Proteinuric and Non-Proteinuric Diabetic Kidney Disease: Different Presentations of the Same Disease?
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
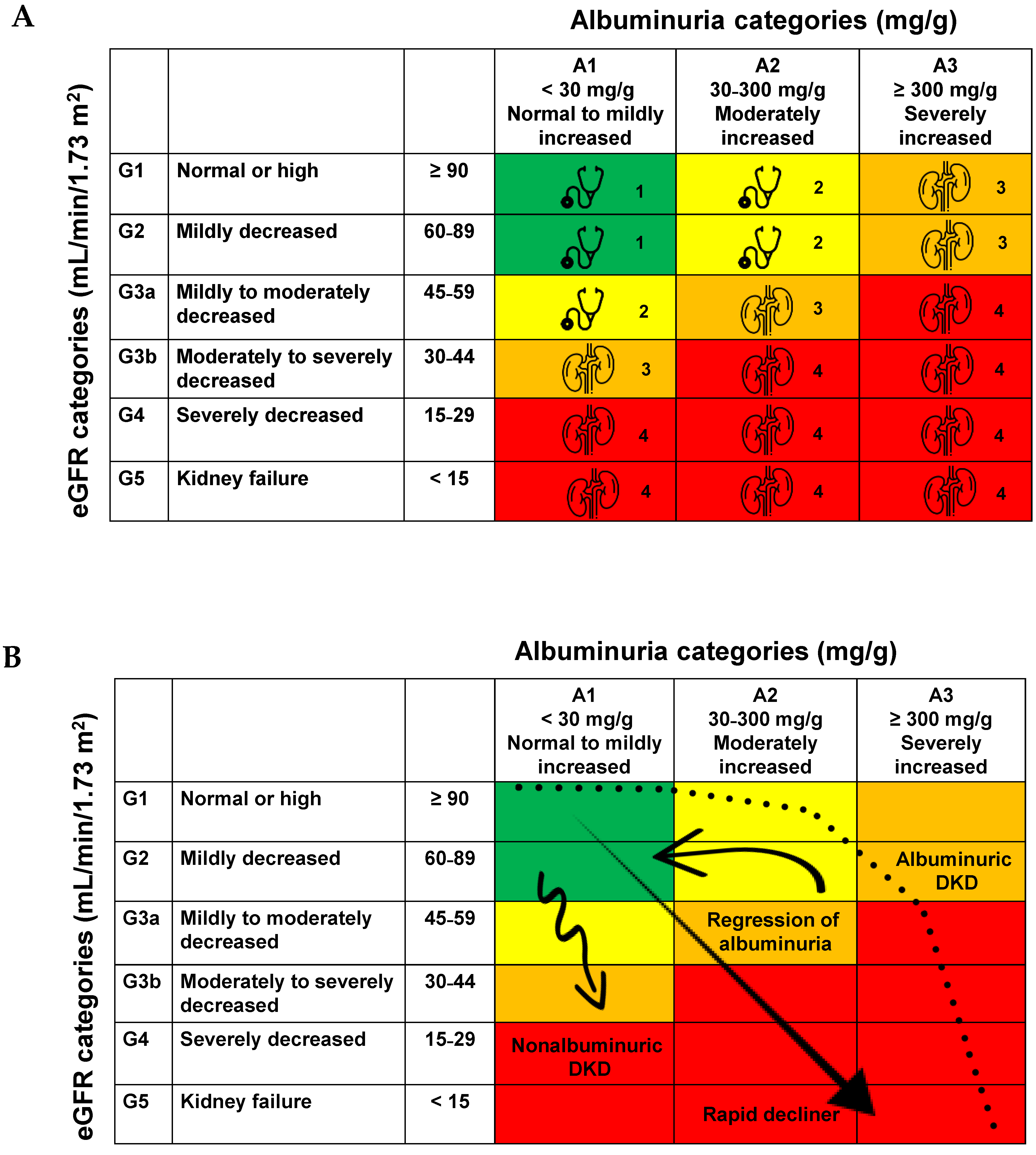
3.1. Natural History of DKD
3.2. NP-DKD
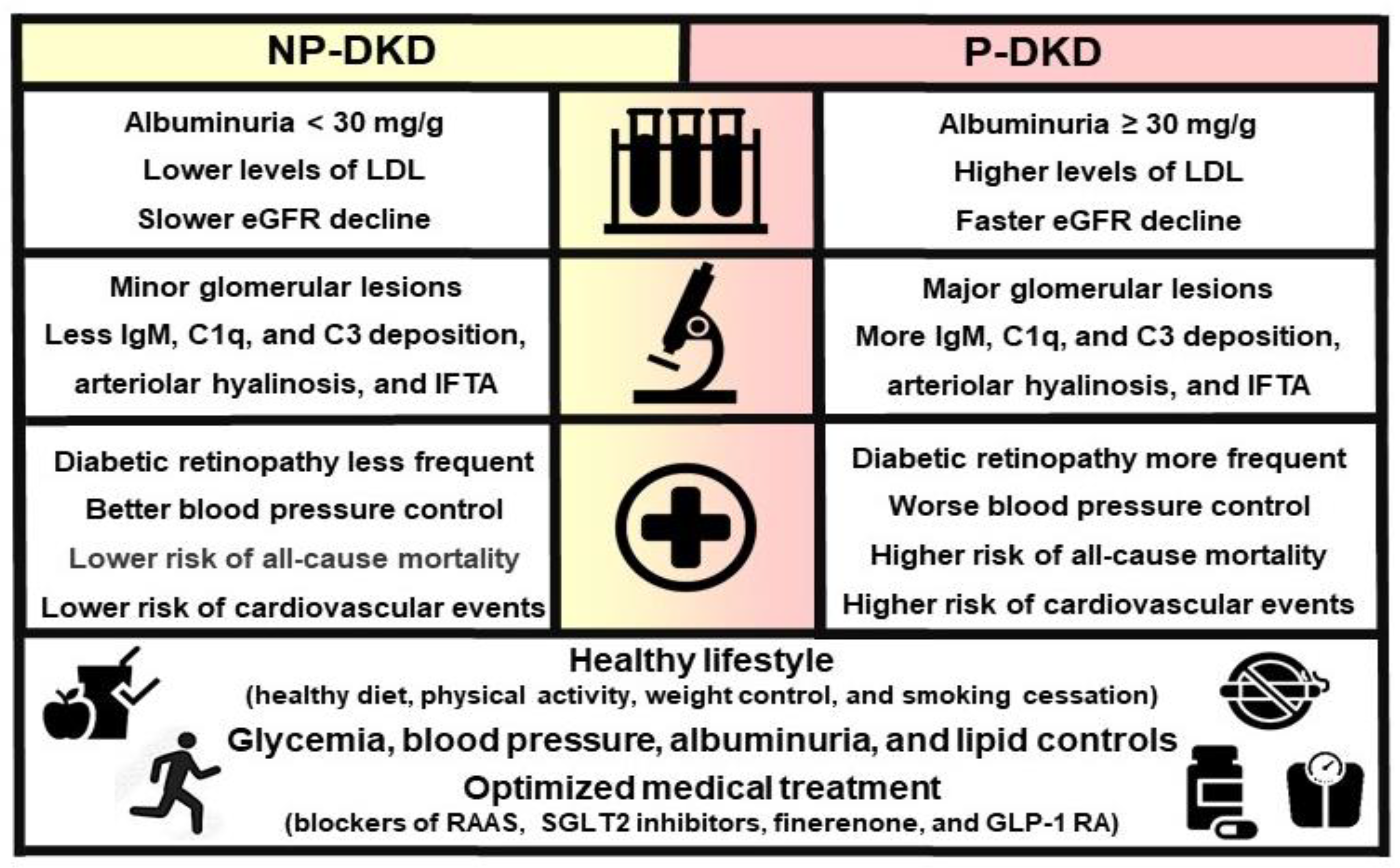
3.3. Clinical, Laboratory, and Morphological Parameters Associated with P-DKD vs. NP-DKD
3.4. Histopathology Associated with P-DKD vs. NP-DKD
3.5. Treatment of P-DKD vs. NP-DKD
- DM (−1.05 vs. −2.73; absolute difference +1.68 and relative difference −62%) compared to non-DM (−1.66 vs. −2.75; absolute difference +1.09 and relative difference −40%);
- eGFR < 30 (−1.84 vs. −2.85; absolute difference +1.01 and relative difference −35%) compared to ≥30–45 (−1.18 vs. −2.50; absolute difference +1.32 and relative difference −53%) and ≥45 (−1.58 vs. −3.60; absolute difference +2.01 and relative difference −56%);
- UACR < 30 mg/g (−0.11 vs. −0.89; absolute difference +0.78 and relative difference −87%) compared to UACR ≥ 30–300 mg/g (−0.49 vs. −1.69; absolute difference +1.20 and relative difference −71%) and UACR ≥ 300 mg/g (−2.35 vs. −4.11; absolute difference +1.76 and relative difference −43%.
