Abstract
Soil is a limited resource that is vital for plant production during the agricultural phase, and consequently, it is a fundamental component of the agro-industrial sector. In a near future, when efficiency in food production will be crucial to feed a growing population, agronomic strategies to ensure food quality need to be tested and optimized with field trials. Taking this into consideration, in 2018, as part of the execution of a fortification workflow for Rocha pears (Pyrus communis L.), field characterization was carried out before the beginning of foliar spraying to identify possible limitations to the increase in calcium in fruits. Thus, in March, soil samples were collected from an orchard (i.e., a parcel with 500 m2) located in the western region of Portugal, where this variety is largely produced. During sample analysis, humidity, organic matter, pH, electrical conductivity and colorimetric parameters were assessed using a CIELab system (with and without organic matter) and mineral analysis was assessed using X-ray fluorescence (of soils and fruits at harvest). Humidity values indicated there was even irrigation in the orchard. Additionally, it was found that organic matter values influenced soil color. The electrical conductivity and pH values were within the recommended range for pomeids. Additionally, higher values of Ca and P prevailed in soils, while K and S contents remained higher in fruits. In conclusion, no major limitations were identified, and field characterization before Ca fortification workflow was useful to assess the orchard’s conditions and possible limitations to nutrient absorption by trees.
1. Introduction
Food is a necessity granted to consumers by agroindustries. However, in the context of an expected increase in global population, set to reach 11 billion people by the end of the century, and likely limitations of water and land resources, maximizing efficiency and reducing waste becomes crucial to achieve sustainability [1,2].
Agricultural land takes up 38% of the global land surface, in which two thirds are meant for livestock, and the remaining third is used as cropland (10% is intended for permanent crops such as fruit trees) [2]. Calcium (Ca) is the third most important element present in soils, but sometimes, its compounds can be unavailable for plant absorption due to its insolubility [3]. Furthermore, besides Ca availability in soils, other factors such as competition with other cations (such as magnesium (Mg) or potassium (K)), transpiration and root growth influence Ca absorption by plants [4].
Currently, in Portugal, over 11,300 ha is used for pear production [5]. This land is mostly occupied with Rocha pear (Pyrus communis L.) orchards, a Portuguese variety, of which more than half of the average annual production of over 170,000 tons is exported [6]. This study hence focused on the physical–chemical assessment of an orchard of pears prior to the execution of a fortification workflow with Ca to identify limiting factors to potential Ca increases in fruits, further considering the mobilization of nutrients from soils to control fruits.
2. Materials and Methods
2.1. Soil Sampling
In an orchard established in Alcobaça, a Portuguese county in the west of Portugal, an agricultural parcel of 20 m× 25 m (thus 500 m2) was selected. From right to left, the tree rows were identified as Control (Ctr), Ca(NO3)2, Null and CaCl2. In late March, using a rectangular grid of 5.70 m× 4 m, a total of 16 soil samples were collected (4 per row). After briefly cleaning the samples of plants and major debris, 600–1000 g from a depth of 30 cm was collected into polyethylene bags for transport.
2.2. Humidity, Organic Matter, Colorimetric and Mineral Analysis
From each sample, humidity and organic matter (OM) were determined as depicted in [7] by firstly drying the soil samples for 24 h at 105 °C, followed by a second step in which samples were heated at 550 °C for 4 h.
After the first and second steps, colorimetric parameters were determined using a Minolta CR 400 colorimeter (Minolta Corp. Ramsey, NJ, USA), as indicated in [8]. Lastly, X-ray fluorescence analysis was performed with an XRF analyzer (Thermo Scientific, Niton model XL3t 950 He GOLDD+, USA) to assess the mineral levels in soils, as described in [9], and in fruits, with minor changes performed regarding sample preparation. After briefly being cleaned with deionized water, fruits were cut and dried (50 °C) until constant weights were achieved, following compaction into pellets.
2.3. Electrical Conductivety and pH Analysis
After the humidity analysis, the electrical conductivity (EC) and the pH of the soil samples were assessed with a potentiometer C6030 (Consort, Turnhout, Belgium), according to [7].
2.4. Statistic
A One-way ANOVA (p ≤ 0.05) was performed to compare tree rows, and a Tukey test was conducted, considering a 95% confidence level.
3. Results
Overall, the agricultural parcel presented values that ranged between 13.0 and 22.2% for humidity, 1.7 and 4.6% for OM, 105.6 and 328.0 µS cm−1 for EC and 5.2 and 7.8 for pH (Figure 1).
