Abstract
Active methylene compounds such as thioamides are widely used in the organic chemistry for the construction of a variety of heterocyclic systems, such as thieno[2,3-b]pyridines, 1,2,4-dithiazoles, isothiazoles, 1,2,3-thiadiazoles, etc. N,N′-Diphenyldithiomalondiamide (dithiomalondianilide) as a compound with methylene active group is also of interest as a starting reagent for the synthesis of new N,S-containing heterocycles with potential pharmacological application. However, the reactions of dithiomalondianilide are poorly studied. In the present study, we report the synthesis of new 4,5,6,7-tetrahydro[1,2]dithiolo[3,4-b]dithiolopyridine-5-carboxamides through the reaction of dithiomalondianilide with 3-aryl-2-cyanoacrylamides. The products were characterized using FTIR and NMR spectroscopy as well as X-ray analysis.
1. Introduction
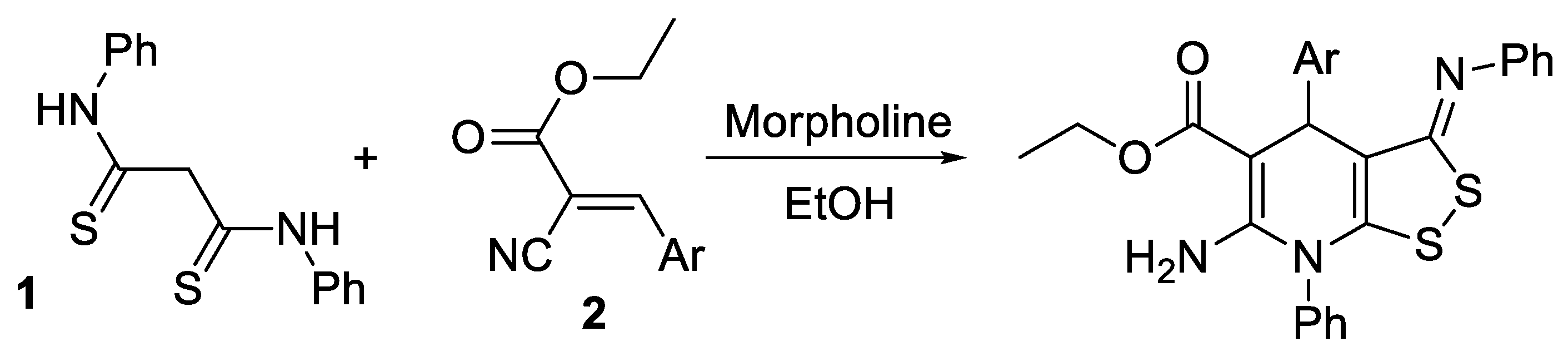
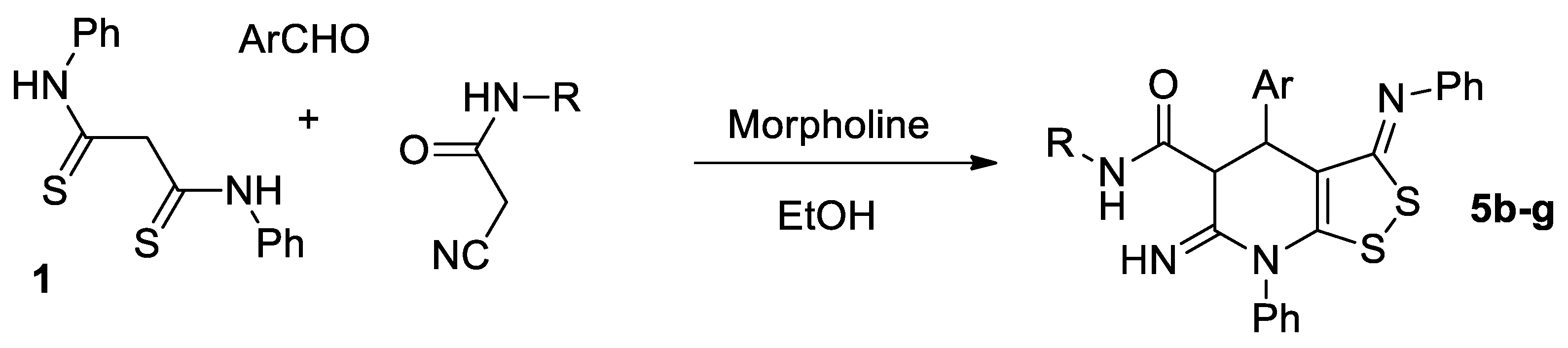
Active methylene compounds such as thioamides are widely used in organic chemistry for the construction of a variety of heterocyclic systems such as thieno[2,3-b]pyridines [1,2,3,4], 1,2,4-dithiazoles [5], isothiazoles [6], 1,2,3-thiadiazoles [7], etc. N,N’-Diphenyldithiomalondiamide (dithiomalondianilide) as a compound with a methylene active group is also of interest as a starting reagent for the synthesis of new N,S-containing heterocycles with potential pharmacological applications. However, the reactions of dithiomalondianilide are poorly studied. Thus, up to date, only a few reactions with dithiomalondianilide have been reported to give heterocyclic compounds. Recently, we reported a new reaction of dithiomalondianilide 1 with 3-aryl-2-cyanoacrylates 2 that resulted in the formation of new dithiolodihydropyridines [8] (Scheme 1):
Scheme 1.
The reaction of N,N’-diphenyldithiomalondiamide with 3-aryl-2-cyanoacrylates.
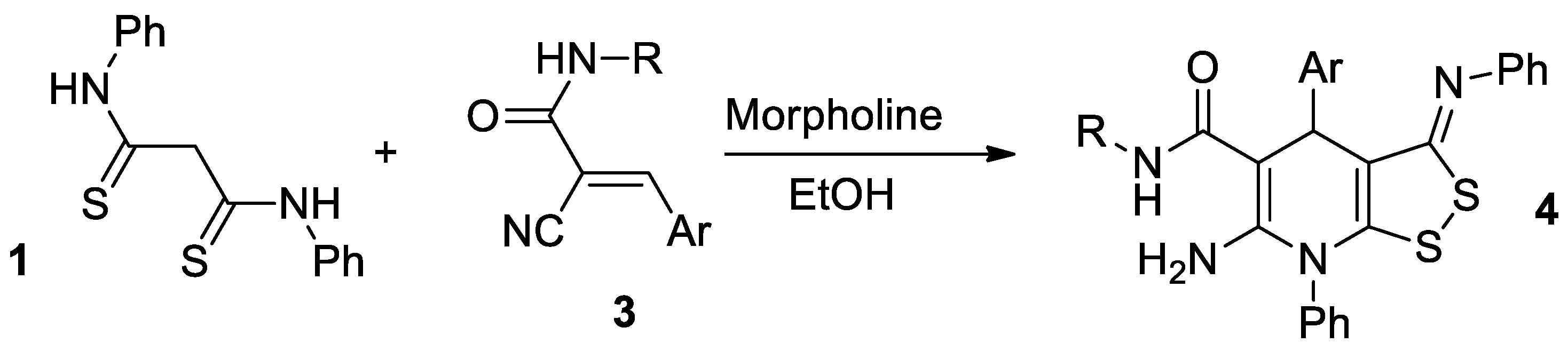
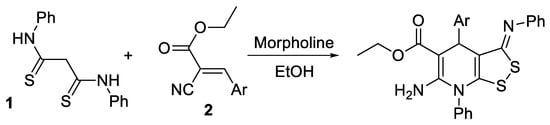
We suggested that the reaction is applicable to a wide range of Michael acceptors. Our assumption is that the interaction of thioamide 1 with substituted cyanoacrylamides 3 representing substituted acriylonitrile has to lead to the formation of related dithiolodihydropyridine-5-carboxamides 4, according the Scheme 2:
Scheme 2.
Expected result of the reaction between dithiomalondianilide with N-substituted 2-cyanoacrylamides.