3.6. Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koye, D.N.; Magliano, D.J.; Nelson, R.G.; Pavkov, M.E. The Global Epidemiology of Diabetes and Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Nordheim, E.; Geir, J.T. Chronic kidney disease in patients with diabetes mellitus. Endocr. Connect. 2021, 10, R151–R159. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; Caramori, M.L.; Chan, J.C.N.; Heerspink, H.J.L.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: An update based on rapidly emerging new evidence. Kidney Int. 2022, 102, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Dominguez, M.; Golestaneh, L. Diabetic Kidney Disease: An Update. Med. Clin. N. Am. 2023, 107, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Yamanouchi, M.; Furuichi, K.; Hoshino, J.; Ubara, Y.; Wada, T. Nonproteinuric diabetic kidney disease. Clin. Exp. Nephrol. 2020, 24, 573–581. [Google Scholar] [CrossRef]
- Molitoris, B.A.; Sandoval, R.M.; Yadav, S.P.S.; Wagner, M.C. Albumin uptake and processing by the proximal tubule: Physiological, pathological, and therapeutic implications. Physiol. Rev. 2022, 102, 1625–1667. [Google Scholar] [CrossRef]
- Sauriasari, R.; Safitri, D.D.; Azmi, N.U. Current updates on protein as biomarkers for diabetic kidney disease: A systematic review. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211049612. [Google Scholar] [CrossRef]
- Oshima, M.; Shimizu, M.; Yamanouchi, M.; Toyama, T.; Hara, A.; Furuichi, K.; Wada, T. Trajectories of kidney function in diabetes: A clinicopathological update. Nat. Rev. Nephrol. 2021, 17, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Pissas, G.; Golfinopoulos, S.; Efthymiadi, M.; Poulianiti, C.; Polyzou Konsta, M.A.; Liakopoulos, V.; Stefanidis, I. Routes of Albumin Overload Toxicity in Renal Tubular Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 9640. [Google Scholar] [CrossRef]
- Kramer, H.J.; Nguyen, Q.D.; Curhan, G.; Hsu, C.Y. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003, 289, 3273–3277. [Google Scholar] [CrossRef]
- Thomas, M.C.; Macisaac, R.J.; Jerums, G.; Weekes, A.; Moran, J.; Shaw, J.E.; Atkins, R.C. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment cO-existing with NIDDM [NEFRON] 11). Diabetes Care 2009, 32, 1497–1502. [Google Scholar] [CrossRef]
- Vistisen, D.; Andersen, G.S.; Hulman, A.; Persson, F.; Rossing, P.; Jorgensen, M.E. Progressive Decline in Estimated Glomerular Filtration Rate in Patients with Diabetes after Moderate Loss in Kidney Function-Even without Albuminuria. Diabetes Care 2019, 42, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Vassalotti, J.A.; Centor, R.; Turner, B.J.; Greer, R.C.; Choi, M.; Sequist, T.D. Practical Approach to Detection and Management of Chronic Kidney Disease for the Primary Care Clinician. Am. J. Med. 2016, 129, 153–162. [Google Scholar] [CrossRef]
- Laranjinha, I.; Matias, P.; Mateus, S.; Aguiar, F.; Pereira, P.; Perneta, S.M.; Costa, R.; Lourenco, A.; Guia, J.; Barata, J.D.; et al. Diabetic kidney disease: Is there a non-albuminuric phenotype in type 2 diabetic patients? Nefrologia 2016, 36, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E.; Steffes, M.; Sun, W.; Rutledge, B.; Cleary, P.; de Boer, I.H.; Zinman, B.; Lachin, J. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care 2010, 33, 1536–1543. [Google Scholar] [CrossRef]
- Porrini, E.; Ruggenenti, P.; Mogensen, C.E.; Barlovic, D.P.; Praga, M.; Cruzado, J.M.; Hojs, R.; Abbate, M.; de Vries, A.P. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2015, 3, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Nosadini, R.; Velussi, M.; Brocco, E.; Bruseghin, M.; Abaterusso, C.; Saller, A.; Dalla, V.M.; Carraro, A.; Bortoloso, E.; Sambataro, M.; et al. Course of renal function in type 2 diabetic patients with abnormalities of albumin excretion rate. Diabetes 2000, 49, 476–484. [Google Scholar] [CrossRef]
- Macisaac, R.J.; Tsalamandris, C.; Panagiotopoulos, S.; Smith, T.J.; McNeil, K.J.; Jerums, G. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 2004, 27, 195–200. [Google Scholar] [CrossRef]
- Retnakaran, R.; Cull, C.A.; Thorne, K.I.; Adler, A.I.; Holman, R.R. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006, 55, 1832–1839. [Google Scholar] [CrossRef]
- Parving, H.H.; Lewis, J.B.; Ravid, M.; Remuzzi, G.; Hunsicker, L.G. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: A global perspective. Kidney Int. 2006, 69, 2057–2063. [Google Scholar] [CrossRef]
- New, J.P.; Middleton, R.J.; Klebe, B.; Farmer, C.K.; de Lusignan, S.; Stevens, P.E.; O’Donoghue, D.J. Assessing the prevalence, monitoring and management of chronic kidney disease in patients with diabetes compared with those without diabetes in general practice. Diabet. Med. 2007, 24, 364–369. [Google Scholar] [CrossRef]
