Abstract
Glucose, fructose, and galactose are widely used in the food industry as sweeteners and food additives. The over-consumption of these carbohydrates has been identified as a possible trigger of non-communicable diseases. These include insulin resistance, obesity, and type 2 diabetes. These sugars induce an energy overload with consequent adipose tissue (AT) expansion, contributing to the development of obesity. Furthermore, a common feature of these non-communicable diseases is the detrimental, chronic, low-grade inflammation contributing to their onset. In the present review, we identify the most widely used dietary free sugars and their direct impacts on AT metabolism and inflammation, as well as their involvement in systemic inflammation and effects on the immune cell phenotype and function. Additionally, we discuss the capacity of the free sugars to induce immune modulation, enhancing inflammation, an underlying hallmark of insulin resistance, obesity, and T2DM. Dietary sugars have an important and deleterious metabolic impact on AT and also on immune cells. More research is needed to effectively understand the impact of chronic exposure to high levels of individual or combined sugars on metabolism, with the impact on immunomodulation being especially important.
1. Introduction
The Western diet is characterized by the presence of highly processed foods, which are particularly rich in salt, saturated fats, poor-quality protein, and simple carbohydrates, such as those deriving from corn, refined cereals, and sugars (glucose, fructose, and sucrose) [1]. Many of these carbohydrates present high glycemic and high insulinemic indices that quickly induce glucose and insulin stimulation peaks for short periods of time [2]. In contrast, diets with large contents of high-quality protein and plant-based foods, such as vegetables, nuts, fruits, and honey, which contain carbohydrates with low glycemic and low insulinemic indices, are generally considered healthier [1]. The energy overload caused by the increased consumption of refined sugars (free sugar) and saturated fats can lead to a drastic expansion of adipose tissue (AT) depots, especially when associated with a lack of physical activity [3]. In addition, a reduction in the fiber content of ingested foods may support metabolic destabilization, since sugars are quickly and freely available in the system [4,5]. Therefore, the Western diet has been identified as a possible trigger of the development of non-communicable diseases from an early stage of life. Insulin resistance (IR), obesity, type 2 diabetes mellitus (T2DM), and metabolic syndrome are some of the most prevalent non-communicable diseases worldwide [1,6,7].
In the present review, we discuss the most widely used dietary sugars and their impacts on metabolism, systemic inflammation, and AT-specific inflammation. Furthermore, we discuss how these sugars are capable of modulating the immune cells and their immunometabolism, promoting inflammation, an underlying hallmark of insulin resistance, obesity, and T2DM.
2. Dietary (Free) Sugars
Free sugars are mono- and disaccharides that are added to food, excluding the naturally present types, such as lactose in milk and sucrose in fruits and vegetables, although these naturally present sugars can be considered as free sugars when they are added to a product (Figure 1) [3,8]. Generally, free sugars are widely used in the food industry as sweeteners and food additives, especially in beverages and during food transformation and preparation [3,8], appearing in large proportions in the Western diet [9]. The monosaccharides, glucose, and fructose, as well as disaccharides, sucrose, and lactose, are used as additives in the Western diet [3,10], with fructose and glucose representing almost 50% of the added sugars [11]. These two monosaccharides are commonly used individually or in the form of sucrose or high-fructose corn syrup (HFCS). Sucrose is extracted and purified from sugar cane and sugar beet, while fructose is obtained by the enzymatic degradation of cornstarch into glucose or glucose polymers to be further isomerized enzymatically into fructose, producing the HFCS [3]. The main difference between sucrose and HFCS lies in the fructose content, which varies between 42% and 55% in HFCS and is 50% in sucrose. Moreover, sucrose is composed of glucose covalently bonded to fructose, while in HFCS, these molecules remain in their free forms, rendering them highly bioavailable and increasing their absorption when consumed [3,12]. Lactose is also widely used not only in the food industry, where it is applied in the form of condensed milk and in caramel flavors, but also in the pharmaceutical industry as a drug carrier [13]. In similarity to sucrose, lactose is a disaccharide of glucose and galactose that is used frequently in the confectionery and bakery industries. The production of caramel flavors using lactose depends on the Maillard reaction [13]. In the bakery industry, this process is important for creating the brown color of products, since lactose is not degraded by yeast [13].
