Abstract
Background: Diabetic kidney disease (DKD) is a leading cause of end-stage kidney disease (ESKD) worldwide. This review examines the potential differences in clinical presentation, outcomes, and management between individuals with proteinuric DKD (P-DKD) and non-proteinuric DKD (NP-DKD). Methods: We analyzed articles published globally from 2000 and 2024. Results: Individuals with NP-DKD generally have lower blood pressure levels and a more favorable lipid profile. In contrast, histological studies show that P-DKD is associated with more severe glomerulosclerosis, mesangial expansion, arteriolar hyalinosis, interstitial-fibrosis/tubular atrophy, and immune complex deposits. Additionally, those with P-DKD are more likely to develop diabetic retinopathy and have a higher risk of all-cause mortality and progression to ESKD. Strategies to slow DKD progression, applicable to both NP-DKD and P-DKD, include non-pharmacologic and pharmacologic interventions such as renin–angiotensin system blockers, sodium-glucose co-transporter-2 inhibitors, finerenone, and glucagon-like protein receptor agonists. Conclusions: NP-DKD and P-DKD represent different presentations of the same underlying disease.
1. Introduction
Diabetic kidney disease (DKD) accounts for nearly half of all chronic kidney disease (CKD) cases [1] and is the leading cause of end-stage renal disease (ESKD) in the general population [2].
DKD is classified into stages based on the progression of albuminuria and the decline in the estimated glomerular filtration rate (eGFR) [3]. Clinically, it is characterized by persistent albuminuria and/or reduced renal function, confirmed by two measurements taken at least three months apart [4]. DKD can be further categorized into proteinuric DKD (P-DKD) and non-proteinuric DKD (NP-DKD), with albumin being the primary protein found in the urine of diabetic patients. Non-proteinuric DKD (NP-DKD) is defined by an eGFR of less than 60 mL/min/1.73 m2 and a urinary albumin–creatinine ratio (UACR) below 300 mg/g [5], or more recently below 30 mg/g [3].
Under normal circumstances, only a small amount of albumin passes through the glomerular filtration barrier, and most of this is reabsorbed by the proximal renal tubular cells. Therefore, albuminuria is commonly associated with kidney diseases that affect the glomerulus or proximal tubule [6].
Following glomerular filtration, an excess of protein in the proximal tubule surpasses its capacity to reabsorb albumin through endocytosis after binding by the megalin–cubilin receptor complex [6].
Albuminuria is a well-established risk factor for the progression of DKD [7], although it has limitations, such as the potential for spontaneous regression [8]. In addition to being a marker of kidney disease severity, albuminuria has a direct toxic effect on the kidneys. It contributes to cellular apoptosis, senescence, overproduction of reactive oxygen species, endoplasmic reticulum stress, and epithelial-mesenchymal transition in proximal renal tubular epithelial cells, leading to an unfolded protein response and DNA damage response [9]. Moreover, excessive albumin levels increase the expression of cell cycle arrest inducers p21 and p16, reduce the level of the cellular proliferation marker Ki-67, and raise the level of the cellular senescence marker β-galactosidase [9].
This review aims to determine if there are clinical, outcome and management differences between individuals with proteinuric DKD (P-DKD) and those with non-proteinuric DKD (NP-DKD).
2. Materials and Methods
To gather information for this review, searches were performed in Scopus, Web of Science, Embase, PubMed, and MEDLINE. The review covered articles published globally from 2000 to 2024, with 50% of the selected articles published within the past 5 years.
3. Results and Discussion
The findings of this review are organized into six sessions, outlined below:
3.1. Natural History of DKD
Traditionally, in the natural progression of DKD, the onset of overt proteinuria usually occurs before a more rapid decline in eGFR. However, over the past decade, numerous studies have shown that many diabetic patients with reduced eGFR do not develop proteinuria [10,11]. For example, a study at the Steno Diabetes Center found that approximately 20% of diabetic patients without albuminuria never developed proteinuria before progressing to ESKD, indicating that proteinuria is not a necessary precursor for ESKD [12]. The risk of CKD progression is depicted in Figure 1A,B, with the frequency of evaluations and referrals to a nephrologist stratified as shown in Figure 1A [13].
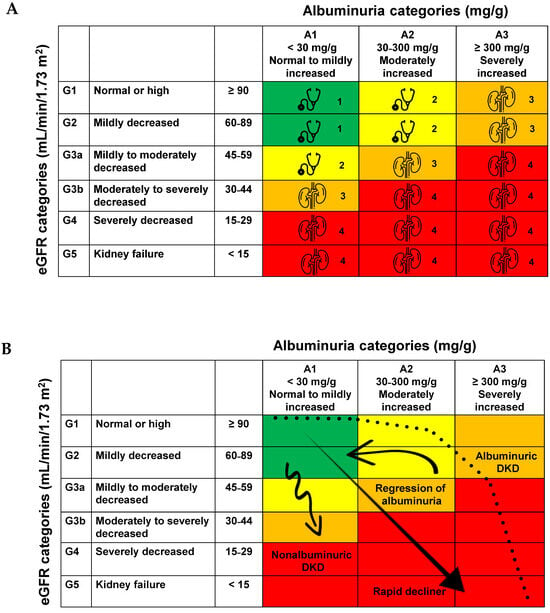
Figure 1.
(A). Risk of chronic kidney disease progression, frequency of visits and referral to nephrology, as indicated by the kidney icon, are stratified based on eGFR and albuminuria. Adapted from [13]. The eGFR and albuminuria grid illustrates the risk through color coding, ranging from favorable to unfavorable (green, yellow, orange, red, deep red). (B). Trajectories of kidney function in DKD. Adapted from [8].
