Abstract
The principal purpose of this study is to determine the prevalence of peripheral arterial disease (PAD), as well as the principal associated risk factors, in patients registered in the IDON-PAD database. PAD is a condition characterized by the narrowing or blockage of arteries in the body’s extremities due to plaque buildup, leading to reduced blood flow and tissue ischemia. While PAD primarily affects the lower extremities, it can lead to symptoms such as intermittent claudication and, in severe cases, ulcers and amputations. Risk factors for PAD are numerous and cumulative, including smoking, age over 50, type 2 diabetes mellitus, and hypertension. The prevalence of PAD increases with age, with rates ranging from 2.5% in those over 50 to 60% in those over 85, varying by ethnicity and study population. Diabetic patients face a higher risk of PAD-related complications and have lower success rates with revascularization procedures. The diagnosis of PAD traditionally relied on physical examination and symptoms, but the Ankle–Brachial Index is now a standard diagnostic tool due to its non-invasive nature and reliability. In Mexico, the prevalence of PAD is estimated at 10%, with significant risk factors being the duration of diabetes, hypertension, hypertriglyceridemia, and smoking. Notably, 70% of PAD cases are asymptomatic, emphasizing the importance of proactive screening. This study aimed to determine the prevalence of PAD and associated risk factors in diabetic patients aged 40 and above. The prevalence was found to be 11.2%, with high-risk waist circumference, elevated triglycerides, positive Edinburgh questionnaire, and weak pulses as significant predictors. The detection and management of PAD in diabetic patients require a comprehensive approach, including lifestyle modifications and regular screenings. Prevention strategies should focus on controlling risk factors, including obesity, hypertension, and dyslipidemia. In conclusion, PAD is a prevalent yet underdiagnosed condition in diabetic patients, necessitating proactive screening and comprehensive management to mitigate associated risks and improve patient outcomes. The principal limitation of this study is that, as it uses a cross-sectional methodology and is not an experimental study, although we can establish the prevalence of PAD as well as the associated risk factors, we cannot define causality or determine the hazard ratio for each of these factors. Special thanks to Dr. Leobardo Sauque Reyna and all participants for their contribution to this research.
1. Introduction
Peripheral arterial disease (PAD) is a syndrome characterized by a multifactorial etiology that manifests as stenosis or obstruction of the arterial lumen (excluding coronary and cerebral arteries) by an atheroma plaque that originates in the intima and proliferates within the lumen, causing hemodynamic changes in blood flow that diminish the perfusion pressure and generate local and distal tissue and organ ischemia.
In medical practice, the arterial obstruction of the lower extremities manifests as the presence of pain in the legs induced by walking—known as intermittent claudication—as well as paleness that, in critical stages, can cause ulcers and non-traumatic amputations that affect patients’ quality of life [1,2].
The origin of the PAD is multifactorial, and its development includes multiple cardiovascular risk factors. The principal factors are cumulative, and when they coexist with factors, such as smoking, age of 50 years or older, type 2 diabetes mellitus T2DM, and hypertension, the severity of PAD increases [3].
The prevalence in patients over 50 years old is 2.5% and increases to 14.5% in patients over 70 years old. In the meta-analysis conducted by Vitalis A et al. [4] examining the prevalence of PAD across different ethnicities, a higher prevalence was found in African Americans and patients with type 2 diabetes mellitus (T2DM). In the latter group, the prevalence was up to four times higher compared to patients without diabetes. Similarly, in the study conducted by Cacoub P et al. in a French population at high risk (N = 5679), a 28% overall prevalence of PAD was observed. This contrasts with the 38% prevalence in the subgroup with the highest cardiovascular risk (subjects with a clinical history of atherothrombotic events) [5].
The prevalence of PAD in patients over 55 years old ranges from 3 to 29%, where the prevalence increases with age and is up to 60% in patients over 85 years old. The gap between these data is associated with ethnicity or race, the type of study population (higher or lower risk), and the methodology used to diagnose PAD [6].
The prognosis of PAD in patients with T2DM is less favorable than in patients without T2DM, as diabetic patients have a lower probability of success with revascularization and major cardiovascular morbimortality once PAD is present, as described in the article by Nativel M. et al. [7].
