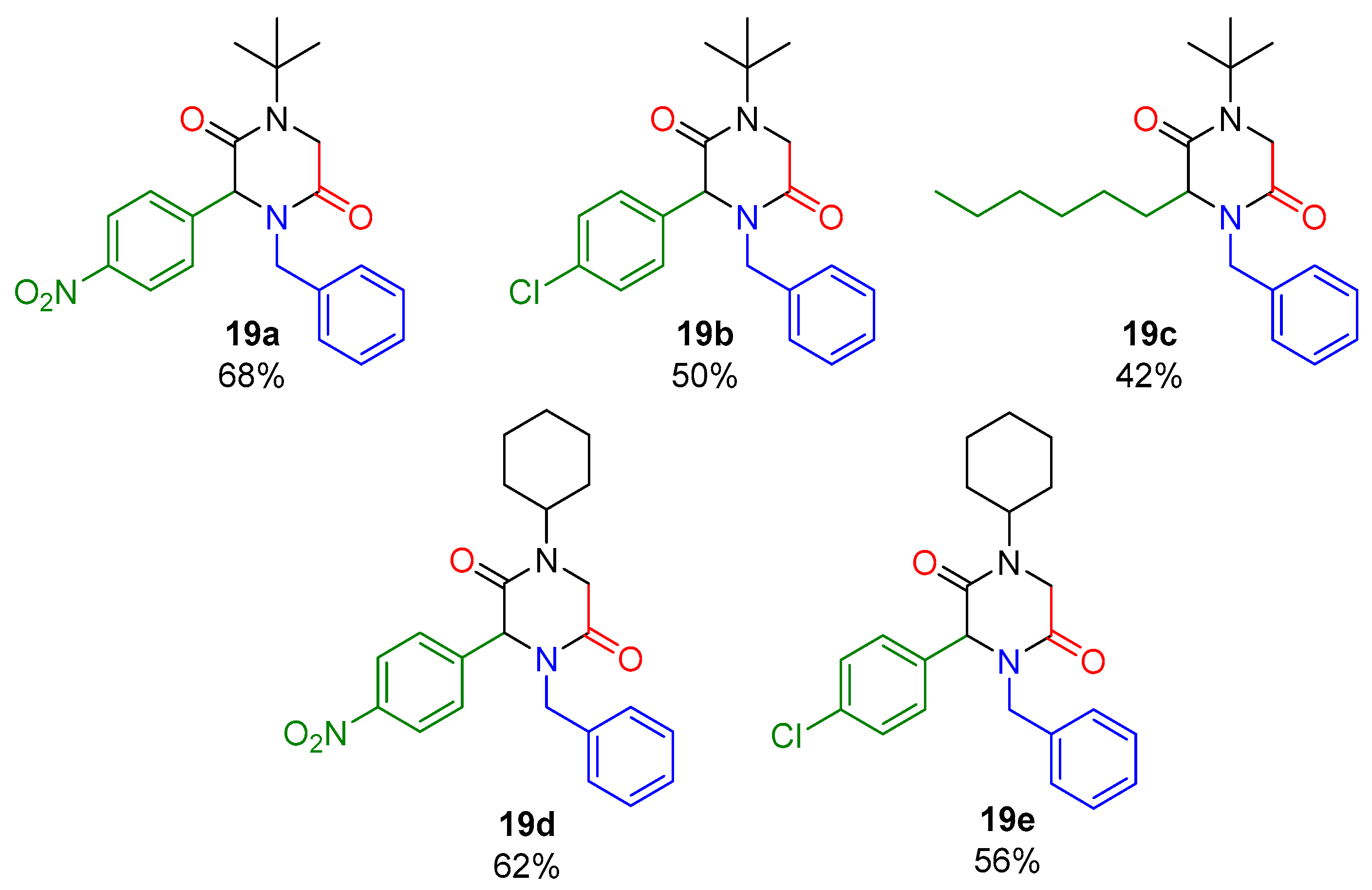
In Scheme 2, a series of 2,5-DKPs (19a–e) is depicted, which was synthesized under the optimized conditions (Table 1, entry 2 and entry 4). The effect of the stereo-electronic nature of the carbonyl component was evaluated, employing aromatic and aliphatic aldehydes. Finally, products were obtained in good yields (42–68%).