In general, carboxamides found an application as steel corrosion inhibitors [9], fungicides with a wide antifungal spectrum [10], and antimicrobials, antibacterial and antimalarial drugs [11]. Therefore, the development of new synthetic approaches towards substituted dithiolopyridine-5-carboxamides seems to be an important task.
2. Result and Discussion
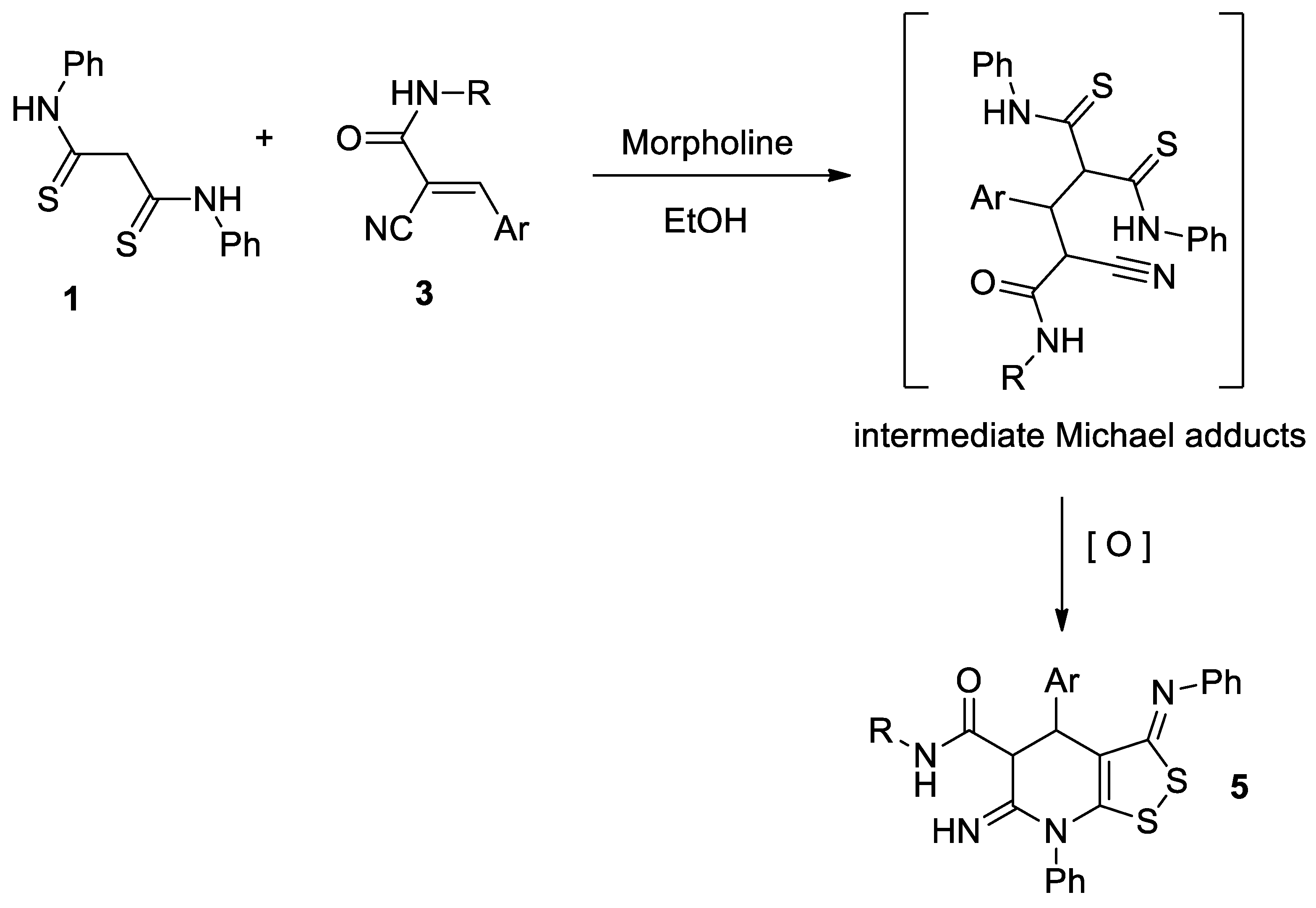
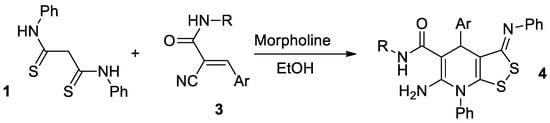
We found that dithiomalondianilide 1 reacts with 3-aryl-2-cyanoacrylamides 3 under mild conditions to create dithiolotetrahydropyridine-5-carboxamides 5 in good yields. Presumably, the reaction proceeds as the morpholine-catalyzed Michael addition is followed by oxidative heterocyclization to give 6-imino-4,5,6,7-tetrahydro-3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxamides 5 (Scheme 3).