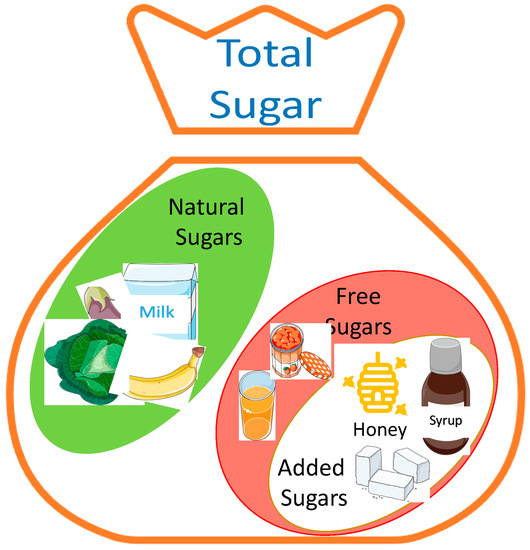
Figure 1.
Total sugar content of a product: The total sugar of a product consists of its natural sugar content and/or the sugar added during its preparation, although, in some cases, natural sugars can be considered as free sugars, since they are easily bioavailable. Additionally, honey can be used as an added sugar in the food industry. The figure was created using pictures from Servier Medical Art (smart.servier.com). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/, accessed on 27 October 2022).
3. Sugar Metabolism and Its Effect on Adipose Tissue
In different proportions, all the sugars described above are widely used in the Western diet, and there is no consensus regarding their potential harmful effects on metabolism [11,14]. Sucrose and HFCS are composed of monomers of fructose and glucose, and despite their similar structures, they follow different metabolic pathways, as described below [15], while galactose, resulting from lactose, follows the Leloir metabolic pathway [16] (Figure 2).
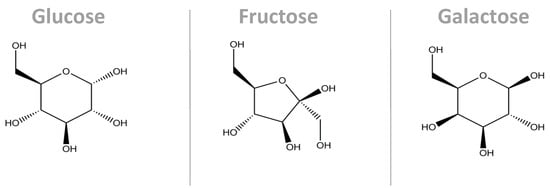
Figure 2.
Molecular structure of the main monosaccharides that compose our diet. Despite their similarity in terms of the molecular structures, their conformations are different, causing them to interact with their microenvironments in different ways. Additionally, their metabolic pathways are different. These three molecules are the carbon skeleton for sucrose (glucose + fructose bonded by a α1,β2-glycosidic bond) and for lactose (glucose + galactose bonded by a β1,4-glycosidic bond) [17].
3.1. Sucrose and HFCS—Fructose and Glucose Metabolism
After ingestion, fructose is passively absorbed by the intestinal apical membrane via the high-affinity transporter glucose transporter (GLUT)5, passed on to the portal circulation by GLUT2. On the other hand, glucose is absorbed by enterocytes, mainly through the co-transporters sodium-glucose linked transporter 1 (SGLT1) and GLUT2. Once in circulation, fructose is metabolized in the tissues by phosphofructokinase and glucokinase, and the latter is also regulated by insulin [9,15,18]. An increase in fructose consumption, associated with an unrestricted pathway, leads to a rapid increase in uric acid synthesis, gluconeogenesis, glycolysis, and de novo lipogenesis [15,19]. Unlike glucose, fructose is not regulated by the activity of phosphofructokinase, an enzyme that limits the glycolytic flux [19]. The hexo-phosphate and trios-phosphate intermediaries, resulting from fructolysis, are used as substrate for the pathways described above [15,19]. Moreover, excessive amounts of fructose will overload the liver’s capacity to oxidize it. Therefore, the fructose remains in circulation and can be used by other tissues, such as AT [20]. Fructose has been described as a potential lipogenic and adipogenic nutrient accelerating lipid deposition, particularly in visceral AT, and ectopic fat deposition in the other insulin-sensitive tissues, such as the liver and muscle [19,20,21]. In addition, fructose has been observed to disrupt insulin sensitivity in adults [21]. Stanhope and colleagues conducted a clinical study with 32 participants aged from 40 to 72 years. The subjects were divided into two groups, including glucose- and fructose-sweetened beverage consumers, for a period of 10 weeks. The study indicated an increase in the plasma lipids and lipoproteins in the fructose consumer group, while the glucose consumer group remained unchanged, except for the triglycerides, which showed an opposite pattern. Furthermore, the authors observed alterations in insulin sensitivity and glucose tolerance after 9 weeks of beverage consumption. The fructose consumer group showed increased insulin and glucose levels during an oral glucose tolerance test compared to baseline, while the glucose consumer group remained unchanged. Moreover, the insulin sensitivity index decreased by approximately 17% in the fructose consumption group [21]. Additionally, the same study indicated that the group who consumed fructose-sweetened beverages showed a higher expression of lipogenic genes in VAT [21].