3.2. NP-DKD
Recent reports suggest that the prevalence of NP-DKD ranges from 20% to 40% [14], with a prevalence of 20% among patients with type 1 diabetes (T1DM) and approximately 40% among those with type 2 diabetes mellitus (T2DM) [15,16]. However, some studies have reported an even higher prevalence of NP-DKD among diabetic patients, with estimates reaching 50–60%, as shown in Table 1. The factors contributing to this DKD phenotype are not fully understood but may include an increase in the number of elderly patients, as well as those with DM, hypertension, dyslipidemia, obesity, hyperuricemia, microangiopathy, or more intensive treatment regimens, including the use of renoprotective agents [15].

Table 1.
Prevalence, clinical and demographic parameters and outcomes in diabetic patients according to proteinuria.
Table 1 summarizes the key aspects of NP-DKD, including its prevalence, associated clinical and demographic parameters, and distinct patterns of eGFR decline over time according to proteinuria [10,11,12,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Notably, patients with T2DM may experience significant renal impairment while remaining normoalbuminuric or microalbuminuric.
Although the risk of progression to ESKD is relatively lower in NP-DKD when compared to P-DKD, analyses of the Kidney Early Evaluation Program cohort revealed that the adjusted incidence rate for ESKD in these populations was 7.8 times higher than that of non-albuminuric patients without DM [31]. Thus, the risks of cardiovascular diseases and other complications of CKD remain high in these populations [32].
3.3. Clinical, Laboratory, and Morphological Parameters Associated with P-DKD vs. NP-DKD
Research shows that clinical factors associated with NP-DKD include female sex, hypertension, smoking, poor glycemic control, absence of diabetic retinopathy, and use of the RAAS inhibitors [10,33]. In a prospective, observational study of 400 patients with T2DM who had significant proteinuria (>500 mg/day) and/or a reduced eGFR < 60 mL/min/1.73 m2, patients were categorized into two groups based on their urine protein-creatinine ratio [34]. Among these, 106 patients (26.5%) were identified with NP-DKD. This group exhibited a higher eGFR at the start of the study, at 6 months, and after 1 year, indicating a slower decline in eGFR compared to those with P-DKD. The NP-DKD group was significantly older (56.5 ± 2.1 vs. 54.7 ± 11.6 years), had a lower prevalence of diabetic retinopathy (46.2% vs. 74.1%), higher hemoglobin levels (11.3 ± 1.7 vs. 10.5 ± 2.0 g/dL), and higher cholesterol levels (169.3 ± 43.3 vs. 157.1 ± 58.1 mg/dL) [34].
The NEFRON study found that T2DM was associated with a higher incidence of albuminuric renal impairment compared to the general non-diabetic population [11]. Diabetic patients with an eGFR < 60 mL/min/1.73 m2 and albuminuria were more likely to have a history of hypertension, retinopathy, macrovascular disease, or a first-degree relative with CKD compared to those with an eGFR ≥ 60 mL/min/1.73 m2. This association was not seen in T2DM patients with normoalbuminuric renal impairment. However, diabetic individuals with an eGFR < 60 mL/min/1.73 m2, both with and without albuminuria, exhibited significantly higher rates of visual impairment, atrial fibrillation, and heart failure compared to diabetic individuals with an eGFR ≥ 60 mL/min/1.73 m2. These findings highlight the importance of regularly assessing both eGFR and proteinuria in diabetic patients.
Conversely, the prevalence of diabetic retinopathy was significantly higher in patients with P-DKD compared to those with NP-DKD (66.4% vs. 38.9%, respectively). NP-DKD patients had better renal outcomes and maintained significantly higher serum albumin levels compared to those with P-DKD (41.11 ± 3.61 g/L vs. 32.65 ± 5.81 g/L, respectively) [35]. NP-DKD patients also had lower levels of LDL and HDL cholesterol compared to P-DKD patients (2.07 [1.71–2.37] mmol/L vs. 2.80 [2.10–3.42] mmol/L; and 0.81 [0.64–0.99] mmol/L vs. 0.92 [0.84–1.12] mmol/L, respectively). To note, there was no significant difference in the use of RAAS inhibitors between these two groups.
Additionally, Yamanouchi et al. [5] found that patients with NP-DKD had better blood pressure control despite less frequent use of RAAS inhibitors. This is consistent with the established link between elevated systolic blood pressure and increased albuminuria [36]. The lower use of anti-RAAS drugs may also be due to factors such as hyperkalemia or renal artery stenosis [3].
Furthermore, a biopsy-based cohort study employing a propensity-score matched analysis not only revealed that patients with NP-DKD exhibited lower levels of systolic and diastolic blood pressure along with a more favorable lipid profile, but also demonstrated a significantly higher 5-year CKD progression-free survival when compared to individuals with P-DKD (86.6% vs. 30.3%) [28]. Importantly, the 5-year death-free survival rates were 98.4% for NP-DKD and 87.5% for the P-DKD group, and these differences remained statistically significant in the propensity-matched cohort (98.3% vs. 82.6%, respectively). These findings emphasize the association of proteinuria as a key indicator of worse renal outcomes and mortality in patients with CKD.
Additionally, urinary levels of MCP-1 [37] and tumor necrosis factor alpha (TNF-α) [38] were found to be higher in P-DKD patients (A2 and A3) compared to NP-DKD patients (A1). However, in a post hoc analysis, urinary CXCL8 levels (a chemokine that regulates acute inflammatory response) exhibited no significant differences among A2 and A3 patients, despite being higher in these groups in contrast to A1 patients [39].
3.4. Histopathology Associated with P-DKD vs. NP-DKD
There are few studies comparing renal biopsy findings between patients with P-DKD and NP-DKD, and no distinct histopathological features are specific to the non-proteinuric phenotype [16]. Compared to P-DKD patients, those with NP-DKD typically show less severe glomerular lesions, such as reduced mesangial expansion and fewer nodular sclerosis (Kimmelstiel–Wilson lesions) [35,36].