- Penno, G.; Solini, A.; Bonora, E.; Fondelli, C.; Orsi, E.; Zerbini, G.; Trevisan, R.; Vedovato, M.; Gruden, G.; Cavalot, F.; et al. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J. Hypertens. 2011, 29, 1802–1809. [Google Scholar] [CrossRef]
- Dwyer, J.P.; Parving, H.H.; Hunsicker, L.G.; Ravid, M.; Remuzzi, G.; Lewis, J.B. Renal Dysfunction in the Presence of Normoalbuminuria in Type 2 Diabetes: Results from the DEMAND Study. Cardiorenal. Med. 2012, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, V.; Zhao, B.; Azar, K.M.; Wang, E.J.; Choi, S.; Wong, E.C.; Fortmann, S.P.; Palaniappan, L.P. Racial/ethnic differences in the prevalence of proteinuric and nonproteinuric diabetic kidney disease. Diabetes Care 2013, 36, 1215–1221. [Google Scholar] [CrossRef]
- Ekinci, E.I.; Jerums, G.; Skene, A.; Crammer, P.; Power, D.; Cheong, K.Y.; Panagiotopoulos, S.; McNeil, K.; Baker, S.T.; Fioretto, P.; et al. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes Care 2013, 36, 3620–3626. [Google Scholar] [CrossRef] [PubMed]
- Mottl, A.K.; Kwon, K.S.; Mauer, M.; Mayer-Davis, E.J.; Hogan, S.L.; Kshirsagar, A.V. Normoalbuminuric diabetic kidney disease in the U.S. population. J. Diabetes Complicat. 2013, 27, 123–127. [Google Scholar] [CrossRef]
- Boronat, M.; Garcia-Canton, C.; Quevedo, V.; Lorenzo, D.L.; Lopez-Rios, L.; Batista, F.; Riano, M.; Saavedra, P.; Checa, M.D. Non-albuminuric renal disease among subjects with advanced stages of chronic kidney failure related to type 2 diabetes mellitus. Ren. Fail. 2014, 36, 166–170. [Google Scholar] [CrossRef]
- Yamanouchi, M.; Furuichi, K.; Hoshino, J.; Toyama, T.; Hara, A.; Shimizu, M.; Kinowaki, K.; Fujii, T.; Ohashi, K.; Yuzawa, Y.; et al. Nonproteinuric Versus Proteinuric Phenotypes in Diabetic Kidney Disease: A Propensity Score-Matched Analysis of a Nationwide, Biopsy-Based Cohort Study. Diabetes Care 2019, 42, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Luk, A.O.; Lau, E.S.H.; Tam, C.H.T.; Ozaki, R.; Lim, C.K.P.; Wu, H.; Jiang, G.; Chow, E.Y.K.; Ng, J.K.; et al. Nonalbuminuric Diabetic Kidney Disease and Risk of All-Cause Mortality and Cardiovascular and Kidney Outcomes in Type 2 Diabetes: Findings From the Hong Kong Diabetes Biobank. Am. J. Kidney Dis. 2022, 80, 196–206. [Google Scholar] [CrossRef]
- Fabre, L.; Rangel, E.B. Age-related markers and predictors of diabetic kidney disease progression in type 2 diabetes patients: A retrospective cohort study. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188241242947. [Google Scholar] [CrossRef]
- Chang, T.I.; Li, S.; Chen, S.C.; Peralta, C.A.; Shlipak, M.G.; Fried, L.F.; Whaley-Connell, A.T.; McCullough, P.A.; Kurella, T.M. Risk factors for ESRD in individuals with preserved estimated GFR with and without albuminuria: Results from the Kidney Early Evaluation Program (KEEP). Am. J. Kidney Dis. 2013, 61, S4–S11. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Amouzegar, A. Diabetic Kidney Disease without Albuminuria: A New Entity in Diabetic Nephropathy. Iran. J. Kidney Dis. 2024, 1, 1–8. [Google Scholar] [PubMed]
- Bash, L.D.; Selvin, E.; Steffes, M.; Coresh, J.; Astor, B.C. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Arch. Intern. Med. 2008, 168, 2440–2447. [Google Scholar] [CrossRef]
- Sangha, S.; Yadav, R.K.; Subbiah, A.; Bagchi, S.; Mahajan, S.; Bhowmik, D.; Agarwal, S.K. Clinical Profile of Nonproteinuric Kidney Disease in Type 2 Diabetic Patients in India. Indian J. Nephrol. 2023, 33, 283–288. [Google Scholar] [PubMed]
- Chang, D.Y.; Li, M.R.; Yu, X.J.; Wang, S.X.; Chen, M.; Zhao, M.H. Clinical and Pathological Characteristics of Patients with Nonproteinuric Diabetic Nephropathy. Front. Endocrinol. 2021, 12, 761386. [Google Scholar] [CrossRef]
- Baltu, D.; Salanci, B.V.; Gulhan, B.; Ozaltin, F.; Duzova, A.; Topaloglu, R. Albuminuria is associated with 24-hour and night-time diastolic blood pressure in urinary tract infection with renal scarring. Turk. J. Pediatr. 2023, 65, 620–629. [Google Scholar] [CrossRef]
- Shoukry, A.; Bdeer, S.; El-Sokkary, R.H. Urinary monocyte chemoattractant protein-1 and vitamin D-binding protein as biomarkers for early detection of diabetic nephropathy in type 2 diabetes mellitus. Mol. Cell Biochem. 2015, 408, 25–35. [Google Scholar] [CrossRef]
- Lampropoulou, I.T.; Stangou, M.; Papagianni, A.; Didangelos, T.; Iliadis, F.; Efstratiadis, G. TNF-alpha and microalbuminuria in patients with type 2 diabetes mellitus. J. Diabetes Res. 2014, 2014, 394206. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Wang, R.; Ma, N.; Liu, L.; Shi, R.; Zhang, B.; Lin, N.; Tian, Y. Potential Role and Expression Level of Urinary CXCL8 in Different Stages of Incipient Diabetic Nephropathy with Undiminished Creatinine Clearance: A Pilot Study. Diabetes Metab. Syndr. Obes. 2023, 16, 1783–1790. [Google Scholar] [CrossRef]