PAD can be diagnosed based on examination (skin color changes, cold extremities, and weak pulses) and symptoms (intermittent claudication and sensitivity alterations) [8], but the Ankle–Brachial Index (ABI) is now considered a standardized diagnostic method. It is a technique with the advantage of being non-invasive and painful, and it has acceptable precision, confidence, and reproducibility [9].
While information about PAD in the Latin American population is limited, we know that the prevalence of PAD in this group is around 12.5% [10].
In Mexico, the study performed by Buitrón-Granados L et al. [11]. followed a cross-sectional methodology and enrolled 400 patients who attended a first-level medical IMSS unit in Mexico City. They gathered information about previous medical history of T2DM, smoking, and hypertension, as well as laboratory variables and ABI index measurement (diagnostic cut point index of <0.9) with Doppler Ultrasound equipment (Doppler Versadopp@ 1000, Medzer Inc., Wilmington, DE, USA). At the end of the study, the global prevalence of PAD was 10% (14% in men and 8.4% in women). It stands out that the principal risk factors found were the duration of diabetes, the presence of hypertension, hypertriglyceridemia, and smoking. Just 30% of the patients with PAD had symptoms, while 70% were asymptomatic.
It is also known that both conditions (PAD and T2DM), alone and together, represent major public health issues and that both are considered cardiovascular risk factors. Many epidemiological studies have reported that patients with PAD have greater cardiovascular morbidity and mortality compared with patients without PAD [11,12]; on the other hand, it is well-known that patients with T2DM have a two-fold increase in cardiovascular risk for macrovascular events (myocardial infarction, stroke, and cardiovascular death) compared with the diabetic population [13,14].
PAD is considered an underdiagnosed condition due to a significant percentage of patients being asymptomatic. It is estimated that only 9% to 11% of patients report classic symptoms and, in a considerably high percentage of patients, the diagnosis is also delayed and is made when there is already severe tissue damage, ulcers, and/or amputation in the feet or pelvic limbs [15].
The poor detection of PAD, especially in asymptomatic populations, suggests that many patients will not receive appropriate treatment, particularly the management of peripheral arterial disease, as a cardiovascular risk factor, similar to the case for hypercholesterolemia and systemic arterial hypertension [16].
Regarding the incidence of PAD, a study by Hooi JD et al., which involved a 7.2-year follow up of a cohort of 2327 asymptomatic subjects in the Netherlands with the risk factors, revealed that the main factors increasing the risk of developing PAD are smoking, hypertension, and T2DM. This incidence study also emphasized the role of these factors in the cumulative risk [17].
A study by Rachael L. Morley et al. examined a population with a positive diagnosis of PAD, where 95% of patients had at least one of the risk factors, such as smoking and/or diabetes. Age was another significant risk factor, as in a population of 2174 patients studied, it was observed that the risk of developing PAD increased by 1% from 40 to 49 years old and by 15% in patients over 70 years old [18].
Patients with diabetes have up to 5.9 times greater risk of experiencing a cardiovascular event, as well as an added risk of developing PAD, which negatively impacts the functionality and quality of life of those who suffer from it. Patients with type 2 diabetes and PAD have a three to six times increased risk of experiencing a myocardial infarction [19].
Due to the high likelihood of developing PAD in patients with T2DM and the negative impact this has on their quality of life, the timely detection, treatment, and management of PAD as a cardiovascular risk factor are paramount for patients diagnosed with PAD. The ABI is the most widely used method for the diagnosis of PAD, as most patients may be asymptomatic [12].
Peripheral arterial disease in patients with diabetes is paramount in Mexico for several critical reasons. First, Mexico is experiencing a significant rise in the prevalence of diabetes, with estimates suggesting that nearly 13 million adults are affected by the disease [20]. Second, diabetes significantly increases the risk of developing PAD, with diabetic individuals being up to four times more likely to develop PAD compared to their non-diabetic counterparts [21]. Third, PAD in diabetic patients is associated with a higher risk of cardiovascular events, including myocardial infarction and stroke [15]. Due to the significance of PAD in the population with T2DM and the scarcity of data, the purpose of this study was to determine the prevalence of PAD, as well as the main associated risk factors, in patients with T2DM receiving care at the IDON, which is a population inherently at higher cardiovascular risk.