The incidence of arteriolar hyalinosis was significantly lower in the NP-DKD group compared to the P-DKD group (66.7% vs. 88.9%) [35]. Additionally, NP_DKD patients had lower deposition of IgM and C1q deposition on direct immunofluorescence compared to P-DKD patients (11.1% vs. 77.8% for IgM, and 0.0% vs. 58.3% for C1q) [35]. Although proteinuric patients had significantly higher C3 deposition overall, C3 and C4 levels serum levels were similar between P-DKD and NP-DKD groups. Complement deposition in the kidney, particularly C1q and C3, correlates with more severe renal damage in DKD, including functional impairment (lower eGFR and higher proteinuria) and structural damage (interstitial fibrosis and tubular atrophy [IFTA], interstitial inflammation, vascular lesions, and global sclerosis) [40]. IFTA is a valuable predictor of kidney prognosis in both NP-DKD and P-DKD [28,41].
Importantly, in a matched-propensity score cohort, P-DKD patients exhibited more severe histological kidney alterations according to several diabetic-based classifications (Fioretto, Tervaert, and Japanese) [28]. All kidney compartments showed significant damage in P-DKD compared to individuals with NP-DKD.
3.5. Treatment of P-DKD vs. NP-DKD
Optimizing the control of hyperglycemia, hypertension and dyslipidemia is crucial for preventing the progression of kidney disease in proteinuric patients [42]. This approach should likely also be considered in the case of NP-DKD; however, there is limited evidence regarding these interventions in non-proteinuric patients [42]. It is noteworthy that clinical trials generally did not include patients with low levels of albuminuria. Additionally, patients already using RAAS blockers to control proteinuria were often randomized into these trials, which contributed to overlooking the potential benefits of adding other drugs when evaluating proteinuria reduction as an outcome.
RAAS blockers have already demonstrated efficacy in slowing DKD be approximately 5–7 mL/min/year across several trials, compared to the normal 0.7–0.9 mL/min/year [43]. As reviewed elsewhere, these drugs reduce the progression of UACR from A1 to A2 (trials BENEDICT, ROADMAP, RASS, and ADVANCE), from A2 to A3 (trials IRMA-2 and INNOVATION), progression to ESKD (trials ADVANCE, RENAAL, IDNT, and ORIENT) and mortality (trials RENAAL, IDNT, and ORIENT) [44].
Therefore, their use is recommended owing to their beneficial hemodynamic effects (reduction in glomerular hypertension) and non-hemodynamic effects (reduction in inflammation, oxidative stress, and fibrosis).
Regarding the use of sodium-glucose cotransporter-2 inhibitors (SGLT2i), the EMPA-REG study evaluated the efficacy of empagliflozin in the treatment of DKD. In this trial, most patients were categorized as A1 based on UACR, both in the group with eGFR less than 60 mL/min/1.73 m2 (A1: 47%, A2: 34%, A3: 19%) and in the group with more than 60 mL/min/1.73 m2 (A1: 64%, A2: 27%, A3: 8%), for both the placebo and the empagliflozin groups. Additionally, 80% of the patients were on RAAS blockers [45]. Importantly, the group treated with empagliflozin showed a 39% reduction in incident or worsening nephropathy or cardiovascular death, a 44% reduction in the doubling of serum creatinine, and a 55% decrease in the need for renal replacement therapy. One key finding of the EMPA-REG study was that the progression from A1–A2 to A3 albuminuria decreased by 39%, underscoring the importance of adding SGLT2i to reduce DKD progression and mortality.
In the DAPA-CKD study (n = 4304), both diabetic (67.5%) and non-diabetic (32.5%) individuals with an eGFR ranging from 25 to 75 mL/min/1.73 m2 and an UACR of 200–5000 mg/g were evaluated [46]. The hazard ratio (HR) for the primary composite outcome-defined as a sustained decline in the eGFR of at least 50%, ESKD, or death from renal or cardiovascular causes- was 0.61 (95% confidence interval [CI], 0.51–0.72). Importantly, the benefit of dapagliflozin was consistent across different UACR levels, with an HR of 0.54 for values ≤ 1000 and an HR of 0.62 for values > 1000 mg/g.
Subsequently, in the EMPA-KIDNEY study, which included patients with eGFR ≥ 20–45 or eGFR ≥ 45 to <90 mL/min/1.73 m2 with UACR ≥ 200 mg/g, with or without DM, empagliflozin was associated with a 28% reduction in the progression of kidney disease or death from cardiovascular causes [47]. Notably, 98% of participants were on RAAS blockers, and the benefits of empagliflozin were consistent across eGFR levels at randomization. The proportional risk reduction varied with UACR levels: HR = 0.67 (0.58–0.79) for ≥300 mg/g, HR = 0.91 (0.65–1.26) for 30–300 mg/g, and HR = 1.01 (0.66–1.55) for ≤30 mg/g, regardless of DM. Over the chronic slopes in eGFR from 2 months to final follow-up, there was a between-group difference of 1.37 mL/min/1.73 m2/year (95% CI 1.16 to 1.59). Prespecified exploratory analyses by subgroups revealed that the rate of decline of eGFR (chronic slope) was slower in the empagliflozin group across all key subgroups, including patients with low UACR [48].
Differences in the rate of eGFR decline were more pronounced in subgroups with faster annual decline, such as patients with DM, higher eGFR, or higher baseline UACR [47]. Specifically, the impact of empagliflozin on the differences in eGFR decline (in mL/min/1.73 m2/year) were more significant in these faster-declining subgroups:
- DM (−1.05 vs. −2.73; absolute difference +1.68 and relative difference −62%) compared to non-DM (−1.66 vs. −2.75; absolute difference +1.09 and relative difference −40%);
- eGFR < 30 (−1.84 vs. −2.85; absolute difference +1.01 and relative difference −35%) compared to ≥30–45 (−1.18 vs. −2.50; absolute difference +1.32 and relative difference −53%) and ≥45 (−1.58 vs. −3.60; absolute difference +2.01 and relative difference −56%);
- UACR < 30 mg/g (−0.11 vs. −0.89; absolute difference +0.78 and relative difference −87%) compared to UACR ≥ 30–300 mg/g (−0.49 vs. −1.69; absolute difference +1.20 and relative difference −71%) and UACR ≥ 300 mg/g (−2.35 vs. −4.11; absolute difference +1.76 and relative difference −43%.