- Sun, Z.J.; Li, X.Q.; Chang, D.Y.; Wang, S.X.; Liu, G.; Chen, M.; Zhao, M.H. Complement deposition on renal histopathology of patients with diabetic nephropathy. Diabetes Metab. 2019, 45, 363–368. [Google Scholar] [CrossRef]
- Fukata, F.; Eriguchi, M.; Tamaki, H.; Uemura, T.; Tasaki, H.; Furuyama, R.; Nishimoto, M.; Kosugi, T.; Tanabe, K.; Morimoto, K.; et al. Differential impact of glomerular and tubule-interstitial histological changes on kidney outcome between non-proteinuric and proteinuric diabetic nephropathy. Clin. Exp. Nephrol. 2024, 28, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Naaman, S.C.; Bakris, G.L. Diabetic Nephropathy: Update on Pillars of Therapy Slowing Progression. Diabetes Care 2023, 46, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- Roscioni, S.S.; Heerspink, H.J.; de Zeeuw, D. The effect of RAAS blockade on the progression of diabetic nephropathy. Nat. Rev. Nephrol. 2014, 10, 77–87. [Google Scholar] [CrossRef]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; Ng, S.Y.A.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar]
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur. Heart J. 2022, 43, 474–484. [Google Scholar] [CrossRef]
- Rossing, P.; Anker, S.D.; Filippatos, G.; Pitt, B.; Ruilope, L.M.; Birkenfeld, A.L.; McGill, J.B.; Rosas, S.E.; Joseph, A.; Gebel, M.; et al. Finerenone in Patients With Chronic Kidney Disease and Type 2 Diabetes by Sodium-Glucose Cotransporter 2 Inhibitor Treatment: The FIDELITY Analysis. Diabetes Care 2022, 45, 2991–2998. [Google Scholar] [CrossRef]
- Mann, J.F.E.; Hansen, T.; Idorn, T.; Leiter, L.A.; Marso, S.P.; Rossing, P.; Seufert, J.; Tadayon, S.; Vilsboll, T. Effects of once-weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: A post-hoc analysis of the SUSTAIN 1-7 randomised controlled trials. Lancet Diabetes Endocrinol. 2020, 8, 880–893. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Apperloo, E.; Davies, M.; Dicker, D.; Kandler, K.; Rosenstock, J.; Sorrig, R.; Lawson, J.; Zeuthen, N.; Cherney, D. Effects of Semaglutide on Albuminuria and Kidney Function in People with Overweight or Obesity with or without Type 2 Diabetes: Exploratory Analysis From the STEP 1, 2, and 3 Trials. Diabetes Care 2023, 46, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Sattar, N.; Pavo, I.; Haupt, A.; Duffin, K.L.; Yang, Z.; Wiese, R.J.; Tuttle, K.R.; Cherney, D.Z.I. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: Post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022, 10, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Phanish, M.K.; Chapman, A.N.; Yates, S.; Price, R.; Hendry, B.M.; Roderick, P.J.; Dockrell, M.E.C. Evaluation of Urinary Biomarkers of Proximal Tubular Injury, Inflammation, and Fibrosis in Patients with Albuminuric and Nonalbuminuric Diabetic Kidney Disease. Kidney Int. Rep. 2021, 6, 1355–1367. [Google Scholar] [CrossRef]
- Liu, Y. Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int. 2006, 69, 213–217. [Google Scholar] [CrossRef]
- Chevalier, R.L. The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomerulotubular junction. Am. J. Physiol. Ren. Physiol. 2016, 311, F145–F161. [Google Scholar] [CrossRef]
- Svensson, M.K.; Cederholm, J.; Eliasson, B.; Zethelius, B.; Gudbjornsdottir, S. Albuminuria and renal function as predictors of cardiovascular events and mortality in a general population of patients with type 2 diabetes: A nationwide observational study from the Swedish National Diabetes Register. Diabetes Vasc. Dis. Res. 2013, 10, 520–529. [Google Scholar] [CrossRef]
- Kamijo-Ikemori, A.; Sugaya, T.; Yasuda, T.; Kawata, T.; Ota, A.; Tatsunami, S.; Kaise, R.; Ishimitsu, T.; Tanaka, Y.; Kimura, K. Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care 2011, 34, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Alter, M.L.; Kretschmer, A.; Von, W.K.; Tsuprykov, O.; Reichetzeder, C.; Simon, A.; Stasch, J.P.; Hocher, B. Early urinary and plasma biomarkers for experimental diabetic nephropathy. Clin. Lab. 2012, 58, 659–671. [Google Scholar]
- Tesch, G.H. Diabetic nephropathy—Is this an immune disorder? Clin. Sci. 2017, 131, 2183–2199. [Google Scholar] [CrossRef]
- Niewczas, M.A.; Pavkov, M.E.; Skupien, J.; Smiles, A.; Md Dom, Z.I.; Wilson, J.M.; Park, J.; Nair, V.; Schlafly, A.; Saulnier, P.J.; et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat. Med. 2019, 25, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Mallamaci, F. Nonproteinuric progressive diabetic kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 227–232. [Google Scholar] [CrossRef] [PubMed]
| Study | Design | Sample Size (n) | Objective | Results |
|---|---|---|---|---|
