Abstract
This study presents a comprehensive analysis of 898 clinical trials conducted between 1999 and 2023, focusing on the interplay of metabolic syndrome, cardiovascular diseases (CVDs), and type 2 diabetes mellitus (T2D). This study draws upon data sourced from the International Clinical Trials Registry Platform (ICTRP) until August 2023. The trials were predominantly interventional (67%) or observational (33%). A geographical distribution reveals that while the United States registered approximately 18% of the trials, other regions like Australia, the United Kingdom, and multicounty trials made substantial contributions. Most studies (84%) included both male and female participants, with adults aged 18 to 65 years predominantly represented. The trials aimed at treatment (21%) and prevention (21%), emphasizing the dual focus on addressing existing CVD risk and preventing its development. Notably, CVDs (29%), T2D (8%), and the coexistence of both (21%) constituted the primary conditions of interest. Key interventions encompassed lifestyle and behavioral modifications, dietary supplementation, and drug therapies, with metformin and statins leading in pharmacological treatments. Interestingly, additional interventions such as glucagon-like peptide-1 agonists and dipeptidyl peptidase IV inhibitors are gaining recognition for their potential in managing metabolic syndrome-related conditions. Moreover, the report highlights a growing focus on inflammation, body mass index, blood pressure, body weight, and major adverse cardiovascular events as primary outcomes. Overall, the study highlights the importance of ICTRP as the source of data for clinical trials targeting metabolic syndrome, CVDs, and T2D and the growing recognition of diverse intervention strategies to address this critical global health concern.
1. Introduction
The World Health Organization (WHO) has progressively highlighted the significance of cardiovascular diseases (CVDs) in driving the global burden of disease, with evidence suggesting this condition claimed about 17.9 million lives in 2021 [1]. Briefly, CVD incorporates a group of complex disorders affecting the heart and blood vessels, with atherosclerosis recognized as a major contributing factor [2]. These conditions encompass a range of complications, including stroke, coronary artery disease, heart failure, arrhythmias, and cerebrovascular disease, among others [3]. Predominantly studied in the elderly through the Framingham Heart Study [4,5], research on CVD now spans multiple generations of participants [6,7]. Within this context, metabolic complications have significantly contributed to the development and progression of cardiovascular complications [8]. Certainly, increasing evidence indicates that most patients with diabetes are likely to succumb to CVD-related abnormalities when compared to nondiabetic counterparts [9,10].
Epidemiological data unequivocally establish diabetes as an independent risk factor for the development of CVDs [11,12]. As a result, research exploring the relationship between CVD and diabetes, particularly type 2 diabetes (T2D), has garnered significant interest among researchers [13,14]. T2D is characterized by persistent hyperglycemia and a state of insulin resistance that is followed by subsequent damage or dysfunction of pancreatic β-cells [15]. While T2D was historically associated with older adults [16], it is now known that many diverse factors are associated with the development and progression of this condition even in children and adolescents [17,18]. Indeed, research continues to highlight the importance of clarifying factors that contribute to the development of CVD risk in people with diabetes mellitus [19,20,21]. This includes understanding the influence of factors such as genetic predisposition, lifestyle choices, socioeconomic status, and comorbidities. In fact, risk prediction models are increasingly explored to give insight into the effectiveness of personalized interventions, targeted lifestyle modifications, medication regimens, or novel therapies and how they could contribute to improving CVD outcomes in patients with diabetes [19,20,21]. Such approaches remain instrumental to a more patient-centered approach to managing CVD risk in individuals with diabetes, potentially leading to more effective prevention and treatment strategies [22,23].
Physical activity and lifestyle modification, when applied consistently, currently remain the most effective interventions to alleviate metabolic complications, including reducing CVD [24,25,26,27]. However, only a few people adhere consistently to such strict interventions. Metformin has remained the leading therapeutic intervention for people with T2D [28,29,30]. This biguanide drug is effective at controlling blood glucose levels, while it is increasingly being investigated for its cardioprotective properties in people with diabetes [28,29,30]. Alternatively, statins are recommended for people with dyslipidemia [31], with some research indicating cardioprotective effects of these drugs [32]. However, emerging research has reported limitations with statins, especially the limitations associated with their long-term use in reducing the intracellular levels of coenzyme Q10 [33], a fat-soluble quinone with a structure similar to that of vitamin K and which is vital to the human body. Beyond the therapeutic effects of metformin and statins [28,29,30,32], increasing research has progressively evaluated the antidiabetic and cardioprotective effects of various nutrients, especially commonly used foods such as vegetables, fruits, herbal teas, and others [34,35,36]. Nonetheless, understanding global trends in disease distribution, especially across global regions, has become important before any recommendations can be accepted. For example, adult patients with diabetes are acknowledged to have two–four times higher risk of developing CVD compared to individuals without this condition, and the risk increases with poor glycemic control [37]. Others highlight the significance of understanding trends in the epidemiology of CVD and cardiovascular risk management in T2D for a more individualized patient-centered approach to manage these conditions [38]. Beyond reducing fasting plasma glucose levels, decreasing blood pressure, as well as triglycerides, and low-density lipoprotein cholesterol remains instrumental to lower CVD risk in individuals with diabetes [39].