Scheme 3.
Preparation of 6-imino-4,5,6,7-tetrahydro-3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxamides 5.
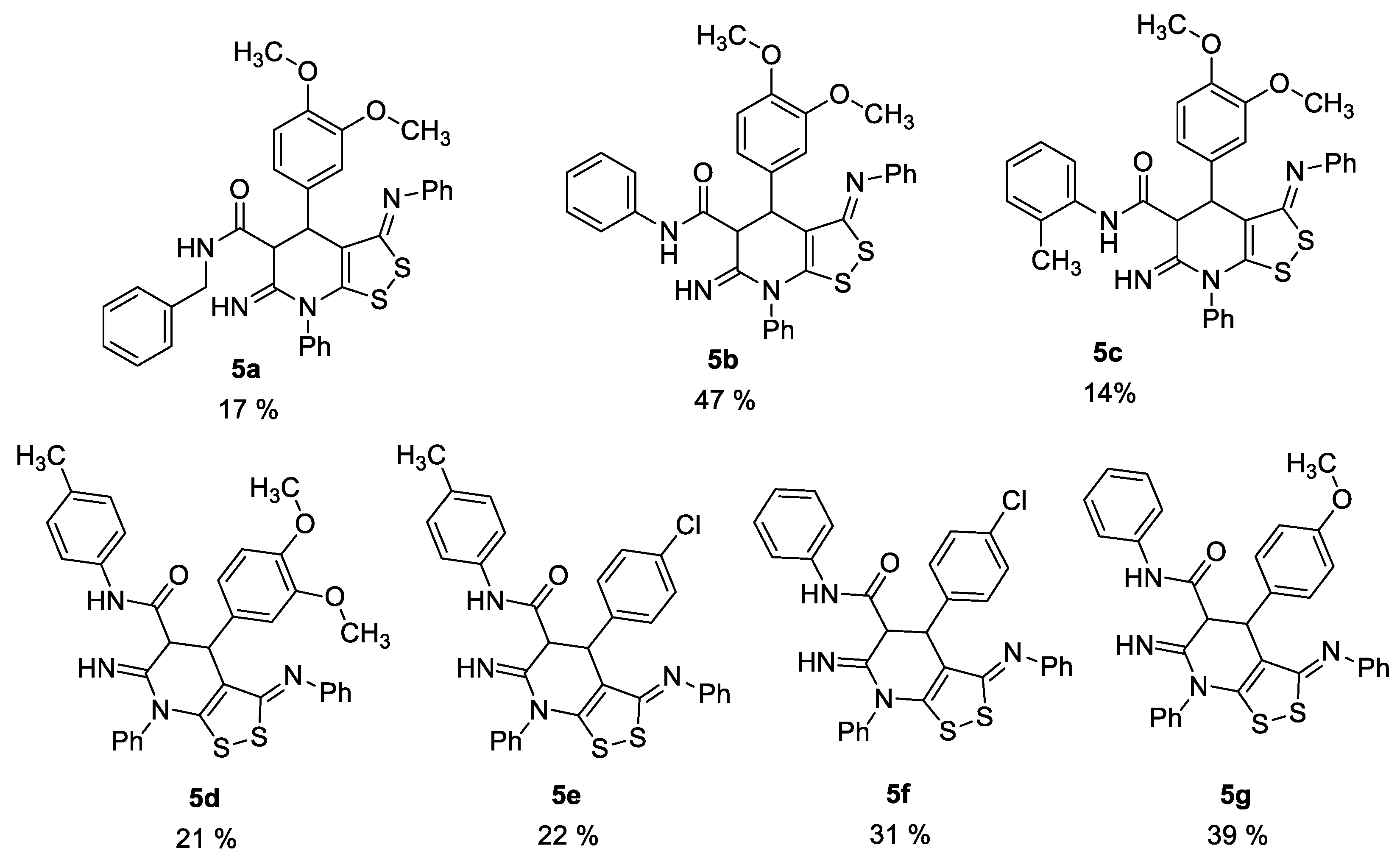
We previously discovered the crucial role of an oxidant in the successful formation of dithiolopyridine core [8], so the synthesis was carried out under air oxygen. Against our expectations, there was no absorption band of amino group in the IR spectra of prepared compounds. Thus, the spectral data indicated the formation of 6-imino-4,5,6,7-tetrahydro-3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxamides 5 (Figure 1), but not 6-amino-4,7-dihydro-3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxamides 4.