The primary objective of this study was to calculate the prevalence of PAD in patients registered in the IDON-PAD database by measuring their ABI using automated equipment from February 2020 to October 2021 and to identify the principal risk factors associated with design strategies of prevention, early treatment, and improved prognosis of patients with T2DM that attend the Instituto de Diabetes, Obesidad y Nutrición S.C. IDON in Cuernavaca, Morelos.
2. Material and Methods
This was a descriptive, comparative, and cross-sectional study.
The object of study was the IDON-PAD secondary database, which has a record of 734 patients with type 2 diabetes mellitus with at least 6 months of evolution who were treated at the IDON. The purpose of this project was to describe the prevalence of PAD in patients with type 2 diabetes registered in the IDON-PAD database who were treated at the IDON, as well as the association of PAD with the main variables of interest as risk factors.
As a first procedure, a deliberate search was conducted for patients at the Institute of Diabetes, Obesity, and Nutrition, located in Cuernavaca, Morelos, México, who had been diagnosed with at least 6 months of the evolution of type 2 diabetes mellitus and were aged at least 40 years. Once these patients were identified, they were invited to participate, following a thorough reading and signing of the informed consent (IC). The diagnosis of type 2 diabetes mellitus was considered when the following criterion was met: a diagnosis in the medical record in at least the last 6 months or receiving antidiabetic treatment in the last 6 months. The diagnostic criteria for T2DM were considered according to the criteria recommended by the American Diabetes Association 2019 ADA: glycated hemoglobin A1C > 6.5%, fasting plasma glucose > 126 mg/dL, and random glucose > 200 mg/dL. The measurement of glucose and hemoglobin was carried out after a minimum fast of 8 h, according to the photocolorimetry method for plasma glucose [22].
Including patients aged 40 and above in this study offers a broader perspective on peripheral arterial disease across various age groups. This approach enhances our understanding of how the disease progresses over time and its prevalence at different life stages. By avoiding age restrictions, a more comprehensive and accurate portrayal of the disease in the general population can be achieved. Despite its lower prevalence in younger individuals, evidence suggests that peripheral arterial disease can manifest in adults as young as 55, indicating that it is not exclusive to older age groups. Studies consistently show a notable increase in the prevalence of the disease with age. This inclusive approach allows for a more precise examination of this trend and insight into disease progression. Given its role as a significant risk factor for severe cardiovascular events, like heart attacks and strokes, grasping its prevalence across diverse age brackets is crucial for crafting effective public health policies. The inclusion of individuals aged 40 and above in this study furnishes valuable insights for identifying high-risk populations and implementing timely preventive measures.
Once informed consent was obtained, patients’ medical records were reviewed, clinical and laboratory information was updated, and a log of clinical laboratory variables was completed. Subsequently, upon reviewing the log of variables and applying applicable inclusion and exclusion criteria, the ABI was measured using the automated vasera 2000 method.
2.1. Population
The population was older than 40 years old and had a diagnosis of at least 6 months of T2DM evolution. Their data were obtained from the IDON-PAD secondary database and the second-level clinic (Instituto de Diabetes, Obesidad y Nutrición S.C.) in Cuernavaca, Morelos, during the time period from February 2020 to October 2021 if they met the relevant criteria.
2.2. Inclusion Criteria
Patients older than 40 years old.
Diagnosis of T2DM of at least 6 months of evolution.
Patients living in the state of Morelos, México.
2.3. Exclusion Criteria
Patients with incomplete information in the database (absence of 1 or more of the clinical and laboratory variables of interest).
2.4. Population Sample
Non-probabilistic convenience sampling was performed by taking all the records available in the IDON-PAD database that were found to be complete (all independent variables complete), met the inclusion criteria, and did not meet any of the exclusion criteria.