The International Clinical Trials Registry Platform (ICTRP) plays a pivotal role in advancing our understanding of metabolic diseases by serving as a centralized repository for clinical trial data. Metabolic diseases, including T2D and CVD, present significant global health challenges. The ICTRP provides a valuable resource for researchers, healthcare practitioners, and policymakers, facilitating the registration and dissemination of data from clinical trials focused on metabolic diseases. Through the comprehensive analysis of data from diverse clinical trials, the ICTRP enables the identification of trends, patterns, and emerging insights in the field of metabolic diseases. Such data are instrumental in informing evidence-based practices, guiding the development of targeted interventions, and ultimately improving the management and prevention of CVD-related complications in patients with diabetes. This study also highlights the importance of the ICTRP as the source of data for clinical trials targeting metabolic syndrome, CVDs, and T2D, including the growing recognition of diverse intervention strategies to address this critical global health concern.
2. Methodology
2.1. Source and Data Description
The ICTRP registry (https://www.who.int/clinical-trials-registry-platform, accessed on 3 September 2023) was accessed for a comprehensive analysis of data on clinical trials reporting on the global trends in CVD risk in people with diabetes [40]. This registry contains clinical data from registries across the globe and has become an important tool to monitor disease surveillance or to evaluate the effects on health outcomes [41,42]. An advanced search function of the ICTRP was used to identify relevant clinical trials, registered on 2 August 2023. This study is a cross-sectional analysis of registered clinical trials for people with diabetes at risk of CVD. We conducted searches in the ICTRP, which is a repository hosted by the WHO that contains regularly updated clinical trial data from primary clinical trial registries of the WHO network. Importantly, a growing number of reports have used this registry to analyze and inform on clinical data that remain significant for public health [43,44,45,46].
2.2. An Approach for Data Analysis and Management
Two researchers (M.N. and N.M.) independently accessed the WHO ICTRP portal to download relevant data on 2 August 2023. The downloaded Excel spreadsheet was checked by other researchers (P.V.D. and D.N.) before data extractions were performed, especially accuracy in collection of information concerning trial registry source, date of registration, retrospective flag, gender, trial phases, and intervention model. Other relevant information that was collected was based on intervention model prominently used, disease condition, and primary outcomes measured, corresponding to CVD risk in people with diabetes. This information allowed for a comprehensive analysis of global trends in CVD risk for people with T2D within an Excel spreadsheet.
3. Results
3.1. Study Inclusion
Briefly, a total of 935 records (registered from 1999 to 2023) of clinical studies were retrieved from the WHO ICTRP registry. After eligibility assessment, including duplicate removal and exclusion of trials that did not focus on CVD and diabetes, 116 studies were excluded (Figure 1). As a result, a total number of 898 studies were suitable for the analysis, predominantly interventional (n = 602), and observational (n = 295) studies (Figure 1).
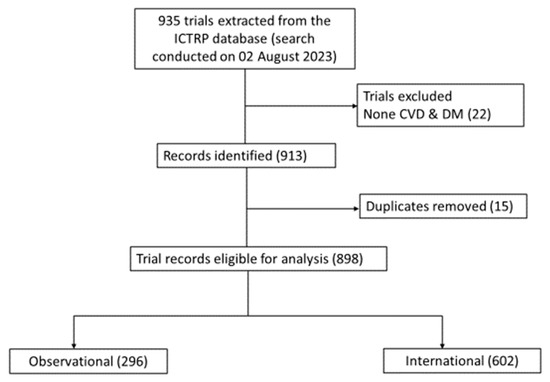
Figure 1.
Flow chart repressing clinical trial selection. Briefly, after eligibility assessment, including duplicate removal and exclusion of trials that did not focus on cardiovascular disease (CVD) and diabetes mellitus (DM), 898 studies were suitable for the analysis, predominantly including interventional (n = 602), and observational (n = 296).
3.2. Data Distribution Based on Country of Origin, Clinical Trial Registry Sources, and Year of Publication
When exploring the regions of registered trials, it was observed that about 18% of trials were registered in the United States, while other regions like Australia, the United Kingdom, and multiple countries trials recorded 10%, 7%, and 9%, respectively (Figure 2A,B). Single-country recruitment centers registered 91% in comparison to multicountry multicenter (9%) trials. The ClinicalTrials.gov registry registered the most trials (56%), followed by ANZCTR (12%), ISRCTN (9%), JPRN (6%), EU-CTR (4%), and CTRI (3%) (Figure 2C). Other registries registered ≤1% of trials related to diabetes and CVDs between 1999 and 2023 (Figure 2C). The highest number of trials recorded per year during the reporting period was 7% in 2016. Even though there was no consistency, in the registration of clinical trials on participants with diabetes and CVD, there was a significant increment from 1999 to 2022, with a probability to rise even in 2023 (Figure 3).