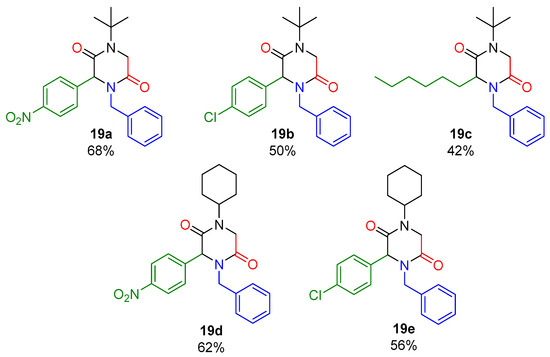
Scheme 2.
Synthesis of 2,5-DKPs scope.
3. Experimental Section
3.1. General Information, Instrumentation, and Chemicals
1H and 13C NMR spectra were acquired on Bruker Advance III spectrometer (500 MHz). The solvent used for NMR spectroscopy was deuterated chloroform (CDCl3). Chemical shifts (d) are given in ppm relative to tetramethylsilane (TMS). Coupling constants are reported in Hertz (Hz). Multiplicities of the signals are reported using standard abbreviations: singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m). NMR spectra were analyzed using MestReNova software version 12.0.0-20080. Reaction progress was monitored using thin-layer chromatography (TLC) on pre-coated silica gel F254 aluminum sheets, and the spots were visualized under UV light at 254 nm. Column chromatography was performed using silica gel (230–400 mesh) as the stationary phase. Mixtures of hexanes and ethyl acetate were used as the mobile phase for column chromatography and in TLC for reaction progress monitoring and measuring retention factors (Rf). All reagents were purchased from Sigma Aldrich and were used without further purification.
3.2. General Procedure
A solution of 1.0 M EtOH, aldehyde (14a–c, 0.13 mmol, 1.0 equiv.), benzylamine (15, 0.13 mmol, 1.0 equiv.), monochloroacetic acid (16, 0.13 mmol, 1.0 equiv.), and isocyanide (17a–b, 0.13 mmol, 1 equiv.) was stirred at room temperature for 18 h. Then, Cs2CO3 (0.24 mmol, 1.5 equiv.) was added, and the reaction mixture was sonicated (45 KHz) for 15 min for base-induced cyclization reaction, which was completed and monitored on TLC. The solvent was removed, and the crude was purified by column chromatography using silica gel and mixtures of ethyl acetate in hexanes, to afford the corresponding 2,5-DKPs (19a–e).
3.3. Spectral Data
- 4-benzyl-1-(tert-butyl)-3-(4-nitrophenyl)piperazine-2,5-dione (19a).
Yellow oil, Rf = 0.32 (30% ethyl acetate in hexanes): 1H NMR (500 MHz, CDCl3, 25 °C, TMS): d 8.24 (d, J = 8,9 Hz, 2H), 7.54 (d, J = 8.9 Hz, 2H), 7.33 (m, 3H), 7.24 (m, 2H), 5.51 (s, 1H), 4.75 (d, J = 15.5 Hz, 1H), 3.73 (d, J = 15.5 Hz, 1H), 3.59 (d, J = 15.5 Hz, 1H), 3.46 (d, J = 15.4 Hz, 1H), 1.09 (s, 9H); 13C NMR (126 MHz, CDCl3): d 168.4, 167.6, 147.9, 143.5, 135.9, 129.4, 128.8, 128.6, 128.3, 124.1, 66.0, 51.9, 51.8, 45.4, 28.1.
- 4-benzyl-1-(tert-butyl)-3-(4-chlorophenyl)piperazine-2,5-dione (19b).