2.5. Data Collections Methods
No additional information was collected from the patients; only the information available in the IDON-PAD secondary database was utilized. The IDON-PAD database was created for research purposes, and patient information was collected after informed consent was obtained, such that the patients authorized the use of their personal and laboratory data. All variables were gathered from the patients’ medical records.
2.6. Ethical Considerations
Before the procedures and collection of data from the participating subjects, the procedures were fully explained, and the informed consent form version 1.0, approved by the research ethics committee on 26 March 2020 and the research committee on 26 March 2020, was read.
This protocol was conducted in accordance with the provisions of the Regulation of the General Heath Law on research, Ministry of Health (1984), specifically in the following sections: Articles 14, 15, 16, 17, 18, 19, 21, 22, and 29. Furthermore, both the informed consent form and the process of obtaining informed consent were carried out in accordance with the Nuremberg Code, the Belmont Report, and the Helsinki Declaration [23].
2.7. Analysis of the Information
Once the complete and correct information was available for the objectives of this study, the population of the database was characterized. The clinical and demographic characteristics were described using descriptive statistics. Continuous variables are expressed as means ± standard deviation using mode and median, and dichotomous variables are expressed as relative frequencies, which were estimated using binomial logistic regression analysis.
The prevalence of peripheral arterial disease detected by ABI measurement was obtained according to the proportion of patients with an ABI of 0.9 compared to patients with a normal ABI (>0.9). This was calculated with a confidence interval of 95%, and the respective prevalence by age and sex was also calculated.
To compare the qualitative variables between the patients with positive peripheral arterial disease vs. the patients with negative peripheral arterial disease, the chi-square test was first used and, for the variables with low frequency, Fisher’s test was used to compare the means and obtain the p-value.
To identify the risk factors associated with PAD in the study population, a multiple logistic regression model was performed. Before the multiple logistic regression was performed, the relationship between the dependent variable and the independent variables was explored through simple logistic regressions. In addition, the correlations between the qualitative and quantitative independent variables were analyzed. For quantitative variables that met the Kolmogorov–Smirnov hypothesis test of a non-normal parametric distribution of the data, Spearman’s coefficient was used, and for normally distributed variables, Pearson’s coefficient was used.
In the single and multiple regressions, the quantitative variables were analyzed as categorical variables, with categories constructed according to their clinical relevance, as shown in the next table. The age variable was classified by decades, and the high variable was in increments of 20. For the somatometry variables—body mass index kg/m2 (BMI), waist circumference, waist–hip ratio, and fat percentage—the WHO classification of risk was used [20]. We decided to add the waist–hip ratio as a variable instead of hip circumference for classification by sex [20], according to the WHO. The percentage of fat was also classified according to sex, including sub-optimal weight, slightly overweight, overweight, and obese. For the blood pressure variables (systolic and diastolic), the AHA [24] classification was used. Laboratory variables, such as HDL cholesterol and triglycerides, were classified according to AHA recommendations [24]. The glycosylated hemoglobin variable was classified according to the recommendations of the ADA [25]. Basal fasting glucose was classified as control, normal–high, slightly high, or very high. The estimated glomerular filtration rate variable, calculated using mdrd, was categorized according to the Chronic Kidney Disease Foundation’s classification [26]. The variables of weight, heart rate, creatinine, total cholesterol, and LDL cholesterol were classified by quartiles as, based on the frequencies, if they were classified by statistical relevance, there would be groups with very low frequencies, which would weaken the statistical analysis.
In the Table 1 below, the way in which quantitative variables were categorized for analysis is shown.

Table 1.
Categorization of quantitative variables.
Finally, the crude contribution of the independent variables as predictor variables was evaluated, all the analyzable variables were analyzed using a multiple logistic regression model with PAD as the dependent variable, and a multiple logistic regression was subsequently performed that was adjusted for the variables with the highest statistically significant correlation coefficients, according to the correlation analysis carried out between the independent variables and the dependent variable.
All p-values were calculated with two tails and were considered significant if they were less than 0.05. To find out the strength and direction of the association between the independent variables and the PAD, the odds ratios and their 95% confidence intervals were calculated using the Stata 16 program.
To guarantee that the research protocol and the techniques used to keep patient data confidential were certified, the protocol was sent to the Research Ethics Committee of the National Institute of Public Health for review and approval.