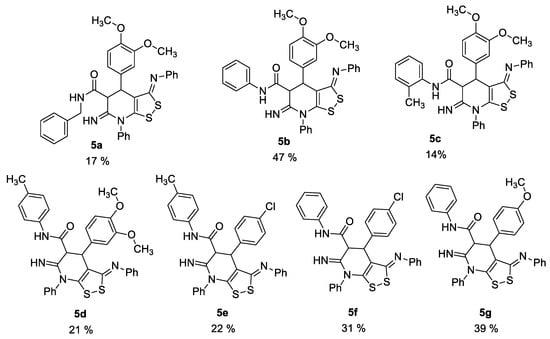
Figure 1.
Structures and yields of the prepared 6-imino-4,5,6,7-tetrahydro-3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxamides 5.
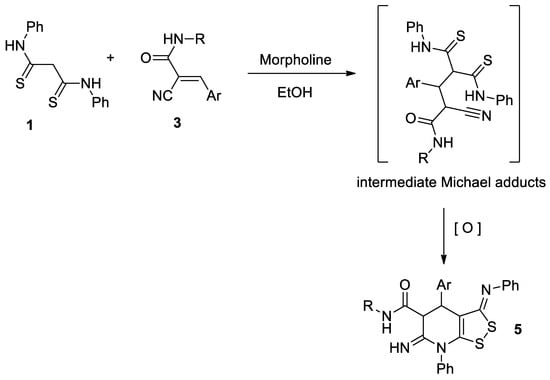
The compounds 5b–g were also prepared by one-pot method involving the formation of cyanoacrylamide 3 in situ from aromatic aldehydes and N-substituted cyanoacetamide, followed by treatment with dithiomalondianilide 1 without isolation of any intermediates (Scheme 4):
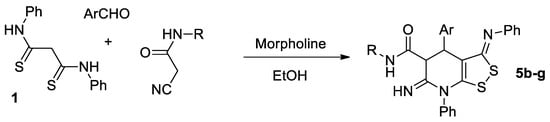
Scheme 4.
One-pot synthesis of 6-imino-4,5,6,7-tetrahydro-3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxamides 5b–g.
3. Experimental
3.1. Procedure for the Preparation of 4,5,6,7-tetrahydro-3H-[1,2]dithiolo[3,4-b]pyridine 5a
Cyanoacrylamide 3a (0.9 mmol) and 0.9 mmol of thioamide 1 were suspended in EtOH, and an excess of morpholine (1.5 mmol) was added. The reaction mixture was then refluxed until thioamide 1 was completely consumed. The reaction was monitored by TLC. Yellow crystalline precipitate was filtered off, washed with ethanol to give [1,2]dithiolo[3,4-b]pyridine 5a.