Colorless oil, Rf = 0.27 (30% ethyl acetate in hexanes): 1H NMR (500 MHz, CDCl3, 25 °C, TMS): d 7.42 (d, J = 8,5 Hz, 2H), 7.33 (m, 3H), 7.28 (d, J = 8.9 Hz, 2H), 7.24 (m, 2H), 5.54 (s, 1H), 4.45 (d, J = 15.6 Hz, 1H), 3.83 (d, J = 15.7 Hz, 1H), 3.77 (d, J = 15.5 Hz, 1H), 3.43 (d, J = 15.4 Hz, 1H), 1.19 (s, 9H); 13C NMR (126 MHz, CDCl3): d 168.8, 165.3, 136.9, 135.9, 134.6, 129.4, 128.8, 128.6, 127.8, 127.1, 68.0, 63.3, 49.8, 42.5, 28.8.
- 4-benzyl-1-(tert-butyl)-3-hexylpiperazine-2,5-dione (19c).
Yellow oil, Rf = 0.43 (10% ethyl acetate in hexanes): 1H NMR (500 MHz, CDCl3, 25 °C, TMS): d 7.30 (m, 3H), 7.19 (m, 2H), 5.43 (s, 1H), 4.70 (d, J = 15.6 Hz, 1H), 4.47 (d, J = 15.5 Hz, 1H), 3.64 (d, J = 15.5 Hz, 1H), 3.39 (d, J = 15.4 Hz, 1H), 1.76 (m, 2H), 1.31 (m, 2H), 1.29 (m, 2H), 1.26 (m, 2H), 1.24 (m,2H), 1.12 (s, 9H), 0.88 (t, J = 6.7 Hz, 3H); 13C NMR (126 MHz, CDCl3): d 166.8, 165.3, 136.9, 128.6, 127.8, 127.1, 70.6, 66.3, 49.6, 47.8, 31.4, 29.6, 28.8, 27.6, 24.7, 23.7, 18.3.
- 4-benzyl-1-cyclohexyl-3-(4-nitrophenyl)piperazine-2,5-dione (19d).
Yellow oil, Rf = 0.38 (30% ethyl acetate in hexanes): 1H NMR (500 MHz, CDCl3, 25 °C, TMS): d 8.22 (d, J = 8.7 Hz, 2H), 7.53 (d, J = 8.8 Hz, 2H), 7.35 (m, 3H), 7.24 (m, 2H), 5.51 (d, J = 8.2 Hz, 1H), 4.77 (d, J = 15.5 Hz, 1H), 3.74 (d, J = 15.4 Hz, 1H), 3.65 (m, 1H), 3.60 (d, J = 15.4 Hz, 1H), 3.47 (d, J = 15.4 Hz, 1H), 1.62 (m, 4H), 1.25 (m, 3H), 1.02 (m, 1H), 0.61 (m, 2H); 13C NMR (126 MHz, CDCl3): d 168.2, 167.5, 147.9, 143.3, 135.9, 129.5, 128.8, 128.6, 128.3, 124.1, 65.9, 52.0, 48.7, 45.4, 32.6, 25.2, 24.8.
- 4-benzyl-1-cyclohexyl-3-(4-chlorophenyl)piperazine-2,5-dione (19e).
Yellow oil, Rf = 0.30 (20% ethyl acetate in hexanes): 1H NMR (500 MHz, CDCl3, 25 °C, TMS): d 7.35 (m, 3H), 7.29 (d, J = 7.3 Hz, 2H), 7.23 (m, 2H), 6.99 (d, J = 7.1 Hz, 2H), 5.63 (s, 1H), 4.77 (d, J = 17.5 Hz, 1H), 4.59 (d, J = 17.8 Hz, 1H), 4.05 (d, J = 13.4 Hz, 1H), 3.97 (d, J = 13.4 Hz, 1H), 3.69–3.62 (m, 1H), 1.68–1.55 (m, 4H), 1.26 (m, 3H), 1.02 (m, 1H), 0.60 (m, 2H); 13C NMR (126 MHz, CDCl3): d 168.4, 167.8, 134.8, 133.2, 130.9, 129.0, 128.9, 128.8, 127.8. 127.6, 65.8, 51.9, 48.7, 45.3, 32.2, 25.1, 24.7.
4. Conclusions
The present work contributes a novel green one-pot synthesis of a series of 2,5-DKP, via the Ugi-4CR/SN2-cyclization strategy using mild conditions.