3. Results
In Table 2, we can observe the baseline characteristics of the qualitative variables of the patients. In Table 3, we have the baseline characteristics of the quantitative variables of the patients, where we observe that the mean of characteristics is an age og 61 years, with a BMI of 28.

Table 2.
Description of the qualitative variables.

Table 3.
Description of the quantitative variables.
In Table 4, we can observe the proportion of patients according to glycated hemoglobin levels, finding the population to be polarized, with the highest proportion of patients in the range of 5–6.9%, followed by a glycated hemoglobin > 10%.

Table 4.
Frequency of patients according to glycated hemoglobin results.
3.1. PAD Prevalence
The prevalence of PAD was 11.2%, with a total of 71 patients, of whom 40 (56.3%) were female and 31 (43.7%) were male.
3.2. Comparative Groups of Qualitative Variables
The variables that had a statistically significant difference between both groups were the presence of systemic arterial hypertension and the presence of a previous vascular event or atherosclerotic cardiovascular disease (specifically, non-traumatic vascular amputation), history of eye surgery, antihypertensive treatment, treatment with insulin, the presence of proteinuria, positive sensitivity alteration, positive Edinburgh questionnaire, the presence of signs or symptoms of peripheral arterial disease, color changes, limp gait, weak pulses, dryness, and the presence of ulcers (see Table 5). The difference in proportions of the statistically significant qualitative variables between the positive PAD and the negative PAD groups was then observed.

Table 5.
Comparison of the qualitative variables with negative PAD vs. positive PAD.
3.3. Comparative Groups of Quantitative Variables
The variables that had a statistically significant difference between the means are shown in Table 6. We can see that patients with positive PAD had a higher mean age, serum creatinine, waist circumference, hip circumference, and systolic blood pressure than patients with negative PAD, while PAD-positive patients had a lower glomerular filtration rate than patients with negative PAD.

Table 6.
Comparison of the quantitative variables with negative PAD vs. positive PAD.
The correlations between the dependent variable PAD and the qualitative independent variables as shown in Table 7 were evaluated, with statistically significant correlations found in the following variables: the presence of systemic arterial hypertension, history of laser treatment, history of eye surgery, treatment with antihypertensive drugs, intermediate insulin treatment, treatment with antiplatelet agents, alteration of sensitivity, Edinburgh questionnaire, signs or symptoms suggestive of PAD, changes in limb coloration, and weak pulses in pelvic extremities.

Table 7.
Correlation between the dependent variable PAD and the qualitative independent variables.
3.4. Positive Cases by Age
Among our population, 65.1% (411) were between 55 and 70 years old, 14.4% (91) were over 70 years old, and 20.5% (130) were less than 55 years old.
In Figure 1, we can see that the largest volume of positive cases for PAD was found in the 60- to 69-year-old group, with 39.4% (28), followed by 28.1% (20) for the 70- to 79-year-old group, 17% (12) for the group from 50 to 59 years, 8.5% (6) for the group from 80 to 90 years, and, finally, 7% (5) for the group from 40 to 49 years. The highest proportion of positive cases of PAD was found in the group of 80 to 90 years at a proportion of 0.28.
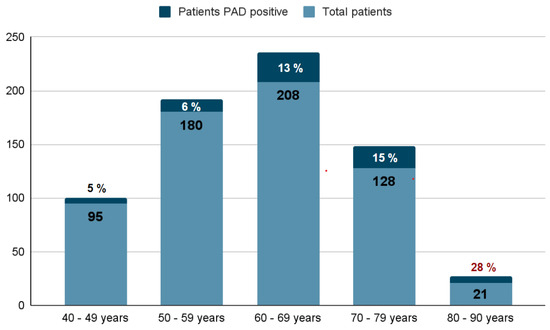
Figure 1.
Frequency and rate of PAD (+) by age group.