Additionally, finerenone, a nonsteroidal mineralocorticoid receptor antagonist, has demonstrated robust evidence of cardiorenal benefits in patients with CKD and T2DM across a broad spectrum of CKD severity. In the FIDELITY study [48], a prespecified pooled analysis of FIDELIO-DKD (more severe CKD; mean eGFR 44.3 mL/min/1.73 m2, median UACR 851 mg/g, and UACR ≥ 300 mg/g in 87.4%) and FIGARO-DKD (less severe CKD; mean eGFR 67.8 mL/min/1.73 m2, median UACR 312 mg/g, and UACR ≥ 300 mg/g in 51.2%), finerenone was associated with a 33% reduction in kidney composite outcomes and a 14% reduction in cardiovascular composite outcomes. It is noteworthy that the benefits of finerenone over a placebo concerning cardiorenal outcomes in patients with both CKD and T2DM were observed, irrespective of SGLT2i usage [49].
Therefore, we now have two evidence-based medications, SGLT2i and finerenone, which, when combined with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, are proven to slow DKD progression to approximately 2.5–3 mL/min/year, provided blood pressure and glucose levels are maintained at guideline goals [43].
Concerning the use of glucagon-like peptide 1 receptor agonists (GLP-1 RA), in the SUSTAIN 1–7 studies, eGFR did not differ between semaglutide and placebo. In the SUSTAIN 1–6 studies, UACR decreased in patients with pre-existing stage 2 or 3 UACR; however, it did not change or increased in those with normoalbuminuria at baseline [50].
Additionally, post hoc analyses of the Semaglutide Treatment Effect in People with obesity (STEP) 2 clinical trial explored the effects of semaglutide associated with lifestyle interventions on renal function [51]. At week 68, the changes in UACR were −14.8% and −20.6% with semaglutide 1.0 mg and 2.4 mg, respectively, and +18.3% with placebo (differences between groups compared to placebo: −28.0% for semaglutide 1.0 mg; −32.9% for semaglutide 2.4 mg). The average percentage difference in UACR compared to placebo at week 68 was −27.2% and −30.5% in the semaglutide 1.0 mg and 2.4 mg groups, respectively (all significant).
In the SURPASS-4 study, participants with T2DM who were treated with a combination of metformin, sulfonylurea, or SGLT2i had a mean eGFR of 81.3 ± 21.1 mL/min/1.73 m2 and a median UACR of 15.0 mg/g (IQR 5.0–55.8) [52]. Participants were randomly assigned in a 1:1:1:3 ratio to receive weekly subcutaneous injection of tirzepatide (5 mg, 10 mg, or 15 mg) or insulin glargine (100 U/mL), with titration to achieve fasting blood glucose levels below 100 mg/dL. During the follow-up, UACR increased by 36.9% in the insulin glargine group, but decreased by 6.8% in the tirzepatide group, resulting in a −31.9% difference between the two groups. Those receiving tirzepatide had a significantly lower occurrence of the composite renal outcome, which included a decline in eGFR ≥ 40% from baseline, renal death, renal failure, or UACR > 300 mg/g, compared to those receiving insulin glargine (HR = 0.58).
In the FLOW trial, patients with type 2 diabetes mellitus (T2DM) were randomly assigned to either a semaglutide or a placebo group [53]. The baseline laboratory data showed a mean estimated glomerular filtration rate (eGFR) of 47 mL/min/1.73 m2 and a median urinary albumin-to-creatinine ratio (UACR) of 567.6 mg/g (A1: 3%, A2: 29%, A3: 68%). The use of renoprotective medications included SGLT2i (16%), angiotensin-converting enzyme inhibitors (35%), and angiotensin II receptor blockers (60%). The main findings in the semaglutide group included a reduction in the first major kidney disease outcome (kidney failure, at least 50% reduction in the eGFR from baseline, or death from kidney-related or cardiovascular causes; HR = 0.76, 95% CI: 0.66–0.88, p = 0.0003), the first kidney-specific component event (HR = 0.79), death from cardiovascular causes (HR = 0.79), death from any cause (HR = 0.80), and a difference in the annual eGFR slope (1.16 mL/min/1.73 m2, 95% CI: 0.86–1.46, p < 0.001). Notably, the UACR was reduced by 40% in the semaglutide group compared to 12% in the placebo group, resulting in a ratio at week 104 that was 32% lower (95% CI: 25–38) in the semaglutide group than in the placebo group. Subgroup analysis of the primary outcome revealed a beneficial effect of semaglutide in patients with UACR ≥ 300 mg/g (HR = 0.74, 95% CI: 0.63–0.87) compared to those with UACR < 300 mg/g (HR = 0.86, 95% CI: 0.60–1.23).
Importantly, further studies are warranted to compare RAAS blockade, including finerenone, and SGLT2i combined with semaglutide. These studies should aim to determine whether patients with lower values of albuminuria would progress at a similar or slower rate compared to patients with higher values of albuminuria or those in the placebo group. These findings also highlight the importance of validating novel biomarkers to assess DKD progression regardless of albuminuria levels.
Therefore, the management of DKD should be grounded in our comprehension of the various interrelated pathophysiological mechanisms encompassing hemodynamic, metabolic, and inflammatory pathways. Given that these pathways play a crucial role in the initiation and progression of DKD, it is essential to prioritize the validation of kidney damage-associated biomarkers alongside both non-pharmacological and pharmacological approaches, in particular RAAS inhibitors, SGLT2i, finerenone, and GLP-1 RA [43]. This holistic approach is of paramount importance in halting the progression of DKD, irrespective of albuminuria levels (Figure 2). It is essential to highlight that the KDIGO guidelines do not recommend different therapeutic approaches between P-DKD and NP-DKD [3]. Additionally, lifestyle modification and self-management should be encouraged for all diabetic patients, including adopting a healthy diet, smoking cessation, weight control, and regular exercise. For patients with DM and CKD who are not on dialysis, it is recommended that protein intake be limited to 0.8 g/kg body weight per day [3].