| Nosadini et al. (2000) [17] | Longitudinal study, kidney biopsy | n = 108 T2DM n = 74 with albuminuria 20–199 μg/min n = 34 with albuminuria > 199 μg/min | To evaluate the course of kidney function, laboratory, and clinical parameters. Follow-up: 4 years | The mean eGFR decreased in both groups, displaying a heterogenous pattern; the odds of being progressors significantly increased across the quartiles of baseline glomerular basement membrane width and mesangial fractional volume. |
| Kramer et al. (2003) [10] | Cross-sectional analysis (Third National Health and Nutrition Examination Survey—NHANES III) | Population estimated in 1.1 million microalbuminuria: ≥17 and ≥25 µg/mg for men and women, respectively microalbuminuria: > 250 µg/mg in men and at least 355 µg/mg in women, respectively | To determine the prevalence of CKD in the absence of micro- or macroalbuminuria and diabetic retinopathy | n = 171/1197 T2DM (13%) had CKD (vs. 7% in non-diabetic population). Adults with T2DM and CKD were more likely to have macroalbuminuria (19% vs. 5%), microalbuminuria (45% vs. 32%), and diabetic retinopathy (28% vs. 15%) compared to those with T2DM but without CKD. Among these adults with T2DM and CKD, both retinopathy and albuminuria (either microalbuminuria or macroalbuminuria) were absent in 30% of cases. |
| MacIsaac et al. (2004) [18] | Cross-sectional survey | n = 301 T2DM | To determine the prevalence and characteristics of patients with T2DM who have CKD normoalbuminuria. GFR evaluated by the plasma disappearance of isotopic (99m)Tc-diethylene-triamine-penta-acetic acid | Prevalence of CKD: 36% The overall prevalence of normoalbuminuria, microalbuminuria, and macroalbuminuria was 39%, 35%, and 26%, respectively. Patients with normoalbuminuria were more likely to be older and female compared to those with macroalbuminuria. The rates of GFR decline (mL/min/1.73 m2/year) were −4.6 ± 1.0 for normoalbuminuric patients, −2.8 ± 1.0 for microalbuminuric patients, and −3.0 ± 0.7 for macroalbuminuric patients, with no significant differences between the groups. |
| Retnakaran et al. (2006) [19] | Longitudinal study (U.K. Prospective Diabetes Study—UKPDS) | n = 5102 T2DM n = 4031 without proteinuria or n = 5032 with normal plasma creatinine at diagnosis | To identify clinical risk factors at diagnosis of T2DM associated with later development of renal dysfunction Follow-up: 15 years | Nearly 40% of T2DM patients developed albuminuria, and approximately 30% developed renal impairment. Among those with renal impairment, 51% did not have preceding albuminuria. Risk factors for developing either albuminuria or renal impairment included higher baseline systolic blood pressure, elevated urinary albumin, increased plasma creatinine, and Indian-Asian ethnicity. Specific risk factors for albuminuria were male sex, larger waist circumference, higher levels of plasma triglycerides, LDL cholesterol, HbA1c, increased white blood cell count, smoking history, and previous retinopathy. Risk factors for renal impairment included female sex, smaller waist circumference, older age, increased insulin sensitivity, and a history of sensory neuropathy. |
| Parving et al. (2006) [20] | Cross-sectional analysis (Developing Education on Microalbuminuria for Awareness of renal and cardiovascular risk in Diabetes study) | n = 32,208 T2DM | To evaluate the global prevalence and determinants of microalbuminuria in T2DM | The overall global prevalence of normoalbuminuria, microalbuminuria, and macroalbuminuria was 51, 39, and 10%, respectively. Risk factors for microalbuminuria or macroalbuminuria included age, HbA1c, systolic and diastolic blood pressure, ethnicity (with higher prevalence in Asians and Hispanics compared to Caucasians), retinopathy, duration of DM, eGFR, diabetic foot lesions, congestive heart failure, body height, and smoking. |
| New et al. (2007) [21] | Cross-sectional analysis | Total population: n = 162,113 | To compare rates of CKD (eGFR < 60 mL/min/1.73 m2) in patients with DM and management of risk factors compared with people without DM | Prevalence of DM: 3.1% CKD: 31% in DM (vs. 6.9% in non-diabetics) CKD in DM: 63% had normoalbuminuria. |
| Thomas et al. (2009) [11] | Cross-sectional analysis (NEPHRON survey) | n = 3893 T2DM n = 11,247 general population (Australian Diabetes, Obesity and Lifestyle—AusDiab) survey | To examine the frequency and predictors of non-albuminuric renal impairment (eGFR < 60 mL/min/1.73 m2) | Among patients with T2DM, 23.1% had renal impairment (eGFR < 60 mL/min/1.73 m2), and 55% of these patients had normoalbuminuria; renal impairment was more common than in the general population only when albuminuria was present (aOR = 1.3), but not for those with normoalbuminuria. Renal impairment without albuminuria was less prevalent in individuals with DM than in the general population, regardless of sex (men: aOR = 0.7; women: aOR = 0.6), ethnicity, or duration of diabetes. |