3.5. Multiple Logistic Regression
Initially, a crude multivariate logistic regression was run using all the independent variables with the dependent variable; however, as it is a crude multiple logistic regression, multicollinearity between the independent variables was present, which reduced its predictive capacity. Therefore, a multivariate logistic regression was subsequently run that did not analyze the strongly correlated variables (see Table 8), and it was adjusted according to the correlations between the independent variables that, in the simple regression, showed a statistically significant correlation with the dependent variable. We can observe that the variables with statistical relevance were high waist circumference, very high waist circumference, triglycerides between 150 and 199 mg/dL, positive Edinburgh questionnaire, and weak pulses in the lower extremities during the examination.

Table 8.
Multiple logistic regression model adjusted for the correlation of independent variables strongly associated with the dependent variable.
With the other variables held constant, subjects with a waist circumference classified as high risk (F: 80–88 cm, M: 95–101 cm) were 4.58 times more likely to present PAD, and subjects with a waist circumference classified as very high risk (F: >88 cm, M: >102 cm) were up to 3.39 times more likely to have PAD than patients with a low-risk waist circumference. Patients with serum triglycerides between 150 and 199 mg/dL were 3.14 times more likely to present PAD than those patients with serum triglycerides < 100 mg/dL. Patients who presented a positive Edinburgh questionnaire were 3.35 times more likely to present PAD than patients who reported a negative Edinburgh questionnaire. Patients who presented weak pulses in the lower extremities during the physical examination were 25.49 times more likely to present PAD than those who presented pulses of adequate intensity during the physical examination.
4. Discussion
This study revealed the prevalence of PAD among diabetic patients to be 11.2%, which is consistent with previous findings in the Mexican population [27]. This underscores the significant comorbidities among these patients, with systemic arterial hypertension, dyslipidemia, and smoking being prevalent risk factors [28], as well as highlights the complex interplay between diabetes and other cardiovascular risk factors, emphasizing the need for comprehensive management strategies [28].
The people in this study varied, with some being larger but having their blood pressure and LDL cholesterol relatively under control. However, many struggled with blood sugar control. This tells us that we cannot just focus on heart issues when treating diabetes; we need a more comprehensive approach to achieve better outcomes for everyone.
It is surprising that only 8% of patients showed signs of having PAD, meaning that many cases could go unnoticed [27]. This reminds us of the importance of having a suspicious mindset in medical consultations and conducting thorough physical examinations, especially given that many PAD patients do not show obvious symptoms. Therefore, we need to make more efforts to detect PAD early and prevent future complications.
People with PAD are at higher risk of serious problems, such as heart attacks or amputations, as well as eye problems [7]. This indicates that diabetes-related vascular issues affect not just the heart but the whole body, highlighting the importance of detecting these problems early and treating them properly. It is vital to focus on maintaining a healthy weight, controlling triglyceride levels, and conducting comprehensive physical examinations in people with diabetes to detect PAD as early as possible and improve daily life and also reduce the risk of serious complications, like foot ulcers or amputations.
Prevention strategies will have to be designed to ensure that patients have the least associated comorbidities, controlling weight, waist circumference, blood pressure levels, and laboratory tests, such as cholesterol (total, HDL, LDL), triglycerides, and serum creatinine.
With the results of this study, it is not possible to accept the hypothesis that the main risk factors associated with PAD are positive smoking and advanced age. When constructing the correlation matrix, we noticed that the sample is probably insufficient for a model with so many variables, such as those included in the database, in addition to the fact that, when trying to classify them, there were categories with very low frequencies, which forced us to classify them by quartiles and not clinical relevance.
5. Conclusions
Peripheral arterial disease is a prevalent and underdiagnosed disease in first- and second-level care. It was found to have a prevalence of 11.2% in patients with type 2 diabetes mellitus who were seen in a second-level care clinic.
The main associated risk factors were waist circumference with high risk (4.5 more times); waist circumference with very high risk (3.3 more times); serum triglycerides greater than 150 mg/dL and less than 199 mg/dL (3.1 more times); and positive Edinburgh questionnaire (3.3 more times).
The main sign that we can observe in patients with positive PAD is the positive Edinburgh questionnaire, as this procedure should be carried out in all visits of patients with type 2 diabetes mellitus.