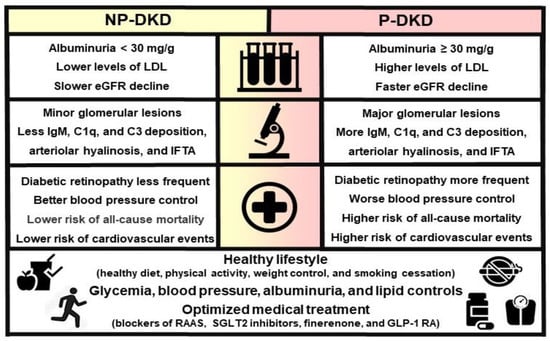
Figure 2.
Laboratory, clinical, histological and prognostic differences between NP-DKD and P-DKD.
3.6. Perspectives
Markers of proximal tubule and glomeruli injury, along with markers of inflammation in DKD could offer valuable clinical insights for evaluating disease progression, identifying different patterns, or verifying the potential therapeutic of pharmacological and non-pharmacological approaches. Notably, the correlation of these markers with varying levels of proteinuria and eGFR could contribute to advancing our understanding of the key mechanistic properties of DKD.
UACR, RBP (retinol-binding protein), and MCP-1 (monocyte chemoattractant protein-1) were associated with the progression of DKD across all levels of albuminuria. In patients with NP-DKD, MCP-1, IL-6, and NGAL (neutrophil gelatinase-associated lipocalin) were linked to progressive CKD, while NAG (N-acetyl-β-d-glucosaminidase), a lysosomal enzyme, was indicative of early kidney damage, showing a significant association with an eGFR below 60 mL/min/1.73 m2 in the UACR < 3 mg/g cohort [54]. This underscores the importance of validating these biomarkers for diagnosing and monitoring DKD progression in conjunction with albuminuria in larger studies. Furthermore, assessing the variability of kidney damage-associated biomarkers is essential to verify the effectiveness of current standard-of-care treatments for DKD, given that its pathophysiological mechanisms involve hemodynamic, metabolic, and inflammatory pathways [43].
In a multivariate analysis, NP-DKD patients exhibited IFTA as risk factors for disease progression [5]. These findings align with prior reports, emphasizing the significant role of interstitial injury in eGFR decline [55,56]. This eGFR decline in the context of NP-DKD has been shown to predict cardiovascular events [57].
In this setting, markers of tubular injury may prove more useful in the future to stratify those whose renal function declines. Liver-type fatty acid-binding protein (L-FABP) and heart-type fatty acid-binding protein (H-FABP) are promising indicators of tubular, but not glomerular, damage.
L-FABP is expressed in the proximal tubules of the human kidney, where it plays a role in fatty acid metabolism and serves as a potential marker for tubular, but not glomerular, damage [58]. In patients with normoalbuminuria, urinary levels of L-FABP and albumin were significantly higher than normal controls. Urinary levels of L-FABP and albumin showed significant differences across the control, normoalbuminuric, microalbuminuric, macroalbuminuric, and ESKD groups, with levels increasing with the severity of DKD. While urinary L-FABP levels were significantly correlated with urinary albumin levels in the overall population, this correlation was absent in the subgroup of patients with an eGFR > 60 mL/min/1.73 m2. Importantly, the area under the curve (AUC) for L-FABP in predicting DKD progression was 0.849. Additionally, Cox regression analysis revealed that elevated urinary L-FABP levels at baseline were significantly associated with DKD progression (adjusted HR [aHR] = 9.45).
In diabetic animals, the urinary excretion of biomarkers such as heart-type fatty acid-binding protein (H-FABP), osteopontin (OPN), nephrin, neutrophil gelatinase-associated lipocalin (NGAL) was significantly elevated compared to non-diabetic animals [59]. Similarly, plasma levels of kidney injury molecule-1 (KIM-1), clusterin, OPN, and tissue inhibitor of metalloproteinases-1 (TIMP-1) were notably higher in diabetic animals. These biomarkers were detectable even before the onset of traditional indicators of DKD, such as albuminuria and urinary protein excretion.
Understanding the inflammatory mechanisms is crucial for comprehending DKD [60]. The Kidney Risk Inflammatory Signature (KRIS), which consists of 17 proteins enriched in tumor necrosis factor receptor superfamily members, has been associated with a 10-year risk of ESKD in both T1DM and T2DM [61]. These proteins have shown significant albuminuria-independent (−9.3 mL/min/1.73 m2 per year per 1 log10 increase in TNF-R1) and albuminuria-mediated (−4.8 mL/min/1.73 m2 per year per 1 log10 increase in TNF-R1) effects on renal function. The total effect of a 1 log10 increase in TNF-R1 on the eGFR slope was −14.1 mL/min/1.73 m2 per year. Notably, 66% of TNF-R1′s impact was albuminuria-independent, suggesting that KRIS proteins significantly contribute to renal function decline, predominantly through pathways independent of albuminuria, though both effects were significant. Therefore, albuminuria might be considered an intermediate phenotype—a risk indicator rather than a direct risk factor in the disease process leading to ESKD.
Additionally, the CKD273 classifier emerges as a promising proteomic biomarker for the early detection of non-proteinuric patients at high risk for progressive DKD [62]. This biomarker outperforms albuminuria in predicting the risk of progression both more effectively and earlier. In a cohort study of patients with T2DM, the CKD273 AUC was 0.92, indicating that it is a more reliable and earlier predictor of macroalbuminuria compared to the microalbuminuria AUC, which was 0.67. The CKD273 classifier predicted the progression to macroalbuminuria 1.5 years earlier than microalbuminuria.