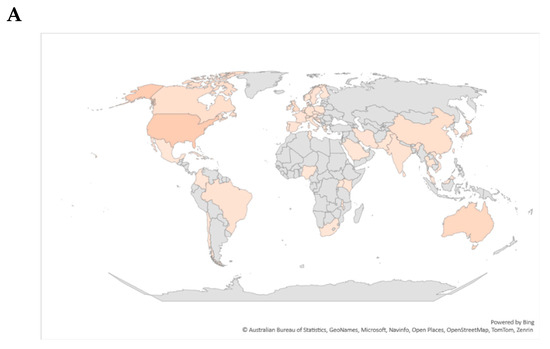
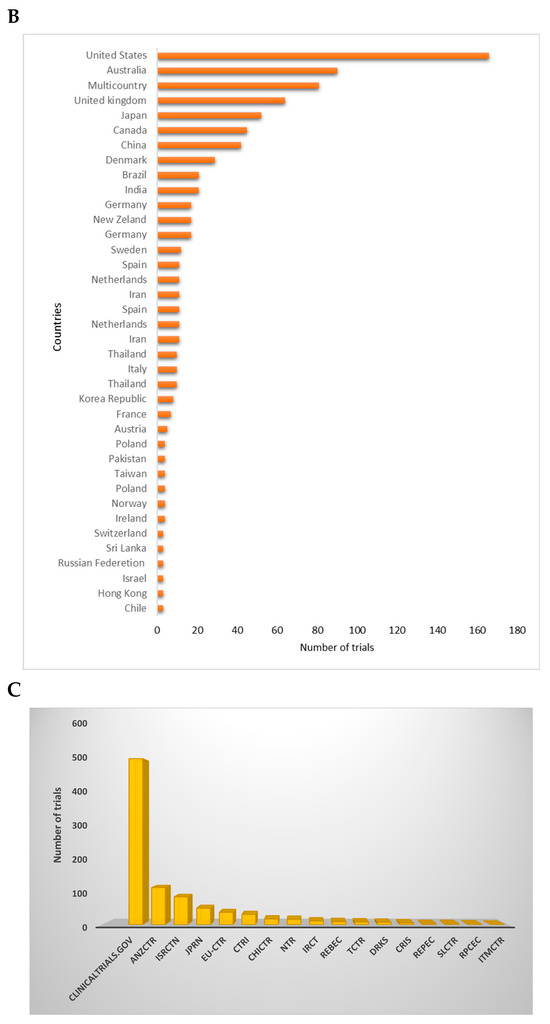
Figure 2.
Global distribution of registered trials per country. (A) depicts a global map color-coded with strong-shaded regions describing a high number of trials registered in that specific region. (B) demonstrates the distribution of trials per country. Briefly, the United States registered the most clinical trials, while other regions like Australia, the United Kingdom, and multiple countries trials recorded 10%, 7%, and 9%, respectively. (C) gives an overview of the distribution of trials across different clinical trial registries. Briefly, some of the listed registries included Australian New Zealand Clinical Trials Registry (ANZCTR), International Standard Randomized Controlled Trial Number (ISRCTN), Japan Primary Registries Network (JPRN), Iranian Registry of Clinical Trials (IRCT), Peruvian Clinical Trials Registry (REPEC), Sri Lanka Clinical Trials Registry (SLCTR), Cuban Public Registry of Clinical Trials (RPCEC), International Traditional Medicine Clinical Trial Registry (ITMCTR), Clinical Research Information Service of the Republic of Korea (CRiS), Thai Clinical Trials Registry (TCTR), Chinese Clinical Trial Register (ChiCTR), and the Clinical Trials Registry India (CTRI).
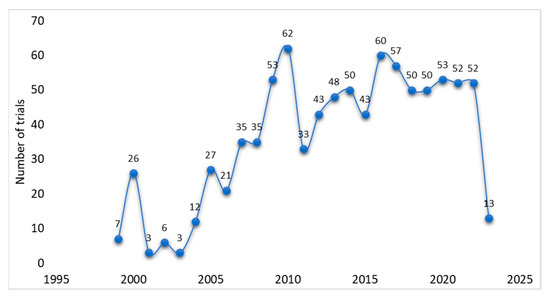
Figure 3.
Distribution of registered trials per year from 1999 to 2023. These data show that a steady increase in trial registration is observed between 1999 and 2022.
3.3. Data Distribution Based on Gender of Participants
Most trials (84%) included both females and males in their studies (Figure 4A). When exploring age differences of those registered, it was observed that most trials recruited adults between 18 and 65 years of age for both minimum and maximum age inclusion (Figure 4B).
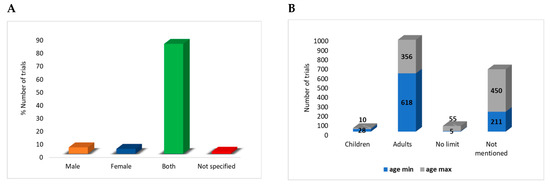
Figure 4.
Overview of gender distribution within the included clinical trials. Most trials, 700 (84%), included both females and males in their studies (A). When exploring age differences of those registered, it is observed that most trials recruited adults (18 to 65 years) for both minimum and maximum age inclusion (B).
3.4. Data Distribution Based on Intervention Model and Disease Type
Mostly, recorded trials focused on different groups of participants receiving different interventions, predominantly of parallel assignment (Figure 5A). Forty-two trials employed cross-over intervention, twenty-eight single group assignments, seventeen factorial, and five sequential. We further explored the primary purpose of individual trials, and we observed that the treatment (21%) and prevention (21%) primary purposes show almost equal percentage numbers of clinical trials (Figure 5A). Other primary purposes of the trials include observation (7%), health service (3%), supportive care (2%), basic science (4%), and diagnostic (1%).
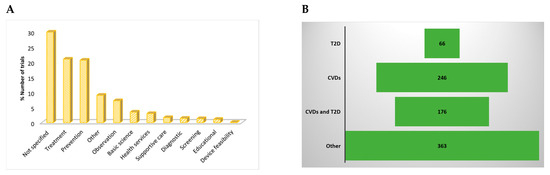
Figure 5.
(A) lists registered trials distributed according to the primary purpose of the study. Except for those that were not specified, most included clinical trials were focused on treatment and prevention. (B) Disease conditions of participants in registered trials included type 2 diabetes (T2D), cardiovascular diseases (CVDs), both conditions (diabetes and CVDs), and others, which were mostly a cluster of metabolic anomalies, such as dyslipidemia, obesity, nonalcoholic fatty liver disease, and hypercholesterolemia.