Adipocytes lack the keto-hexokinase enzyme that converts fructose into fructose-1-phosphate. In these cells, fructose is converted to fructose-6-phosphate by hexokinase stimulating the conversion of pyruvate into acetyl-CoA, thus increasing the synthesis of fatty acids, and leading to consequent palmitate release. In this case, fructose is mostly used in anabolic pathways, in contrast to glucose [22]. Interestingly, Varma and collaborators (2015) postulated that fructose can trigger the oxidation of glucose to lactate in a dose-dependent manner while reducing the utilization of glucose in the glutamate and fatty acid synthesis pathways, using a 10% 13C2-D-glucose trace in cultured adipocytes [23]. Furthermore, the study also indicated a reduction in glucose conversion to glycogen. Consequently, glucose is driven to the one-carbon cycle and the glycine cleavage pathway (SOG pathway) through 3-phosphoglycerate to synthesize serine and other intermediates that are important for the generation of NADPH and ATP [23,24].
A treatment with 10% fructose solution in the drinking water of rats for 24 weeks induced the upregulation of genes related to the insulin-signaling pathway, particularly phosphoinositol-3-kinase (PI3K), protein kinase-B (AKT), insulin receptor(IR)-β, insulin receptor substrate (IRS)-1, and the mammalian target of rapamycin (mTOR), but also those related to adipocyte homeostasis, such as peroxisome proliferator -activated receptor (PPAR)γ and nuclear factor erythroid-2-related factor 2 (NrF2) [25], although the authors did not find any correlation between insulin impairment and adipose tissue inflammation [25]. On the other hand, different studies have suggested that fructose-rich diets affect insulin action and AT metabolism, inducing changes in the secretory patterns of resistin, adiponectin, leptin, and specific adipokines, which, in turn, are linked to inflammation and insulin resistance [26,27]. Furthermore, it has been shown that fructose induces an increase in leptin secretion and, consequently, leptin resistance [28]. However, the mechanism determining how leptin resistance is established is not well understood. Despite its different functions in the organism, leptin has a significant impact on inflammation and the inflammatory onset, not only locally at the tissue level but also systemically, perpetuating further inflammation [25,28]. In addition, Marek et al. (2015) provided important insights into the effects of fructose on the adipocyte endoplasmic reticulum (RE) redox status in mice. Moreover, increased levels of monocyte chemoattractant protein (MCP)-1, intercellular adhesion molecule (ICAM)-1, and tumor necrosis factor (TNF)-α expression by AT have already been described in response to fructose metabolism. The expression of these genes leads to an increase in macrophage infiltration on AT [28,29] but also the release of other pro-inflammatory cytokines by the adipocytes [28]. Interestingly, this inflammatory process caused by the excessive consumption of fructose-rich foods seems to have a gender-dependent impact, particularly regarding the expression of inflammatory markers in VAT [25,30]. Considering the above, Kovačević and collaborators tested the impacts of the ingestion of 10% (w/v) fructose solution on female and male Wistar rats for 9 weeks and noticed that, despite the diet used, there was no impact on the glucose or insulin levels. However, the fructose-treated females showed a significant reduction in the Akt and pAkt-Ser473 levels in VAT and an increase in the PTP1B protein levels in comparison to the standard chow, while the male rats only showed a decreased pAkt-Ser473/Akt ratio. Furthermore, the fructose-treated female showed increased levels of nuclear factor (NF)κB in VAT, followed by increased levels of TNF-α, interleukin (IL)-6, and IL-1β mRNA, as well as increased levels of F4/80, a macrophage marker. In contrast, the male fructose-treated rats showed no differences in the NFκB expression levels [30].