4. Conclusions
P-DKD and NP-DKD are two different types of kidney involvement in DM with respect to histological, biological and outcome aspects, both requiring the same multifactorial therapeutic approach. Therefore, standard-of-care approaches should be applied in both scenarios. Ongoing advances in molecular studies are essential to pinpoint distinct therapeutic targets for NP-DKD and P-DKD. Additionally, there is a critical need for studies that validate new biomarkers, beyond albuminuria, capable of predicting the progression and therapeutic response of DKD across its various presentations.
Author Contributions
L.F., conceptualization, investigation, methodology, project administration, validation, visualization, writing—original draft, and writing—review and editing. J.F.P.-M., conceptualization, investigation, methodology, project administration, validation, visualization, writing—original draft, and writing—review and editing. É.B.R., conceptualization, investigation, methodology, project administration, supervision, validation, visualization, writing—original draft, writing—review and editing, and final approval. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo/São Paulo Research Foundation; 2021/02216-7) to É.B.R.
Institutional Review Board Statement
This work complies with the Declaration of Helsinki.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Koye, D.N.; Magliano, D.J.; Nelson, R.G.; Pavkov, M.E. The Global Epidemiology of Diabetes and Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Nordheim, E.; Geir, J.T. Chronic kidney disease in patients with diabetes mellitus. Endocr. Connect. 2021, 10, R151–R159. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; Caramori, M.L.; Chan, J.C.N.; Heerspink, H.J.L.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: An update based on rapidly emerging new evidence. Kidney Int. 2022, 102, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Dominguez, M.; Golestaneh, L. Diabetic Kidney Disease: An Update. Med. Clin. N. Am. 2023, 107, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Yamanouchi, M.; Furuichi, K.; Hoshino, J.; Ubara, Y.; Wada, T. Nonproteinuric diabetic kidney disease. Clin. Exp. Nephrol. 2020, 24, 573–581. [Google Scholar] [CrossRef]
- Molitoris, B.A.; Sandoval, R.M.; Yadav, S.P.S.; Wagner, M.C. Albumin uptake and processing by the proximal tubule: Physiological, pathological, and therapeutic implications. Physiol. Rev. 2022, 102, 1625–1667. [Google Scholar] [CrossRef]
- Sauriasari, R.; Safitri, D.D.; Azmi, N.U. Current updates on protein as biomarkers for diabetic kidney disease: A systematic review. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211049612. [Google Scholar] [CrossRef]
- Oshima, M.; Shimizu, M.; Yamanouchi, M.; Toyama, T.; Hara, A.; Furuichi, K.; Wada, T. Trajectories of kidney function in diabetes: A clinicopathological update. Nat. Rev. Nephrol. 2021, 17, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Pissas, G.; Golfinopoulos, S.; Efthymiadi, M.; Poulianiti, C.; Polyzou Konsta, M.A.; Liakopoulos, V.; Stefanidis, I. Routes of Albumin Overload Toxicity in Renal Tubular Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 9640. [Google Scholar] [CrossRef]
- Kramer, H.J.; Nguyen, Q.D.; Curhan, G.; Hsu, C.Y. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003, 289, 3273–3277. [Google Scholar] [CrossRef]
- Thomas, M.C.; Macisaac, R.J.; Jerums, G.; Weekes, A.; Moran, J.; Shaw, J.E.; Atkins, R.C. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment cO-existing with NIDDM [NEFRON] 11). Diabetes Care 2009, 32, 1497–1502. [Google Scholar] [CrossRef]
- Vistisen, D.; Andersen, G.S.; Hulman, A.; Persson, F.; Rossing, P.; Jorgensen, M.E. Progressive Decline in Estimated Glomerular Filtration Rate in Patients with Diabetes after Moderate Loss in Kidney Function-Even without Albuminuria. Diabetes Care 2019, 42, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Vassalotti, J.A.; Centor, R.; Turner, B.J.; Greer, R.C.; Choi, M.; Sequist, T.D. Practical Approach to Detection and Management of Chronic Kidney Disease for the Primary Care Clinician. Am. J. Med. 2016, 129, 153–162. [Google Scholar] [CrossRef]
- Laranjinha, I.; Matias, P.; Mateus, S.; Aguiar, F.; Pereira, P.; Perneta, S.M.; Costa, R.; Lourenco, A.; Guia, J.; Barata, J.D.; et al. Diabetic kidney disease: Is there a non-albuminuric phenotype in type 2 diabetic patients? Nefrologia 2016, 36, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E.; Steffes, M.; Sun, W.; Rutledge, B.; Cleary, P.; de Boer, I.H.; Zinman, B.; Lachin, J. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care 2010, 33, 1536–1543. [Google Scholar] [CrossRef]
- Porrini, E.; Ruggenenti, P.; Mogensen, C.E.; Barlovic, D.P.; Praga, M.; Cruzado, J.M.; Hojs, R.; Abbate, M.; de Vries, A.P. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2015, 3, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Nosadini, R.; Velussi, M.; Brocco, E.; Bruseghin, M.; Abaterusso, C.; Saller, A.; Dalla, V.M.; Carraro, A.; Bortoloso, E.; Sambataro, M.; et al. Course of renal function in type 2 diabetic patients with abnormalities of albumin excretion rate. Diabetes 2000, 49, 476–484. [Google Scholar] [CrossRef]