3.5. Data on Disease Classification and Interventions Being Used in People with Diabetes and CVD
The most common conditions investigated in the trials were CVDs (29%) and T2D (8%) (Figure 5B). Other trials included participants with both T2D and CVDs (21%). Other conditions included a cluster of metabolic complications, characterizing metabolic syndrome (43%) (Figure 5B). Correspondingly, it was obvious that measurements of blood lipid profiles, like cholesterol, low-density lipoprotein, high-density lipoproteins, as well as glucose and insulin or glycated hemoglobin levels, were predominant in these trials reflecting primary outcomes in people with diabetes or those at CVD risk (Table 1). Notably, inflammation, body mass index, blood pressure, body weight, and major adverse cardiovascular events were also evaluated and were also increasingly evaluated as primary outcomes in people with diabetes at risk of CVD (Table 1).

Table 1.
Selective reporting for primary outcomes per condition.
3.6. Data on Disease Classification and Interventions Being Used in People with Diabetes at Risk of CVDs
We further evaluated a variety of interventions that are being tested in trials being conducted. In Table 2, the interventions being currently explored are distributed according to the health conditions, including T2D, CVDs, both CVDs and T2D, and other conditions. Notably, lifestyle and behavioral modifications as well as dietary supplementation are the interventions implemented in trials across different disease conditions. Drug intervention for both CVDs and T2D included the use of statins, metformin, and pioglitazone (Table 2). Most interestingly, some trials evaluated drug combinations and drug plus physical activity interventions. For intervention against T2D, metformin, dapagliflozin, and empagliflozin were among the drug interventions implemented. Similarly, lifestyle, physical exercise, and behavioral changes were also encouraged. Against cardiovascular diseases, statin (atorvastatin, simvastatin, rosuvastatin, pravastatin) therapy was among the drug interventions implemented. Drug combination therapy, such as ezetimibe plus statins, was investigated by other trials.

Table 2.
Selective reporting for intervention per health condition.
4. Discussion
The comprehensive analysis of clinical trial data on global trends in risk factors associated with diabetes and CVD provides valuable insights into the state of research in this critical area of public health concern. One of the notable aspects of the study is the diversity in trial registration and recruitment patterns. The analysis of the ICTRP data revealed that while the United States accounted for approximately 18% of registered trials, other regions like Australia, the United Kingdom, and multicountry trials also made substantial contributions. This geographic distribution highlights the global relevance and collaborative nature of research addressing CVD risk in individuals with diabetes. This also corroborates information from other studies, showing that ClinicalTrials.gov is the predominant source for clinical trial data, informing on many disease conditions [43,47]. This also aligns with increasing trends in data on the prevalence of metabolic disease in developed nations, including the United States [48]. However, the limited clinical data available underscore the need for research to be conducted in developing nations, like those within the sub-Saharan Africa region where there is an increasing surge in metabolic diseases [49].
The study identified a consistent increase in the registration of clinical trials from 1999 to 2022, with indications of continued growth in 2023. Consistent with growing reports [9,50], this trend reflects the growing recognition of the significance of CVD risk in individuals with diabetes and the urgent need for research in this field. This is in agreement with a growing number of studies [12,51,52] while also pointing to the need to contain diabetes-related abnormalities in order to minimize long-term CVD complications [9] This also does not exclude a gap in prospective and active research, which could provide more timely and actionable insights into CVD risk management in this population. A commendable aspect of the analyzed trials is the inclusion of both genders, with 84% of trials incorporating both males and females. This balanced representation is crucial for generating results that are applicable to a broader population. Furthermore, the trials predominantly recruited adults aged between 18 and 65 years, which is relevant given that diabetes and CVD risk often develop in adulthood [53]. However, it is essential to continue assessing the impact of interventions on pediatric and elderly populations, as these age groups also face significant health challenges related to diabetes and CVD [54].
In examining the primary purposes of the trials, the study found that treatment and prevention were the most common objectives, each accounting for 21% of the trials. This balance suggests that researchers are not only focused on managing existing CVD risk but also on preventing its development in individuals with diabetes. These findings highlight the comprehensive approach to managing this dual health burden. More so, the analysis revealed that trials predominantly investigated CVDs (29%) and T2D (8%), with a substantial proportion focusing on both conditions (21%). Currently, there is conflicting evidence in terms of CVD burden in developing nations. For example, some research has suggested that incidence, prevalence, and mortality rates remain high in low- and middle-income countries [55], while others have seen a decline in trends for CVD incidence and mortality rates and stable survival rates in patients with CVD [56]. Policymakers have predominantly highlighted the importance of focusing on the management of ischemic heart disease, stroke, and congestive heart failure, which contribute most to the burden of CVD globally [22,57]. Other CVD-related conditions, like diabetic cardiomyopathy, are also a concern [58], primarily due to the increasing prevalence of diabetes [17]. Anyway, there is an urgent need to channel resources toward implementing cost-effective policies and interventions to reduce premature mortalities due to noncommunicable diseases.