| Penno et al. (2011) [22] | Cross-sectional analysis (Renal Insufficiency and Cardiovascular Events –RIACE—Italian Multicenter Study) | n = 15,773 T2DM | To evaluate the association of non-albuminuric renal impairment with cardiovascular risk factors and other complications | Among patients with CKD (eGFR < 60 mL/min/1.73 m2), 56.6% were normoalbuminuric, 30.8% were microalbuminuric, and 12.6% were macroalbuminuric. Non-albuminuric renal impairment was associated with a higher prevalence of cardiovascular disease and a greater likelihood of being female. In contrasts, albuminuric renal impairment was linked to higher HbA1c levels, retinopathy, and male sex. |
| Dwyer et al. (2012) [23] | Cross-sectional analysis (Developing Education on Microalbuminuria for Awareness of Renal and Cardiovascular Risk in Diabetes—DEMAND) | n = 11,573 T2DM normoalbuminuria: UACR < 30 mg/g, microalbuminuria: UACR 30–299 mg/g, and macroalbuminuria UACR > 300 mg/g | To describe the prevalence and risk factors for albuminuria | CKD (stage 3–5): 17% with normoalbuminuria, 27% with microalbuminuria and 31% with macroalbuminuria. Creatinine clearance < 60 mL/min was observed in 20.5% of patients with normoalbuminuria, 30.7% with microalbuminuria, and 35.0% with macroalbuminuria. |
| Bhalla et al. (2013) [24] | Cross-sectional analysis | n = 15,683 persons of non-Hispanic white (NHW), Asian (Asian Indian, Chinese, and Filipino), Hispanic, and non-Hispanic black (NHB) race/ethnicity with T2DM | To examine racial/ethnic differences in the prevalence of DKD, with and without proteinuria | Racial and ethnic minorities had higher rates of P-DKD compared to NHWs (24.8–37.9% vs. 24.8%) and lower rates of NP-DKD (6.3–9.8% vs. 11.7%). Chinese individuals (OR= 1.39 for women and 1.56 for men), Filipinos (OR = 1.57 for women and 1.85 for men), Hispanics (OR = 1.46 for women and 1.34 for men), and NHBs (OR = 1.50 for women) had significantly higher odds of developing P-DKD than NHWs. In contrast, Chinese, Hispanic, and NHB women, as well as Hispanic men, had significantly lower odds of developing NP-DKD compared to NHWs. |
| Ekinci et al. (2013) [25] | Case–control, kidney biopsy | n = 8 with normoalbuminuria n = 6 with microalbuminuria n = 17 with macroalbuminuria | To compare renal biopsy findings in patients with T2DM and eGFR < 60 mL/min/1.73 m2 associated with either normoalbuminuria, microalbuminuria, or macroalbuminuria | The mesangial area increased progressively from normal controls to patients with T2DM and normoalbuminuria, microalbuminuria, and macroalbuminuria. Glomerular changes were more common in patients with microalbuminuria and macroalbuminuria compared to those with normoalbuminuria, while interstitial or vascular changes were more frequent in normoalbuminuric patients compared to those with microalbuminuria and macroalbuminuria. |
| Mottl et al. (2013) [26] | Cross-sectional analysis (NHANES 2001–2008) | n = 2798 T2DM n = 15,743 non-diabetics | To compare the prevalence and modifying factors of normoalbuminuria (NA) vs. albuminuria (ALB) CKD in diabetics and non-diabetics | The prevalence of NA-CKD increased with age, with 9.7% of diabetics and 4.3% in non-diabetics. NA-CKD was less common in diabetic men (OR = 0.58; 95% CI: 0.39–0.87) and less frequent among Black individuals and those from other groups compared to Whites (OR = 0.44; 95% CI 0.29–0.68 and OR = 0.57; 95% CI: 0.34–0.96, respectively). It was more commonly observed in individuals with well- controlled blood pressure and glycemia (OR = 0.25, 95% CI: 0.13–0.50 and OR = 0.48, 95% CI: 0.31–0.74, respectively). Similar patterns were observed in non-diabetic participants. |
| Boronat et al. (2014) [27] | Prospective, observational survey (CERCA-Diabetes Study) | n = 78 normoalbuminuria: UACR < 30 mg/g, microalbuminuria: UACR 30–299 mg/g, and macroalbuminuria UACR > 300 mg/g | To estimate the prevalence and characteristics of non-albuminuric CKD associated with T2DM in individuals who progress to advanced stages of renal failure Follow-up: 2 years | Among patients with T2DM, 21.8% had normoalbuminuria; 20.5% had microalbuminuria, and 57.7% had macroalbuminuria. Patients with normoalbuminuria were more likely to be women and had lower rates of smoking and polyneuropathy. They also had higher body mass index and waist circumference measurements, elevated levels of total and LDL cholesterol, and lower values of HbA1c and serum creatinine compared to those with microalbuminuria or macroalbuminuria. |