- Macisaac, R.J.; Tsalamandris, C.; Panagiotopoulos, S.; Smith, T.J.; McNeil, K.J.; Jerums, G. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 2004, 27, 195–200. [Google Scholar] [CrossRef]
- Retnakaran, R.; Cull, C.A.; Thorne, K.I.; Adler, A.I.; Holman, R.R. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006, 55, 1832–1839. [Google Scholar] [CrossRef]
- Parving, H.H.; Lewis, J.B.; Ravid, M.; Remuzzi, G.; Hunsicker, L.G. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: A global perspective. Kidney Int. 2006, 69, 2057–2063. [Google Scholar] [CrossRef]
- New, J.P.; Middleton, R.J.; Klebe, B.; Farmer, C.K.; de Lusignan, S.; Stevens, P.E.; O’Donoghue, D.J. Assessing the prevalence, monitoring and management of chronic kidney disease in patients with diabetes compared with those without diabetes in general practice. Diabet. Med. 2007, 24, 364–369. [Google Scholar] [CrossRef]
- Penno, G.; Solini, A.; Bonora, E.; Fondelli, C.; Orsi, E.; Zerbini, G.; Trevisan, R.; Vedovato, M.; Gruden, G.; Cavalot, F.; et al. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J. Hypertens. 2011, 29, 1802–1809. [Google Scholar] [CrossRef]
- Dwyer, J.P.; Parving, H.H.; Hunsicker, L.G.; Ravid, M.; Remuzzi, G.; Lewis, J.B. Renal Dysfunction in the Presence of Normoalbuminuria in Type 2 Diabetes: Results from the DEMAND Study. Cardiorenal. Med. 2012, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, V.; Zhao, B.; Azar, K.M.; Wang, E.J.; Choi, S.; Wong, E.C.; Fortmann, S.P.; Palaniappan, L.P. Racial/ethnic differences in the prevalence of proteinuric and nonproteinuric diabetic kidney disease. Diabetes Care 2013, 36, 1215–1221. [Google Scholar] [CrossRef]
- Ekinci, E.I.; Jerums, G.; Skene, A.; Crammer, P.; Power, D.; Cheong, K.Y.; Panagiotopoulos, S.; McNeil, K.; Baker, S.T.; Fioretto, P.; et al. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes Care 2013, 36, 3620–3626. [Google Scholar] [CrossRef] [PubMed]
- Mottl, A.K.; Kwon, K.S.; Mauer, M.; Mayer-Davis, E.J.; Hogan, S.L.; Kshirsagar, A.V. Normoalbuminuric diabetic kidney disease in the U.S. population. J. Diabetes Complicat. 2013, 27, 123–127. [Google Scholar] [CrossRef]
- Boronat, M.; Garcia-Canton, C.; Quevedo, V.; Lorenzo, D.L.; Lopez-Rios, L.; Batista, F.; Riano, M.; Saavedra, P.; Checa, M.D. Non-albuminuric renal disease among subjects with advanced stages of chronic kidney failure related to type 2 diabetes mellitus. Ren. Fail. 2014, 36, 166–170. [Google Scholar] [CrossRef]
- Yamanouchi, M.; Furuichi, K.; Hoshino, J.; Toyama, T.; Hara, A.; Shimizu, M.; Kinowaki, K.; Fujii, T.; Ohashi, K.; Yuzawa, Y.; et al. Nonproteinuric Versus Proteinuric Phenotypes in Diabetic Kidney Disease: A Propensity Score-Matched Analysis of a Nationwide, Biopsy-Based Cohort Study. Diabetes Care 2019, 42, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Luk, A.O.; Lau, E.S.H.; Tam, C.H.T.; Ozaki, R.; Lim, C.K.P.; Wu, H.; Jiang, G.; Chow, E.Y.K.; Ng, J.K.; et al. Nonalbuminuric Diabetic Kidney Disease and Risk of All-Cause Mortality and Cardiovascular and Kidney Outcomes in Type 2 Diabetes: Findings From the Hong Kong Diabetes Biobank. Am. J. Kidney Dis. 2022, 80, 196–206. [Google Scholar] [CrossRef]
- Fabre, L.; Rangel, E.B. Age-related markers and predictors of diabetic kidney disease progression in type 2 diabetes patients: A retrospective cohort study. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188241242947. [Google Scholar] [CrossRef]
- Chang, T.I.; Li, S.; Chen, S.C.; Peralta, C.A.; Shlipak, M.G.; Fried, L.F.; Whaley-Connell, A.T.; McCullough, P.A.; Kurella, T.M. Risk factors for ESRD in individuals with preserved estimated GFR with and without albuminuria: Results from the Kidney Early Evaluation Program (KEEP). Am. J. Kidney Dis. 2013, 61, S4–S11. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Amouzegar, A. Diabetic Kidney Disease without Albuminuria: A New Entity in Diabetic Nephropathy. Iran. J. Kidney Dis. 2024, 1, 1–8. [Google Scholar] [PubMed]
- Bash, L.D.; Selvin, E.; Steffes, M.; Coresh, J.; Astor, B.C. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Arch. Intern. Med. 2008, 168, 2440–2447. [Google Scholar] [CrossRef]
- Sangha, S.; Yadav, R.K.; Subbiah, A.; Bagchi, S.; Mahajan, S.; Bhowmik, D.; Agarwal, S.K. Clinical Profile of Nonproteinuric Kidney Disease in Type 2 Diabetic Patients in India. Indian J. Nephrol. 2023, 33, 283–288. [Google Scholar] [PubMed]
- Chang, D.Y.; Li, M.R.; Yu, X.J.; Wang, S.X.; Chen, M.; Zhao, M.H. Clinical and Pathological Characteristics of Patients with Nonproteinuric Diabetic Nephropathy. Front. Endocrinol. 2021, 12, 761386. [Google Scholar] [CrossRef]
- Baltu, D.; Salanci, B.V.; Gulhan, B.; Ozaltin, F.; Duzova, A.; Topaloglu, R. Albuminuria is associated with 24-hour and night-time diastolic blood pressure in urinary tract infection with renal scarring. Turk. J. Pediatr. 2023, 65, 620–629. [Google Scholar] [CrossRef]
- Shoukry, A.; Bdeer, S.; El-Sokkary, R.H. Urinary monocyte chemoattractant protein-1 and vitamin D-binding protein as biomarkers for early detection of diabetic nephropathy in type 2 diabetes mellitus. Mol. Cell Biochem. 2015, 408, 25–35. [Google Scholar] [CrossRef]