Additionally, a significant number of trials examined a cluster of metabolic complications characterizing metabolic syndrome (43%). This reflects the strong link between diabetes and CVD risk within a broader metabolic context. As expected, primary outcomes measured in these trials often included blood lipid profiles, glucose levels, and insulin or glycated hemoglobin levels. These biomarkers are central to understanding CVD risk in individuals with diabetes, as elevated levels are associated with increased risk. For example, while management of blood glucose is essential for people with diabetes [59], ongoing research has strongly reported on the importance of assessing blood lipid profiles, especially low-density lipoprotein cholesterol to predict CVD risk in individuals with T2D [60,61]. The interventions being explored in the trials encompassed a wide range of approaches, including lifestyle and behavioral modifications, dietary supplementation, and drug interventions. Although physical activity and lifestyle medications are widely favored to improve the metabolic state [24,25,26,27], the use of statins, metformin, and pioglitazone in drug interventions underscores the importance of both glycemic control and lipid management in mitigating CVD risk [62,63]. In fact, metformin is being increasingly investigated for its potential benefits in alleviating CVD-related complications, beyond its well-known properties to improve glycemic levels in patients with T2D [28,29,30]. A previously published systematic review and meta-analysis involving nine clinical trials with 12,026 participants presented results supporting the potential benefits of pioglitazone in reducing major adverse cardiovascular events in people with insulin resistance, pre-diabetes, and diabetes mellitus [64]. However, these results further indicated that pioglitazone may potentially increase the risk of heart failure, edema, and weight gain. This also highlights the emerging use of nutraceuticals and dietary supplements as alternative or combination therapy to manage metabolic disease, including reducing CVD risk in people with diabetes [65,66]. Nutraceuticals and dietary supplements may encompass food sources rich in antioxidants and anti-inflammatory properties that are necessary to diffuse the harmful effects of oxidative stress and inflammation to reduce CVD risk. Both oxidative stress and inflammation remain the predominant pathological features implicated in the progression of many metabolic diseases; this extends to their link with damage to the cardiovascular system [15,67,68]. Beyond the management of blood glucose levels within a diabetic state, many dietary interventions, such as turmeric, herbal teas, cinnamon, mango, blueberries, red wine, chocolate, fish, and extra virgin olive oil, are increasingly being studied for their potential benefits in alleviating CVD risk [34,35,36]. In fact, the growth in clinical trials on the potential benefits of these interventions may be more pronounced when used in combination with currently used therapeutic interventions, like metformin or even statins [69,70,71]. However, as demonstrated by limited data within our reporting, clinical evidence demonstrating the potential benefits of nutraceuticals and dietary interventions against metabolic diseases remains scarce.
Strengths of the report include its comprehensive analysis of clinical trial data, which offers valuable insights into the global trends in risk factors associated with diabetes and CVD. The study’s notable strength lies in the diversity of trial registration and recruitment patterns, with contributions from regions worldwide, highlighting the global relevance and collaborative nature of research addressing CVD risk in individuals with diabetes. The increasing trend in the registration of clinical trials underscores the growing recognition of the significance of CVD risk in this population, emphasizing the urgent need for more research. The balanced representation of both genders and the focus on adults aged between 18 and 65 years in the analyzed trials ensures the applicability of the results to a broader population.
However, the report also has limitations, particularly the lack of clinical data from developing nations, where there is a rising surge in metabolic diseases [72,73]. This geographic gap in research highlights the need for more studies in these regions. Additionally, while the report addresses a broad spectrum of metabolic complications characterizing metabolic syndrome, it may benefit from further exploration of interventions on pediatric and elderly populations, as these age groups also face significant health challenges related to diabetes and CVD [74]. Furthermore, the focus on specific biomarkers, such as blood lipid profiles and glucose levels, might not encompass the full range of relevant indicators for understanding CVD risk. In fact, determination of cholesterol efflux capacity is also another important indicator for those potentially at increased risk of CVD. Nonetheless, the report’s emphasis on the comprehensive approach to managing both existing CVD risk and its prevention in individuals with diabetes is commendable, offering a valuable perspective on this dual health burden.
5. Conclusions
The analysis of clinical trial data from the ICTRP provides a comprehensive overview of research trends in CVD risk among individuals with diabetes. The findings reflect a global effort to understand and address this critical health issue. The study’s insights into trial registration, participant inclusion, trial phases, intervention models, primary purposes, disease classification, and interventions serve as a valuable resource for guiding future research and public health strategies aimed at reducing CVD risk in this vulnerable population. Limitations associated with statistical analysis or analysis of only one clinical trial registry, well beyond research on interventions informing on the status of CVD risk in the pediatric population, are well acknowledged and should be addressed in future studies.
Author Contributions
Conceptualization and data analysis, M.N., N.M., P.V.D. and D.N.; writing—original draft preparation, review, and editing, M.N., N.M., P.V.D., Y.N., A.M., N.L., N.H., A.K.B., S.E.M., S.H., S.E.M.-M., B.B.N. and D.N. All authors have read and agreed to the published version of the manuscript.
Funding
The South African Medical Research Council supports this work on project code: 43500. The content hereof is the sole responsibility of the authors and do not necessarily represent the official views of the funders.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ANZCTR, Australian New Zealand Clinical Trials Registry; ChiCTR, Chinese Clinical Trial Registry; CTRI, Clinical Trials Registry India; CVD, cardiovascular disease; DRKS, German Clinical Trials Register; EU-CTR, EU Clinical Trials Register; GMT, geometric mean titers; ICTRP, International Clinical Trials Registry Platform; IRCT, Iranian Registry of Clinical Trials; ISRCTN, International Standard Randomised Controlled Trial Number; ITMCTR, International Traditional Medicine Clinical Trial Registry; JPRN, Japan Primary Registries Network; Dutch Trial Registry; MMSE, Mini Mental State Examination; NaT, neutralizing antibody titers; PACTR, Pan African Clinical Trials Registry; PSQI, Pittsburgh Sleep Quality Index; ReBec, Brazilian Clinical Trials Registry; REPEC, Peruvian Clinical Trials Registry; RPCEC, Cuban Public Registry of Clinical Trials; SLCTR, Sri Lanka Clinical Trials Registry; T2D, type 2 diabetes; TCTR, Thai Clinical Trials Registry; WHO, World Health Organization.