3.2. Procedure for One-Pot Preparation of 4,5,6,7-tetrahydro-3H-[1,2]dithiolo[3,4-b]pyridines 5b–g
An aromatic aldehyde (1.5 mmol) and corresponding N-substituted cyanoacetamide (1.5 mmol) were dissolved in ethanol (10 mL), and an excess of morpholine (10 mmol) was added. The reaction mixture was heated until cyanoacetamide was consumed completely. Then, an equimolar amount of thioamide 1 was added, and the heating was continued until cyanoacrylamide intermediate was exhausted. The crystalline precipitate was filtered off, washed with ethanol and recrystallized from ethylacetate.
4. Conclusions
Here we report the first example of the synthesis of dithiolotetrahydropyridine-5-carboxamides through the reaction of dithiomalondianilide with N-substituted 3-aryl-2-cyanoacrylamides. A series of new dithiolotetrapyridines was prepared in modest yields (17–47%).
Author Contributions
E.A.V.—investigation, writing (original draft); A.E.S.—investigation; V.V.D.—conceptualization, supervision, investigation, data analysis, funding acquisition, writing (review and editing); N.A.A.—data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 22-23-00458, https://rscf.ru/en/project/22-23-00458 (accessed on 9 October 2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dotsenko, V.V.; Buryi, D.S.; Lukina, D.Y.; Krivokolysko, S.G. Recent advances in the chemistry of thieno[2,3-b]pyridines 1. Methods of synthesis of thieno[2,3-b]pyridines. Russ. Chem. Bull. Int. Ed. 2020, 69, 1829–1858. [Google Scholar] [CrossRef]
- Bakhite, E.A.-G. Recent trends in the chemistry of thienopyridines. Phosphorus Sulfur Silicon Relat. Elem. 2003, 178, 929–992. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Dotsenko, V.V.; Krivokolysko, S.G. The chemistry of thienopyridines. Adv. Heterocycl. Chem. 2007, 93, 117–178. [Google Scholar]
- Sajadikhah, S.S.; Marandi, G. Recent approaches to the synthesis of thieno[2,3-b]pyridines (microreview). Chem. Heterocycl. Compd. 2019, 55, 1171–1173. [Google Scholar] [CrossRef]
- Metwally, M.A.; Abdel-Latif, E.; Bondock, S. Thiocarbamoyl derivatives as synthons in heterocyclic synthesis. J. Sulfur Chem. 2007, 28, 431–466. [Google Scholar] [CrossRef]
- Taubert, K.; Kraus, S.; Schulze, B. Isothiazol-3(2H)-Ones, Part I: Synthesis, reactions and biological activity. J. Sulfur Chem. 2002, 23, 79–121. [Google Scholar] [CrossRef]
- Shafran, Y.; Glukhareva, T.; Dehaen, W.; Bakulev, V. Recent developments in the chemistry of 1,2,3-thiadiazoles. Adv. Heterocycl. Chem. 2018, 126, 109–172. [Google Scholar]
- Dotsenko, V.V.; Sinotsko, A.E.; Strelkov, V.D.; Varzieva, E.A.; Russkikh, A.A.; Levchenko, A.G.; Temerdashev, A.Z.; Aksenov, N.A.; Aksenova, I.V. Alkyl 4-Aryl-6-amino-7-phenyl-3-(phenylimino)-4,7-dihydro- 3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxylates: Synthesis and Agrochemical Studies. Molecules 2023, 28, 609. [Google Scholar] [CrossRef] [PubMed]
- Erami, R.S.; Amirnasr, M.; Meghdadi, S.; Talebian, M.; Farrokhpour, H.; Raeissi, K. Carboxamide derivatives as new corrosion inhibitors for mild steel protection in hydrochloric acid solution. Corros. Sci. 2019, 151, 190–197. [Google Scholar] [CrossRef]
- Luo, B.; Ning, Y. Comprehensive overview of carboxamide derivatives as succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2022, 70, 957–975. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Demir, E.; Bekci, H. Synthesis, characterization and antimicrobial activity of novel xanthene sulfonamide and carboxamide derivatives. J. Enzym. Inhib. Med. Chem. 2013, 28, 885–893. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).