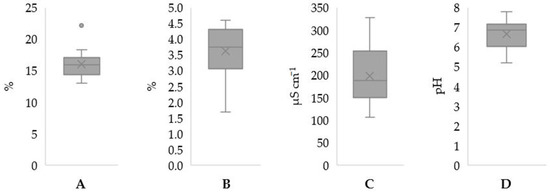
Figure 1.
Boxplots of humidity (A), organic matter (B), electrical conductivity (C) and pH (D) of soil samples (n = 16) from the orchard of Pyrus communis var. Rocha from Alcobaça, Portugal.
For the colorimetric parameters, before the first step (Figure 2), the average values of L, a* and b* varied between 38 and 53, 3 and 5 and 12 and 16, respectively, with the CaCl2 row being significantly higher than the Ctr row and the Null row being significantly inferior to the Ca(NO3)2 and Ctr rows in terms of parameters L and a*, respectively. After the second step (Figure 2), values varied between 40 and 51, 13 and 16 and 18 and 24, respectively, with significant differences occurring for L and b* parameters between rows CaCl2, Ca(NO3)2 and Ctr, with CaCl2 being significantly higher than Ctr.

Figure 2.
Average (n = 3) of colorimetric parameters (L, a* and b*) for each tree row (n = 4) from the orchard before (A) and after (B) organic matter removal. Asterisk (*) represents significant differences between tree rows for each color parameter.
Regarding the mineral content of soils and fruits at harvest (without any foliar sprays) (Figure 3), Ca and phosphorus (P) were superior in soils, while K and sulfur (S) presented higher values in fruits.

Figure 3.
Average content of Ca, K, S and P from soil (light gray), and fruits without foliar sprays at harvest (dark gray), collected from the orchard of Pyrus communis var. Rocha from Alcobaça, Portugal.
4. Discussion
Humidity can depend on precipitation, hydric needs (for the adequate functioning of plants’ metabolism), field drainage and irrigation methods [10]. For this agricultural parcel, and excluding the outlier value (Figure 1), there was a variation of 5.3% between samples, suggesting an adequate drainage system that prevented excessive water accumulation or demonstrated good surface water runoff, overall indicating the even irrigation of the trees [11]. This could be due to the drop-to-drop irrigation system used in the orchard, which is advised for orchards in general to ensure nutrient assimilation from soils and the consequent healthy development of fruits [11].
Organic matter is an indicator of soil quality, influencing nutrient availability, water drainage and color [12], and low values are typical for Portuguese soils [11]. Our values were on average in accordance with another study [13], where the OM values of eight different Rocha pear orchards located in the western region of Portugal varied between 2.46% and 4.68%, with only one sample from our parcel being outside this range. Regarding soil color, in general, the L parameter indicated soil samples with a predominance of darker color (less brightness) and a slight contribution of red (a*) and yellow (b*). This tendency increased after OM removal, except for the L parameter, and on average, a* had a higher difference than the b* parameter. This indicates that OM contributes to the color of soils, which in accordance with other authors [12] as previously mentioned, and its presence caused a decrease in a* and b* parameters, while L was not majorly affected.
Ion accumulation in soils varies with the application of fertilizers and evapotranspiration processes. Additionally, soils with higher salinity values could lead to an increase in energy spent by plants for water absorption, and they could consequently influence nutrient absorption and transport [14]. The EC values from this field were inferior to 600 µS cm−1, which is in accordance with the recommended value for orchards of these trees (pomeids) [11]. Overall, pH values were between 6 and 8 (except three values), which is an adequate interval for agricultural practices [15], since most nutrients are easily absorbed by vegetation. However, Ca and S tend to be less available at a pH of 5 or lower. Although the same can also occur for K, for this nutrient and P, their availability also decreases for higher values of pH [16].
Plants’ appropriate development and growth can be related to 17 elements, which can be acquired from soils, and P, K, Ca and S are required in larger quantities [17]. When considering these four elements and their usual content in soils, the literature [18] indicates a prevalence of K (0.2–3%) and Ca (0.2–1.5%) in comparison to P and S (0.01–0.1%), with our soil values (Figure 3) being in accordance with these proportions. In soils, organic compounds can comprise P and S, while Ca and K are a part of inorganic particles [16]. The higher value of K in soils can be related to its capacity of adsorption to soil particles, resulting in a reserve, and thus, remaining available for plant absorption since it is not easily leached [16]. However, at higher concentrations, this nutrient has an antagonist impact on the absorption of two other cations, namely Mg2+ and Ca2+ [19]. As previously mentioned, Ca is the third most important mineral in soils [3], therefore being a common soil mineral, with dolomite, calcite and gypsum being indicated as the main sources of this mineral [20]. The decline in S content in soils can be associated with the decrease in its content in fungicides, fertilizers, pesticides and industrial activity (SO2 emissions) [21]. In soils, S content fluctuates, cycling between organic (95%) and inorganic forms, from which the presence of sulfate (SO42−) accounts for less than 5% of the total S in soils [17,21]. Phosphorus scarcity in agricultural soils is also increasing; however, like K and nitrogen (N) (accordingly, NPK fertilization), these nutrients are crucial for crops to reach their reproductive stage, with P and N revealing a synergetic interaction and having had a crucial role in food productivity increases [17]. Phosphorus (HPO42−, H2PO4−), S (SO42−), Ca (Ca2+) and K (K+) are acquired by plants in their ionic form [17,18].