References
- World Health Organization. The Leading Causes of Death Globally. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 3 October 2023).
- Wong, N.D.; Budoff, M.J.; Ferdinand, K.; Graham, I.M.; Michos, E.D.; Reddy, T.; Shapiro, M.D.; Toth, P.P. Atherosclerotic cardiovascular disease risk assessment: An American Society for Preventive Cardiology clinical practice statement. Am. J. Prev. Cardiol. 2022, 10, 100335. [Google Scholar] [CrossRef] [PubMed]
- Kereliuk, S.M.; Dolinsky, V.W. Recent Experimental Studies of Maternal Obesity, Diabetes during Pregnancy and the Developmental Origins of Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 4467. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Johnson, A.D.; Benjamin, E.J.; Levy, D.; Vasan, R.S. 70-year legacy of the Framingham Heart Study. Nat. Rev. Cardiol. 2019, 16, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Damaskos, C.; Garmpis, N.; Kollia, P.; Mitsiopoulos, G.; Barlampa, D.; Drosos, A.; Patsouras, A.; Gravvanis, N.; Antoniou, V.; Litos, A.; et al. Assessing Cardiovascular Risk in Patients with Diabetes: An Update. Curr. Cardiol. Rev. 2020, 16, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, A.M. Metabolic syndrome and cardiovascular risk. J. Fam. Community Med. 2010, 17, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef]
- Fan, W. Epidemiology in diabetes mellitus and cardiovascular disease. Cardiovasc. Endocrinol. 2017, 6, 8–16. [Google Scholar] [CrossRef]
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979, 241, 2035–2038. [Google Scholar] [CrossRef]
- Martín-Timón, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Cañizo-Gómez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Ntzani, E.E.; Kavvoura, F.K. Genetic risk factors for type 2 diabetes: Insights from the emerging genomic evidence. Curr. Vasc. Pharmacol. 2012, 10, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Mabhida, S.E.; Ziqubu, K.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hanser, S.; Basson, A.K.; Pheiffer, C.; Kengne, A.P. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J. Diabetes 2023, 14, 130–146. [Google Scholar] [CrossRef]
- Bradley, D.; Hsueh, W. Type 2 Diabetes in the Elderly: Challenges in a Unique Patient Population. J. Geriatr. Med. Gerontol. 2016, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. 10th Diabetes Atlas. Available online: https://diabetesatlas.org/ (accessed on 5 October 2023).
- Ma, C.X.; Ma, X.N.; Guan, C.H.; Li, Y.D.; Mauricio, D.; Fu, S.B. Cardiovascular disease in type 2 diabetes mellitus: Progress toward personalized management. Cardiovasc. Diabetol. 2022, 21, 74. [Google Scholar] [CrossRef]
- Kee, O.T.; Harun, H.; Mustafa, N.; Abdul Murad, N.A.; Chin, S.F.; Jaafar, R.; Abdullah, N. Cardiovascular complications in a diabetes prediction model using machine learning: A systematic review. Cardiovasc. Diabetol. 2023, 22, 13. [Google Scholar] [CrossRef]
- Galbete, A.; Tamayo, I.; Librero, J.; Enguita-Germán, M.; Cambra, K.; Ibáñez-Beroiz, B. Cardiovascular risk in patients with type 2 diabetes: A systematic review of prediction models. Diabetes Res. Clin. Pract. 2022, 184, 109089. [Google Scholar] [CrossRef]
- Liang, J.; Li, Q.; Fu, Z.; Liu, X.; Shen, P.; Sun, Y.; Zhang, J.; Lu, P.; Lin, H.; Tang, X.; et al. Validation and comparison of cardiovascular risk prediction equations in Chinese patients with Type 2 diabetes. Eur. J. Prev. Cardiol. 2023, 30, 1293–1303. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Mthembu, S.X.H.; Mazibuko-Mbeje, S.E.; Ziqubu, K.; Nyawo, T.A.; Obonye, N.; Nyambuya, T.M.; Nkambule, B.B.; Silvestri, S.; Tiano, L.; Muller, C.J.F.; et al. Impact of physical exercise and caloric restriction in patients with type 2 diabetes: Skeletal muscle insulin resistance and mitochondrial dysfunction as ideal therapeutic targets. Life Sci. 2022, 297, 120467. [Google Scholar] [CrossRef] [PubMed]
- Nyawo, T.A.; Pheiffer, C.; Mazibuko-Mbeje, S.E.; Mthembu, S.X.H.; Nyambuya, T.M.; Nkambule, B.B.; Sadie-Van Gijsen, H.; Strijdom, H.; Tiano, L.; Dludla, P.V. Physical Exercise Potentially Targets Epicardial Adipose Tissue to Reduce Cardiovascular Disease Risk in Patients with Metabolic Diseases: Oxidative Stress and Inflammation Emerge as Major Therapeutic Targets. Antioxidants 2021, 10, 1758. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, I.; Solomon, T.P.; Karstoft, K. The Acute Effects of Interval-Type Exercise on Glycemic Control in Type 2 Diabetes Subjects: Importance of Interval Length. A Controlled, Counterbalanced, Crossover Study. PLoS ONE 2016, 11, e0163562. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yuan, S.; Zhao, X.; Qiao, M.; Li, S.; He, N.; Huang, L.; Lyu, J. Metformin Protects Cardiovascular Health in People With Diabetes. Front. Cardiovasc. Med. 2022, 9, 949113. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nyambuya, T.M.; Johnson, R.; Silvestri, S.; Orlando, P.; Mazibuko-Mbeje, S.E.; Gabuza, K.B.; Mxinwa, V.; Mokgalaboni, K.; Tiano, L.; et al. Metformin and heart failure-related outcomes in patients with or without diabetes: A systematic review of randomized controlled trials. Heart Fail. Rev. 2021, 26, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xie, H.; Liu, Y.; Gao, P.; Yang, X.; Shen, Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: A systematic review and an updated meta-analysis. Cardiovasc. Diabetol. 2019, 18, 96. [Google Scholar] [CrossRef]