Good overall yields (42–68%) were obtained for the synthesized products when aromatic and aliphatic aldehydes were employed. This is another example of the effect of the stereo-electronic nature of the components in the outcome of the IMCR/post-transformation strategy.
Finally, the methodology provides the sustainable synthesis of peptidomimetic molecules of interest to medicinal chemistry that have been reported for their pharmacological and biological properties.
Author Contributions
Conceptualization, R.G.-M.; methodology, D.G.-G., R.R.H. and M.A.R.-G.; software, D.G.-G.; validation, R.G.-M.; formal analysis, R.G.-M.; investigation, D.G.-G. and M.A.R.-G.; resources, R.G.-M.; data curation, D.G.-G.; writing—original draft preparation, D.G.-G.; writing—review and editing, R.G.-M.; visualization, R.G.-M.; supervision, R.G.-M.; project administration, R.G.-M.; funding acquisition, R.G.-M. All authors have read and agreed to the published version of the manuscript.
Funding
D.G.-G. is grateful to CONAHCYT-Mexico for the scholarship (824233). R.G.-M. is grateful for the financial support from UG CIIC (005/2022-132/2023) and CONAHCYT-Mexico (CB-2016-28562).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data can be obtained from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jia, J.; Yao, J.; Kong, J.; Yu, A.; Wei, J.; Dong, Y.; Song, R.; Shan, D.; Zhong, X.; Lv, F.; et al. 2,5-Diketopiperazines: A Review of Source, Synthesis, Bioactivity, Structure, and MS Fragmentation. Curr. Med. Chem. 2023, 30, 1060–1085. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.L.; Varejão, J.O.S.; de Souza, J.A.; de Carneiro, J.W.M.; Valdo, A.K.S.M.; Martins, F.T.; Ferreira, B.W.; Barreto, R.W.; da Silva, T.I.; Kohlhoff, M. 2,5-Diketopiperazines via Intramolecular N-Alkylation of Ugi Adducts: A Contribution to the Synthesis, Density Functional Theory Study, X-ray Characterization, and Potential Herbicide Application. J. Agric. Food Chem. 2022, 70, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Bojarska, J.; Mieczkowski, A.; Ziora, Z.M.; Skwarczynski, M.; Toth, I.; Shalash, A.O.; Parang, K.; El-Mowafi, S.A.; Mohammed, E.H.M.; Elnagdy, S. Cyclic Dipeptides: The Biological and Structural Landscape with Special Focus on the Anti-Cancer Proline-Based Scaffold. Biomolecules 2021, 11, 1515. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, A.D. 2,5-diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, Q.; Wang, M.-X. Multicomponent Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2015; pp. 1–12. [Google Scholar]
- Orru, R.V.A.; Ruijter, E. Synthesis of Heterocycles via Multicomponent Reactions I; Springer: Berlin/Heidelberg, Germany, 2010; pp. 99–107. [Google Scholar]
- Pharande, S.G.; Rentería-Gómez, M.A.; Gámez-Montaño, R. Mechanochemical IMCR and IMCR-Post Transformation Domino Strategies: Towards the Sustainable DOS of Dipeptide-like and Heterocyclic Peptidomimetics. New J. Chem. 2022, 46, 9298–9303. [Google Scholar] [CrossRef]
- Pharande, S.G.; Corrales Escobosa, A.R.; Gámez-Montaño, R. Endogenous Water-Triggered and Ultrasound Accelerated Synthesis of 1,5-Disubstituted Tetrazoles via a Solvent and Catalyst-Free Ugi-Azide Reaction. Green Chem. 2017, 19, 1259–1262. [Google Scholar] [CrossRef]
- Bohn, C.; Westermann, B.; Wessjohann, L. One-Pot Multicomponent Synthesis of N-Substituted Tryptophan-Derived Diketopiperazines. Synthesis 2008, 13, 2077–2082. [Google Scholar]
- Kaveti, B.; Ramírez-López, S.C.; Gámez Montaño, R. Ultrasound-Assisted Green One-Pot Synthesis of Linked Bis-Heterocycle Peptidomimetics via IMCR/Post-Transformation/Tandem Strategy. Tetrahedron Lett. 2018, 59, 4355–4358. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).