For plants, an adequate proportion of elements should pass by K > Ca > P > S [16], and regarding mineral content in pear fruits, K is present in larger quantities in comparison to P and Ca [22], with the same tendency occurring in our data (Figure 3). These results can not only be related to absorption and translocation processes, and hence mobility in plants, but also the physiological functions of these minerals. In plants, Ca and S are classified as immobile, while K and P are classified as mobile [16], and although K is present in larger quantities in the phloem, it is also present in the xylem, whereas Ca mobilization to fruits is mainly associated with the xylemic tissues [23]. Both function as co-factors for enzymes, with Ca performing not only structural roles, but also functioning as a secondary messenger, while K influences cell turgor and electroneutrality [16]. During the early stages, mineral apport to fruits is achieved via the xylem, but with maturity, photoassimilate transport via the phloem increases. Thus, Ca content in fruits tends to be dependent on the early stages, remaining stable or even slightly diminishing, while mobile ions such as K+ and HPO42− are transported to the fruit during all growing seasons (via the xylem and phloem) [24]. Phosphorus is also a component of enzymes, cell membranes and other macromolecules such as nucleic acids, thus being involved in energy production (ATP and ADP), photosynthesis and carbohydrate metabolism [16,17]. Sulfur is involved in amino acid (being a part of two) and protein synthesis, and plants can acquire it not only from soils but also from leaves [16]. Thus, S translocation to protein synthesis tissues (such as fruits) as glutathione has also been reported [16].
5. Conclusions
Considering this parcel’s characteristics, no major limitations were identified, deeming it adequate for the implementation of a foliar spray workflow. The presence of organic matter influenced the colorimetric parameters of soils. The results further showed that there were no constraints to nutrient absorption due to the presence of an adequate irrigation system, no necessity of additional energy spent by trees during nutrient absorption from soil, and pH values adequate for nutrients availability. Hence, no concerns arose regarding water limitations or salinity during nutrient absorption by roots. The mineral values from soil and fruit were in accordance with values in the literature, and availability, interaction, transport and physiological functions contribute to their proportions.
Author Contributions
Conceptualization, J.C.R., P.S.C. and F.C.L.; methodology, P.L., M.S. and F.C.L.; formal analysis, C.C.P., A.R.F.C., D.D., I.C.L. and A.C.M.; resources, M.M.S., P.L., F.H.R., M.F.P., M.S. and F.C.L.; writing—original draft preparation, C.C.P.; writing—review and editing, C.C.P. and F.C.L.; supervision, F.C.L.; project administration, F.C.L.; funding acquisition, F.C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PDR2020, grant number 101-030734. Funding from Fundação para a Ciência e Tecnologia (FCT) UI/BD/150718/2020 is also greatly acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thanks to Tiago Peralta and Ribamaior—Produção e Comércio de Frutas Lda. for technical assistance. We also give thanks to the research centers (GeoBioTec) UIDB/04035/2020 and (CEF) UIDB/00239/2020 for support facilities.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- FAO—Food and Agriculture Organization of the United Nations. The Future of Food and Agriculture—Trends and Challenges; FAO: Rome, Italy, 2017; pp. 3–16. ISBN 978-92-5-109551-5. [Google Scholar]
- FAO—Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/sustainability/news/detail/en/c/1274219/ (accessed on 22 September 2021).
- D’Imperio, M.; Renna, M.; Cardinali, A.; Buttaro, D.; Serio, F.; Santamaria, P. Calcium biofortification and bioaccessibility in soilless “baby leaf” vegetable production. Food Chem. 2016, 213, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Bonomelli, C.; Gil, P.M.; Schaffer, B. Effect of soil type on calcium absorption and partitioning in young avocado (Persea americana Mill.) Trees. Agronomy 2019, 9, 837. [Google Scholar] [CrossRef] [Green Version]
- INE—Instituto Nacional de Estatística. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_publicacoes&PUBLICACOESpub_boui=437147278&PUBLICACOESmodo=2 (accessed on 22 September 2021).