- Clark, L.T. Treating dyslipidemia with statins: The risk-benefit profile. Am. Heart J. 2003, 145, 387–396. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, J.K. Statins and cardiovascular diseases: From cholesterol lowering to pleiotropy. Curr. Pharm. Des. 2009, 15, 467–478. [Google Scholar] [CrossRef]
- Mthembu, S.X.H.; Orlando, P.; Silvestri, S.; Ziqubu, K.; Mazibuko-Mbeje, S.E.; Mabhida, S.E.; Nyambuya, T.M.; Nkambule, B.B.; Muller, C.J.F.; Basson, A.K.; et al. Impact of dyslipidemia in the development of cardiovascular complications: Delineating the potential therapeutic role of coenzyme Q(10). Biochimie 2023, 204, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.; Keshavamurthy, S.; Saha, S. Nutraceuticals in the Management of Cardiovascular Risk Factors: Where is the Evidence? Cardiovasc. Hematol. Disord. Drug Targets 2021, 21, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Sosnowska, B.; Penson, P.; Banach, M. The role of nutraceuticals in the prevention of cardiovascular disease. Cardiovasc. Diagn. Ther. 2017, 7, S21–S31. [Google Scholar] [CrossRef]
- Carrizzo, A.; Izzo, C.; Forte, M.; Sommella, E.; Di Pietro, P.; Venturini, E.; Ciccarelli, M.; Galasso, G.; Rubattu, S.; Campiglia, P.; et al. A Novel Promising Frontier for Human Health: The Beneficial Effects of Nutraceuticals in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 8706. [Google Scholar] [CrossRef]
- Dal Canto, E.; Ceriello, A.; Rydén, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 2019, 26, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.S.; Ko, S.H. Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes. Metabolism 2021, 123, 154838. [Google Scholar] [CrossRef]
- Malekzadeh, H.; Lotfaliany, M.; Ostovar, A.; Hadaegh, F.; Azizi, F.; Yoosefi, M.; Farzadfar, F.; Khalili, D. Trends in cardiovascular risk factors in diabetic patients in comparison to general population in Iran: Findings from National Surveys 2007–2016. Sci. Rep. 2020, 10, 11724. [Google Scholar] [CrossRef] [PubMed]
- Ghersi, D.; Pang, T. En route to international clinical trial transparency. Lancet 2008, 372, 1531–1532. [Google Scholar] [CrossRef]
- World Health Organization. International Standards for Clinical Trial Registries: The Registration of all Interventional Trials is a Scientific, Ethical and Moral Responsibility, version 3.0 ed.; World Health Organization: Geneva, Switzerland, 2018; Available online: https://iris.who.int/bitstream/handle/10665/274994/9789241514743-eng.pdf?sequence=1 (accessed on 20 September 2023).
- Ndwandwe, D.E.; Runeyi, S.; Pienaar, E.; Mathebula, L.; Hohlfeld, A.; Wiysonge, C.S. Practices and trends in clinical trial registration in the Pan African Clinical Trials Registry (PACTR): A descriptive analysis of registration data. BMJ Open 2022, 12, e057474. [Google Scholar] [CrossRef]
- Mathebula, L.; Malinga, T.; Mokgoro, M.; Ndwandwe, D.; Wiysonge, C.S.; Gray, G. Cholera vaccine clinical trials: A cross-sectional analysis of clinical trials registries. Hum. Vaccines Immunother. 2023, 19, 2261168. [Google Scholar] [CrossRef]
- He, Y.; Yang, J.; Lv, Y.; Chen, J.; Yin, F.; Huang, J.; Zheng, Q. A Review of Ginseng Clinical Trials Registered in the WHO International Clinical Trials Registry Platform. BioMed Res. Int. 2018, 2018, 1843142. [Google Scholar] [CrossRef] [PubMed]
- Feizabadi, M.; Fahimnia, F.; Mosavi Jarrahi, A.; Naghshineh, N.; Tofighi, S. Iranian clinical trials: An analysis of registered trials in International Clinical Trial Registry Platform (ICTRP). J. Evid. Based Med. 2017, 10, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Merson, L.; Ndwandwe, D.; Malinga, T.; Paparella, G.; Oneil, K.; Karam, G.; Terry, R.F. Promotion of data sharing needs more than an emergency: An analysis of trends across clinical trials registered on the International Clinical Trials Registry Platform. Wellcome Open Res. 2022, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Mayo-Wilson, E.; Heyward, J.; Keyes, A.; Reynolds, J.; White, S.; Atri, N.; Alexander, G.C.; Omar, A.; Ford, D.E.; Atri, N.; et al. Clinical trial registration and reporting: A survey of academic organizations in the United States. BMC Med. 2018, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Okafor, C.I. The metabolic syndrome in Africa: Current trends. Indian J. Endocrinol. Metab. 2012, 16, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Benjamin, I.J.; Burke, G.L.; Chait, A.; Eckel, R.H.; Howard, B.V.; Mitch, W.; Smith, S.C., Jr.; Sowers, J.R. Diabetes and cardiovascular disease: A statement for healthcare professionals from the American Heart Association. Circulation 1999, 100, 1134–1146. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef]