It is essential to actively search for signs or symptoms indicative of peripheral arterial disease in all patients with type 2 diabetes mellitus during regular medical consultations, as well as to incorporate the Edinburgh questionnaire into each visit. Additionally, regular measurement of the ABI should be encouraged for the early detection of PAD.
Encouraging healthy lifestyle habits among patients with T2DM is crucial to prevent and control the associated comorbidities. Keeping these conditions within control targets can significantly reduce the risk of both macrovascular and microvascular complications.
Author Contributions
Conceptualization; A.I.P.A., E.M.C. and L.S.R., methodology; A.I.P.A., E.M.C. and L.S.R., software; A.I.P.A. and E.M.C., validation; L.S.R. and E.M.C., formal analysis: A.I.P.A., E.M.C. and L.S.R., investigation; A.I.P.A., J.M.F., D.S.G.M., R.G.S., resources; L.S.R., data curation; A.I.P.A., E.M.C. and L.S.R., writing—original draft preparation; A.I.P.A., E.M.C. and L.S.R., writing—review and editing; A.I.P.A. and L.S.R., visualization, A.I.P.A., supervision, E.M.C. and L.S.R., project administration; A.I.P.A. and L.S.R., funding acquisition; L.S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Instituto de Diabetes, Obesidad and Nutrición S.C, in Cuernavaca Morelos.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Ethics Committee of RIO MAYO MEDICA, local version No. 1.0 8MX and date of approval 14 feb for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The available informationfrom the complete database used in this study is on Zenodo, with the DOI: 10.5281/zenodo.11176030.
Acknowledgments
Thanks to Leobardo Sauque Reyna, director of the Institute of Diabetes, Obesity, and Nutrition, for giving us all the support and tools to make this research possible. Thank you to all the participants and subjects of the sample who gladly contributed to the research. Thanks to the entire multidisciplinary team that contributed in very specific and varied ways to generate this scientific research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Regensteiner, J.G.; Hiatt, W.R.; Coll, J.R.; Criqui, M.H.; Treat-Jacobson, D.; McDermott, M.M.; Hirsch, A.T. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc. Med. Lond. Engl. 2008, 13, 15–24. [Google Scholar] [CrossRef]
- Ouriel, K. Peripheral arterial disease. Lancet 2001, 13, 57–64. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.; Dormandy, J.; Nehler, M.; Harris, K.; Fowkes, F.; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, 5–67. [Google Scholar] [CrossRef]
- Antonios, V.; Gregory, Y.H.L.; Mark, K.; Rajiv, K.V.; Alena, S. Ethnic differences in the prevalence of peripheral arterial disease: A systematic review and meta-analysis. Expert Rev. Cardiovasc. Ther. 2017, 15, 328–338. [Google Scholar]
- Cacoub, P.; Cambou, J.-P.; Kownator, S.; Belliard, J.-P.; Beregi, J.-P.; Branchereau, A.; Carpentier, P.; Léger, P.; Luizy, F.; Maïza, D.; et al. Prevalence of peripheral arterial disease inhigh-risk patients using ankle-brachial index in general practice: Across-sectional study. Int. J. Clin. Pract. 2009, 63, 63–70. [Google Scholar] [CrossRef]
- Darling, J.; Bodewes, T.; Deery, S.; Guzman, R.; Wyers, M.; Hamdan, A.; Verhagen, H.J.; Schermerhorn, M.L. Outcomes after frst-time lower extremity revascularization for chronic limb-threatening ischemia between patients with and without diabetes. Vasc. Surg. 2018, 67, 59–69. [Google Scholar] [CrossRef]
- Nativel, M.; Potier, L.; Alexandre, L.; Baillet-Blanco, L.; Ducasse, E.; Velho, G.; Marre, M.; Roussel, R.; Rigalleau, V.; Mohammedi, K. Lower extremity arterial disease in patients with diabetes: A contemporary narrative review. Cardiovasc. Diabetol. 2018, 23, 17–138. [Google Scholar] [CrossRef]
- Burk, H. Das Hunderttage-Stadion Entstehungsgeschichte des Bad Nauheimer Kunsteisstadions unter Colonel Paul R. Knight; Stadt Bad Nauheim: Bad Nauheim, Germany, 1999. [Google Scholar]