- ANP—Associação Nacional de Produtores de Pera Rocha. Available online: https://perarocha.pt/anp/ (accessed on 22 September 2021).
- Marques, A.C.; Lidon, F.C.; Coelho, A.R.F.; Pessoa, C.C.; Luís, I.C.; Scotti-Campos, P.; Simões, M.; Almeida, A.S.; Legoinha, P.; Pessoa, M.F.; et al. Quantification and tissue localization of selenium in rice (Oryza sativa L., Poaceae) grains: A perspective of agronomic biofortification. Plants 2020, 9, 1670. [Google Scholar] [CrossRef] [PubMed]
- Luís, I.C.; Lidon, F.C.; Pessoa, C.C.; Marques, A.C.; Coelho, A.R.F.; Simões, M.; Patanita, M.; Dôres, J.; Ramalho, J.C.; Silva, M.M.; et al. Zinc enrichment in two contrasting genotypes of Triticum aestivum L grains: Interactions between edaphic conditions and foliar fertilizers. Plants 2021, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.R.F.; Lidon, F.C.; Pessoa, C.C.; Marques, A.C.; Luís, I.C.; Caleiro, J.C.; Simões, M.; Kullberg, J.; Legoinha, P.; Brito, G.; et al. Can foliar pulverization with CaCl2 and Ca(NO3)2 trigger Ca enrichment in Solanum Tuberosum L. tubers? Plants 2021, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Santos, W.; Oliveira, G.; Lima, J.; Curi, N.; Marques, J. Soil moisture space-time analysis to support improved crop management. Ciência Agrotecnologia 2015, 39, 39–47. [Google Scholar] [CrossRef] [Green Version]
- DGADR (Direção-Geral de Agricultura e Desenvolvimento Rural). Normas técnicas para a produção integrada de pomóideas (Volume II). Available online: https://www.dgadr.gov.pt/mediateca/send/8-protecao-e-producao-integradas/110-normas-tecnicas-para-producao-integrada-de-pomoideas-vol-ii (accessed on 17 September 2021).
- Margesin, R.; Schinner, F. (Eds.) Manual of Soil Analysis—Monitoring and Assessing Soil Bioremediation; Springer: Berlin/Heidelberg, Germany, 2005; p. 366. [Google Scholar] [CrossRef]
- Mendes, R. Pools de Nutrientes em Pomares e sua Relação com a Incidência de Acastanhamentos Internos em pera Rocha. Master’s Thesis, Instituto Superior de Agronomia, Universidade de Lisboa, Lisbon, Portugal, 2017. Available online: http://hdl.handle.net/10400.5/14841 (accessed on 23 September 2021).
- Visconti, F.; de Paz, J.M. Electrical conductivity measurements in agriculture: The assessment of soil salinity. In New Trends and Developments in Metrology; Cocco, L., Ed.; IntechOpen: London, UK, 2016; pp. 99–126. [Google Scholar]
- Läuchli, A.; Grattan, S. Soil pH extremes. In Plant Stress Physiology; Shabala, S., Ed.; CAB International: Cambridge, UK, 2012; pp. 194–209. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates: Sunderland, UK, 2002; p. 623. [Google Scholar]
- Kumar, A.; Kumar, S.; Mohapatra, T. Interaction between macro- and micro-nutrients in plants. Front. Plant Sci. 2021, 12, 665583. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.R.; Alshaal, T.A.; Amer, M.; Domokos-Szabolcsy, É.; Elhawat, N.; Prokisch, J.; Fári, M. Soil quality and plant nutrition. In Sustainable Agriculture Reviews 14; Ozier-Lafontaine, H., Lesueur-Jannoyer, M., Eds.; Springer: Cham, Switzerland, 2014; Volume 14, pp. 345–447. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient interactions in crop plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 73. [Google Scholar]
- Scherer, H.W. Sulfur in soils. J. Plant Nutr. Soil Sci. 2009, 172, 326–335. [Google Scholar] [CrossRef]
- PortFIR. PortFIR—Pear Dehydrated. Available online: http://portfir.insa.pt/foodcomp/food?21322 (accessed on 18 October 2021).
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, R.G.; Brummell, D.A.; Burdon, J.N.; Patterson, K.J.; Schaffer, R.J. Chapter 11—Fruit growth, ripening and post-harvest physiology. In Plants in Action, 2nd ed.; Brummel, D.A., Ed.; (Chapter Editor); Australian Society of Plant Scientists: Melbourne, Australia, 2017; Available online: http://plantsinaction.science.uq.edu.au (accessed on 23 September 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).