- De Rosa, S.; Arcidiacono, B.; Chiefari, E.; Brunetti, A.; Indolfi, C.; Foti, D.P. Type 2 Diabetes Mellitus and Cardiovascular Disease: Genetic and Epigenetic Links. Front. Endocrinol. 2018, 9, 2. [Google Scholar] [CrossRef]
- Joseph, J.J.; Deedwania, P.; Acharya, T.; Aguilar, D.; Bhatt, D.L.; Chyun, D.A.; Di Palo, K.E.; Golden, S.H.; Sperling, L.S. Comprehensive Management of Cardiovascular Risk Factors for Adults With Type 2 Diabetes: A Scientific Statement From the American Heart Association. Circulation 2022, 145, e722–e759. [Google Scholar] [CrossRef]
- Jaul, E.; Barron, J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front. Public Health 2017, 5, 335. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, T.A.; Bitton, A.; Anand, S.; Abrahams-Gessel, S.; Murphy, A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr. Probl. Cardiol. 2010, 35, 72–115. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Zayeri, F.; Salehi, M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: Results from global burden of disease study 2017. BMC Public Health 2021, 21, 401. [Google Scholar] [CrossRef]
- Gaziano, T.A. Cardiovascular disease in the developing world and its cost-effective management. Circulation 2005, 112, 3547–3553. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tian, C.; Gao, Z.; Zhang, B.; Zhao, L. Research status and trends of the diabetic cardiomyopathy in the past 10 years (2012–2021): A bibliometric analysis. Front. Cardiovasc. Med. 2022, 9, 1018841. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, R.S.; Aleppo, G.; Bailey, T.S.; Bergenstal, R.M.; Fisher, W.A.; Greenwood, D.A.; Young, L.A. The Role of Blood Glucose Monitoring in Diabetes Management; American Diabetes Association Clinical Compendia: Arlington, VA, USA, 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Lammi-Keefe, C.J.; Hou, L.; Hu, G. Impact of low-density lipoprotein cholesterol on cardiovascular outcomes in people with type 2 diabetes: A meta-analysis of prospective cohort studies. Diabetes Res. Clin. Pract. 2013, 102, 65–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khil, J.; Kim, S.M.; Chang, J.; Choi, S.; Lee, G.; Son, J.S.; Park, S.M.; Keum, N. Changes in total cholesterol level and cardiovascular disease risk among type 2 diabetes patients. Sci. Rep. 2023, 13, 8342. [Google Scholar] [CrossRef] [PubMed]
- van Stee, M.F.; de Graaf, A.A.; Groen, A.K. Actions of metformin and statins on lipid and glucose metabolism and possible benefit of combination therapy. Cardiovasc. Diabetol. 2018, 17, 94. [Google Scholar] [CrossRef]
- Gandhi, G.Y.; Mooradian, A.D. Management of Hyperglycemia in Older Adults with Type 2 Diabetes. Drugs Aging 2022, 39, 39–58. [Google Scholar] [CrossRef]
- Liao, H.W.; Saver, J.L.; Wu, Y.L.; Chen, T.H.; Lee, M.; Ovbiagele, B. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: A systematic review and meta-analysis. BMJ Open 2017, 7, e013927. [Google Scholar] [CrossRef]
- Le, Y.; Wang, B.; Xue, M. Nutraceuticals use and type 2 diabetes mellitus. Curr. Opin. Pharmacol. 2022, 62, 168–176. [Google Scholar] [CrossRef]
- Derosa, G.; Limas, C.P.; Macías, P.C.; Estrella, A.; Maffioli, P. Dietary and nutraceutical approach to type 2 diabetes. Arch. Med. Sci. 2014, 10, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2018, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Ragheb, S.R.; El Wakeel, L.M.; Nasr, M.S.; Sabri, N.A. Impact of Rutin and Vitamin C combination on oxidative stress and glycemic control in patients with type 2 diabetes. Clin. Nutr. ESPEN 2020, 35, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.F.; Thyfault, J.P. Exercise-Pharmacology Interactions: Metformin, Statins, and Healthspan. Physiology 2020, 35, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Colletti, A. Statins and nutraceuticals/functional food: Could they be combined? In Combination Therapy in Dyslipidemia; Adis: Cham, Switzerland, 2015; pp. 127–142. [Google Scholar] [CrossRef]
- Bowo-Ngandji, A.; Kenmoe, S.; Ebogo-Belobo, J.T.; Kenfack-Momo, R.; Takuissu, G.R.; Kengne-Ndé, C.; Mbaga, D.S.; Tchatchouang, S.; Kenfack-Zanguim, J.; Lontuo Fogang, R.; et al. Prevalence of the metabolic syndrome in African populations: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0289155. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.J.; Larson, H.J.; Moloi, M.H.; Tshatsinde, E.A.; Meheus, A.; Paterson, P.; François, G. Addressing public questioning and concerns about vaccination in South Africa: A guide for healthcare workers. Vaccine 2012, 30 (Suppl. S3), C72–C78. [Google Scholar] [CrossRef]
- Sahoo, K.; Sahoo, B.; Choudhury, A.K.; Sofi, N.Y.; Kumar, R.; Bhadoria, A.S. Childhood obesity: Causes and consequences. J. Fam. Med. Prim. Care 2015, 4, 187–192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).