- Lampropoulou, I.T.; Stangou, M.; Papagianni, A.; Didangelos, T.; Iliadis, F.; Efstratiadis, G. TNF-alpha and microalbuminuria in patients with type 2 diabetes mellitus. J. Diabetes Res. 2014, 2014, 394206. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Wang, R.; Ma, N.; Liu, L.; Shi, R.; Zhang, B.; Lin, N.; Tian, Y. Potential Role and Expression Level of Urinary CXCL8 in Different Stages of Incipient Diabetic Nephropathy with Undiminished Creatinine Clearance: A Pilot Study. Diabetes Metab. Syndr. Obes. 2023, 16, 1783–1790. [Google Scholar] [CrossRef]
- Sun, Z.J.; Li, X.Q.; Chang, D.Y.; Wang, S.X.; Liu, G.; Chen, M.; Zhao, M.H. Complement deposition on renal histopathology of patients with diabetic nephropathy. Diabetes Metab. 2019, 45, 363–368. [Google Scholar] [CrossRef]
- Fukata, F.; Eriguchi, M.; Tamaki, H.; Uemura, T.; Tasaki, H.; Furuyama, R.; Nishimoto, M.; Kosugi, T.; Tanabe, K.; Morimoto, K.; et al. Differential impact of glomerular and tubule-interstitial histological changes on kidney outcome between non-proteinuric and proteinuric diabetic nephropathy. Clin. Exp. Nephrol. 2024, 28, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Naaman, S.C.; Bakris, G.L. Diabetic Nephropathy: Update on Pillars of Therapy Slowing Progression. Diabetes Care 2023, 46, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- Roscioni, S.S.; Heerspink, H.J.; de Zeeuw, D. The effect of RAAS blockade on the progression of diabetic nephropathy. Nat. Rev. Nephrol. 2014, 10, 77–87. [Google Scholar] [CrossRef]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; Ng, S.Y.A.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar]
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur. Heart J. 2022, 43, 474–484. [Google Scholar] [CrossRef]
- Rossing, P.; Anker, S.D.; Filippatos, G.; Pitt, B.; Ruilope, L.M.; Birkenfeld, A.L.; McGill, J.B.; Rosas, S.E.; Joseph, A.; Gebel, M.; et al. Finerenone in Patients With Chronic Kidney Disease and Type 2 Diabetes by Sodium-Glucose Cotransporter 2 Inhibitor Treatment: The FIDELITY Analysis. Diabetes Care 2022, 45, 2991–2998. [Google Scholar] [CrossRef]
- Mann, J.F.E.; Hansen, T.; Idorn, T.; Leiter, L.A.; Marso, S.P.; Rossing, P.; Seufert, J.; Tadayon, S.; Vilsboll, T. Effects of once-weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: A post-hoc analysis of the SUSTAIN 1-7 randomised controlled trials. Lancet Diabetes Endocrinol. 2020, 8, 880–893. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Apperloo, E.; Davies, M.; Dicker, D.; Kandler, K.; Rosenstock, J.; Sorrig, R.; Lawson, J.; Zeuthen, N.; Cherney, D. Effects of Semaglutide on Albuminuria and Kidney Function in People with Overweight or Obesity with or without Type 2 Diabetes: Exploratory Analysis From the STEP 1, 2, and 3 Trials. Diabetes Care 2023, 46, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Sattar, N.; Pavo, I.; Haupt, A.; Duffin, K.L.; Yang, Z.; Wiese, R.J.; Tuttle, K.R.; Cherney, D.Z.I. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: Post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022, 10, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Phanish, M.K.; Chapman, A.N.; Yates, S.; Price, R.; Hendry, B.M.; Roderick, P.J.; Dockrell, M.E.C. Evaluation of Urinary Biomarkers of Proximal Tubular Injury, Inflammation, and Fibrosis in Patients with Albuminuric and Nonalbuminuric Diabetic Kidney Disease. Kidney Int. Rep. 2021, 6, 1355–1367. [Google Scholar] [CrossRef]
- Liu, Y. Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int. 2006, 69, 213–217. [Google Scholar] [CrossRef]
- Chevalier, R.L. The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomerulotubular junction. Am. J. Physiol. Ren. Physiol. 2016, 311, F145–F161. [Google Scholar] [CrossRef]
- Svensson, M.K.; Cederholm, J.; Eliasson, B.; Zethelius, B.; Gudbjornsdottir, S. Albuminuria and renal function as predictors of cardiovascular events and mortality in a general population of patients with type 2 diabetes: A nationwide observational study from the Swedish National Diabetes Register. Diabetes Vasc. Dis. Res. 2013, 10, 520–529. [Google Scholar] [CrossRef]
- Kamijo-Ikemori, A.; Sugaya, T.; Yasuda, T.; Kawata, T.; Ota, A.; Tatsunami, S.; Kaise, R.; Ishimitsu, T.; Tanaka, Y.; Kimura, K. Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care 2011, 34, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Alter, M.L.; Kretschmer, A.; Von, W.K.; Tsuprykov, O.; Reichetzeder, C.; Simon, A.; Stasch, J.P.; Hocher, B. Early urinary and plasma biomarkers for experimental diabetic nephropathy. Clin. Lab. 2012, 58, 659–671. [Google Scholar]
- Tesch, G.H. Diabetic nephropathy—Is this an immune disorder? Clin. Sci. 2017, 131, 2183–2199. [Google Scholar] [CrossRef]
- Niewczas, M.A.; Pavkov, M.E.; Skupien, J.; Smiles, A.; Md Dom, Z.I.; Wilson, J.M.; Park, J.; Nair, V.; Schlafly, A.; Saulnier, P.J.; et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat. Med. 2019, 25, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Mallamaci, F. Nonproteinuric progressive diabetic kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 227–232. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).