- Ko, S.; Bandyk, D. Interpretation and significance of ankle-brachial systolic pressure index. Semin. Vasc. Surg. 2013, 26, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Rosero, E.; Kane, K.; Clagett, G.; Timaran, C.H. A systematic review of the limitations and approaches to improve detection and management of peripheral arterial disease in Hispanics. Vasc. Surg. 2010, 51, 27–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buitrón-Granados, L.; Martínez-López, C.; Escobedo-de la Peña, J. Prevalence of peripheral arterial disease and related risk factors in an urban Mexican population. Angiology 2004, 55, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Shemanski, L.; Manolio, T.; Cushman, M.; Mittelmark, M.; Polak, J.; Powe, N.R.; Siscovick, D. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arter. Vasc. Biol. 1999, 19, 38–45. [Google Scholar]
- Emerging Risk Factors Collaboration; Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 15–22. [Google Scholar]
- Resnick, H.; Lindsay, R.; McDermott, M.; Devereux, R.; Jones, K.; Fabsitz, R.; Howard, B.V. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: The Strong Heart Study. Circulation 2004, 17, 109–733. [Google Scholar]
- Hirsch, A.; Criqui, M.; Treat-Jacobson DRegensteiner, J.; Creager, M.; Olin, J.; Krook, S.; Krook, S.H.; Hunninghake, D.B.; Comerota, A.J.; Walsh, M.E.; et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001, 19, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Rada, C.; Oummou, S.; Merzouk, F.; Amarir, B.; Boussabnia, G.; Bougrini, H.; Benzaroual, D.; Elkarimi, S.; Elhattaoui, M. Ankle-brachial index screening for peripheral artery disease in high cardiovascular risk patients. Prospective observational study of 370 asymptomatic patients at high cardiovascular risk. J. Mal. Vasc. 2016, 41, 353–357. [Google Scholar] [CrossRef]
- Hooi, J.D.; Kester, A.D.; Stoffers, H.E.; Overdijk, M.M.; van Ree, J.W.; Knottnerus, J.A. Incidence of and risk factors for asymptomatic peripheral arterial occlusive disease: A longitudinal study. Am. J. Epidemiol. 2001, 153, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Morley, R.L.; Sharma, A.; Horsch, A.D.; Hinchliffe, R.J. Peripheral artery disease. BMJ 2018, 360, j5842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ran, X.; Xu, Z.; Cheng, Z.; Shen, F.; Yu, Y.; Gao, L.; Chai, S.; Wang, C.; Liu, J.; et al. Epidemiological characteristics of lower extremity arterial disease in Chinese diabetes patients at high risk: A prospective, multicenter, cross-sectional study. J. Diabetes Its Complicat. 2018, 32, 150–156. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes. Diabetes Care 2019, 42, S13–S28. [Google Scholar]
- Asociación Médica Mundial. Declaración de Helsinki de la AMM—Principios Éticos Para las Investigaciones Médicas en Seres Humanos. 2013. Available online: https://www.wma.net/es/policies-post/declaracion-de-helsinki-de-la-amm-principios-eticos-para-las-investigaciones-medicas-en-seres-humanos/ (accessed on 13 April 2024).
- WHO. The Use and Interpretation of Anthropometry. In Physical Status: The Use of and Interpretation of Anthropometry; Report of a WHO Expert Committee; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef]
- National Kidney Foundation. Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease; National Kidney Foundation, Inc.: New York, NY, USA, 2013; pp. 1–150. [Google Scholar]
- Smith, A.B.; Maltais, S.; Kisilevsky, M.; Gagne, E. Peripheral Artery Disease: Epidemiology, Pathophysiology, and Clinical Presentation. Curr. Cardiol. Rep. 2022, 24, 1–12. [Google Scholar]
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Erlinger, T.P. Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 2004, 110, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Langer, R.D.; Fronek, A.; Feigelson, H.S.; Klauber, M.R.; McCann, T.J.; Browner, D. Mortality over a period of 10 years in patients with peripheral arterial disease. N. Engl. J. Med. 1992, 326, 381–386. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).