| Yamanouchi et al. (2019) [28] | Repeated longitudinal analysis, propensity-score analysis of two cohorts: proteinuria vs. non-proteinuria, kidney biopsy | n = 526 T2DM with eGFR < 60 mL/min/1.73 m2 Non-proteinuria: UACR < 300 mg/g); poteinuria: UACR ≥ 300 mg/g) After the propensity-score matching: n = 82 in the non-proteinuric group and n = 164 in the proteinuric group | To assess the clinicopathological features, renal outcomes, and mortality in patients with T2DM and CKD without overt proteinuria Follow-up: median 1.9 years (IQR, 0.9–5.0 years) | Patients with NP-DKD had lower systolic blood pressure and less severe pathological lesions. CKD progression-free survival from the date of renal biopsy was 86.6% (95% CI 72.5–93.8) for the NP-DKD group and 30.3% (95% CI 22.4–38.6) for those with P-DKD. This lower renal risk was consistent across all subgroup analyses. Additionally, the 5-year death-free survival was 98.3% for the NP-DKD group and 82.6% for the P-DKD group. |
| Vistisen et al. (2019) [12] | Repeated longitudinal analysis | n = 935 T1DM n = 1984 T2DM | To quantify the impact of albuminuria status on the progression of eGFR trajectories following CKD stage 3 (CKD3) and to evaluate potential variations in progression patterns among the subgroup with normoalbuminuria Follow-up: 16 years | Over the first 10 years following CKD3, the mean annual declines in eGFR were as follows: 1.9, 2.3, and 3.3 mL/min/1.73 m2 for normoalbuminuria, microalbuminuria and macroalbuminuria in T1DM, and 1.9, 2.1, and 3.0 in T2DM, respectively. Albuminuria status significantly influenced eGFR changes in both types of diabetes, with the most pronounced decline in kidney function observed in patients with macroalbuminuria. Normoalbuminuric patients displayed two distinct eGFR decline patterns, one of which showed accelerated deterioration. This accelerated decline was associated with lower usage of lipid-lowering treatments, renin–angiotensin system blockers, and other antihypertensive therapies. |
| Jin et al. (2022) [29] | Multicenter prospective cohort (Hong King Diabetes Biobank) | n = 19,025 T2DM 4 groups defined by baseline eGFR and albuminuria: no DKD (no decreased eGFR or albuminuria), albuminuria without decreased eGFR, decreased eGFR without albuminuria, and albuminuria with decreased eGFR | To compare the risks of adverse outcomes between patients with non-albuminuric phenotype and other phenotypes in the context of DKD | Non-albuminuric DKD was associated with increased risks of all-cause mortality (HR = 1.59), hospitalization for heart failure (HR = 3.08), and CKD progression (HR = 2.37) compared to individuals without DKD, regardless of baseline eGFR. However, the risk of CVD was not significantly elevated. In contrast, the risks of death, CVD, hospitalization for heart failure, and CKD progression were higher in the presence of albuminuria, whether or not accompanied by decreased eGFR. |
| Fabre et al. (2024) [30] | Repeated longitudinal analysis | n = 304 T2DM | To assess the trajectory of eGFR and albuminuria concerning age (<75 and ≥75 years old) in individuals with DKD and identify predictive factors for the decline in eGFR decline, variation in albuminuria, mortality, and progression to renal replacement therapy Follow-up: 3 years | Comparable declines in eGFR were observed across both age groups during the follow-up period. During the first year, 24 h albuminuria was higher in patients with T2DM < 75 years of age (median 457 mg vs. 181.5 mg), but no differences were observed in subsequent years. In contrast, albuminuria increased more severely in T2DM with ≥75 years old (50% vs. 30.4%) from the first to second year of follow-up. Overall, 60.1% of patients experienced an increase in albuminuria, and 76.4% showed a decrease in eGFR. Age and smoking were the main predictors of increased albuminuria. Age, average SBP, average DBP, and smoking were associated with eGFR decline. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabre, L.; Pedregosa-Miguel, J.F.; Rangel, É.B. Proteinuric and Non-Proteinuric Diabetic Kidney Disease: Different Presentations of the Same Disease? Diabetology 2024, 5, 389-405. https://doi.org/10.3390/diabetology5040030
Fabre L, Pedregosa-Miguel JF, Rangel ÉB. Proteinuric and Non-Proteinuric Diabetic Kidney Disease: Different Presentations of the Same Disease? Diabetology. 2024; 5(4):389-405. https://doi.org/10.3390/diabetology5040030
Chicago/Turabian StyleFabre, Larissa, Juliana Figueredo Pedregosa-Miguel, and Érika Bevilaqua Rangel. 2024. "Proteinuric and Non-Proteinuric Diabetic Kidney Disease: Different Presentations of the Same Disease?" Diabetology 5, no. 4: 389-405. https://doi.org/10.3390/diabetology5040030
APA StyleFabre, L., Pedregosa-Miguel, J. F., & Rangel, É. B. (2024). Proteinuric and Non-Proteinuric Diabetic Kidney Disease: Different Presentations of the Same Disease? Diabetology, 5(4), 389-405. https://doi.org/10.3390/diabetology5040030