Data suggest that fructose-rich diets induce chronic inflammation in a dose-dependent manner [31]. Wang et al. (2020) fed six-week-old Sprague Dawley male rats with low (2.6 g/kg/day), medium (5.3 g/kg/day), and high doses (10.5 g/kg/day) of fructose for 20 weeks and identified a dose-dependent increase in the circulating levels of IL-6, TNF-α, and macrophage inflammatory protein (MIP)-2 when compared to the controls, while the opposite was observed for IL-10 in an inverse pattern. In the same study, the highest fructose dose led to an increase in the number of inflammatory cells in the pancreas, a 10% increase in liver steatosis, colon inflammation, and gut microbiota alterations. Furthermore, the acute inflammatory response to a fructose-rich diet during the postprandial state was recently studied in healthy subjects and in patients with T2DM. The data showed that the levels of IL-6 and ICAM-1 were increased in the healthy subjects in the postprandial state, while MCP-1 was increased in both the healthy subjects and in the patients with T2DM [18].
The glycemic load caused by the consumption of sucrose and HFCS has also been suggested to be a possible trigger of the inflammatory processes [32,33,34]. In addition, a recent study conducted by Patkar et al. (2021) indicated that the long-term (3 months) consumption of 5% (w/v) sucrose could be a trigger of the onset of systemic low-grade inflammation, without the induction of obesity, in male Wistar rats. Furthermore, they observed an increase in some of the immune cell populations in circulation, such as lymphocytes, basophils, and neutrophils [11].
3.2. Lactose—Galactose and Glucose
Lactose is a disaccharide composed of galactose and glucose, and it is metabolized in the intestinal lumen by lactase. A complementary mechanism of lactose metabolism is through the colonic microbiota, primarily in adults [35]. This sugar differs from the other mono- and disaccharides since it has no specific transporter to pass through the intestinal barrier. However, concentrations of around 0.02 mmol/L of lactose have already been found in circulation in healthy young adult men, contrary to what had been postulated [35]. Despite not being metabolized in other tissues, lactose seems to play a role in systemic inflammation, a topic that will be discussed further in this review. On the other hand, lactose monomers are easily metabolized by the organism.
Like fructose, galactose is absorbed by the endothelial cells, released into the blood stream, and transported to the liver via the portal vein [36]. A large amount of galactose is retained and metabolized in the liver, but small amounts remain in circulation and reach other tissues, such as AT and the skeletal muscle [36]. It follows the Leloir pathway once it enters the adipocytes [16]. Krycer et al. (2020) treated 3T3-L1 adipocytes with either 25 mM of glucose or 25 mM of galactose, and they found a reduction in lactate production by the galactose-treated cells, even after insulin stimulation [37]. On the other hand, mitochondrial respiration was increased upon treatment with galactose and upon insulin stimulation [37]. Thus, galactose appears to be used to feed a different pathway, rather than glycolysis, in the adipocytes. To test this, the authors used tracers to differentiate the galactose and glucose carbons using 13C-labels (both 25 mM) [37]. They found a reduction in glucose-6-phosphate after the galactose treatment [37]. The glucose-6-phosphate is considered a common point between the glucose and galactose oxidation pathways and how galactose enters the glycolytic pathway. The data indicate that galactose follows a different pathway from glycolysis and is a poor substrate for energy metabolism [16]. Interestingly, similar results were found in a study on mature adipocytes isolated from rats [37]. However, Krishna et al. (2020) did not identify any effect of 25 mM of galactose or 25 mM of lactose treatment on adipocyte differentiation [38]. Considering the low degradation rate of galactose compared to glucose, high amounts of galactose in circulation can lead to galactosemia and, consequently, to the glycation of different macromolecules, including amino acids, creating advanced glycation end products (AGEs) and reactive oxygen species (ROS) [5,39]. These molecules are responsible for tissue damage that, in turn, leads to accelerated aging [40,41]. Furthermore, studies have identified that high levels of galactose also induce an accelerated aging process, possibly due to the production of ROS and AGEs [41]. Additionally, it has been reported that both AGEs and ROS are involved in the inflammation onset through the action of the nuclear factor (NF)-κB gene [5]. On the other hand, a study conducted in cultured mammalian cells, HEK293 and HepG2, indicated that galactose induces the accumulation of fructose-6-phosphate that is necessary for the N-glycosylation process. This contributes to a reduction in starvation-induced endoplasmic reticulum stress, emphasizing the contribution of galactose to other pathways rather than energy production [42]. Interestingly, a recent study indicated that in the diet of 3-week-old, postweaning mice, the substitution of glucose for galactose (1:1, mimicking lactose) instead of glucose alone for 3 weeks, prior to an HFD for 9 weeks, was enough to reduce the levels of circulating leptin when compared to glucose alone, although no differences were found in white AT leptin receptor expression, especially in the female mice [43].
Despite the widespread use of these sugars in the Western diet, more studies are required to understand the impacts of lactose and galactose on human metabolism and tissue physiology. It is crucial to elucidate their underlying physiologic mechanisms of action, especially those of tissue-specific metabolism and inflammation. Furthermore, clinical trials are necessary to observe the impacts of these sugars on the organism, since the majority of the published studies were performed in vitro or on animal models.
4. The Impact of Sugar on Immunometabolism
In addition to AT and the other insulin-sensitive organs, dietary sugars can impact metabolism in different organs, including the immune system, by affecting the function and regulatory capacity of the immune cells [11,34]. The impacts of different dietary sugars (e.g., sucrose, lactose, fructose, and glucose) on specific immune cell populations, regarding their metabolism and function, have been reported (Figure 3 and Table 1). Evidence suggests that the consumption of sucrose- or fructose-containing foods leads to an increase in the leukocytes in animal models [11,26]. A significant increase in the lymphocytes, basophils, and neutrophils was reported after the treatment of Wistar rats with 5% (w/v) sucrose in water for 12 weeks [11]. Similarly, Rodrigues et al. (2014) evaluated the impact of fasting with 20% sucrose- (control) and 20% fructose-containing diets on postprandial male BALB/c mice. The team showed that the sucrose- and fructose-containing diets induced an increase in the total leukocyte populations in the post-prandial state [26]. Specifically, the fructose-containing diet showed the highest impact on leukocyte proliferation, followed by an increase in the pro-inflammatory cytokines and chemokines in the liver and AT, namely IL-6, TNF-α, and CCL2, and a reduction in IL-10. This pro-inflammatory state was accompanied by an increase in neutrophil recruitment and infiltration into the liver [26]. This may have been triggered by an increase in ROS, the activity of inducible nitric oxide synthase (iNOS), and TNF-α secretion during Kuppfer cell activation, as hypothesized by Kanuri et al. (2011). Interestingly, a 30% fructose drink apparently had a stronger influence on immune cell function and activation, leading to an increase in the number of neutrophils (approx. 5.5-fold higher), as well as the expression of ICAM-1 (approx. 1.8-fold) by the neutrophils, compared to plain water after treating C57BL/6j mice for 8 weeks [44]. Other sugars, such as lactose, can also induce alterations in the immune cells leading to their immunomodulation [45,46]. Lactose is a β-galactoside that can interact with proteins, such as the galectin family, leading to changes in the microenvironment of the immune cells [46]. In particular, the binding of lactose to galactin-9 (Gal-9) reduced the engagement of Gal-9 with its receptor, T-cell immunoglobulin and mucin domain 3 (TIM-3, also known as CD366), avoiding the immune suppression of the pro-inflammatory profile of T cells [46].
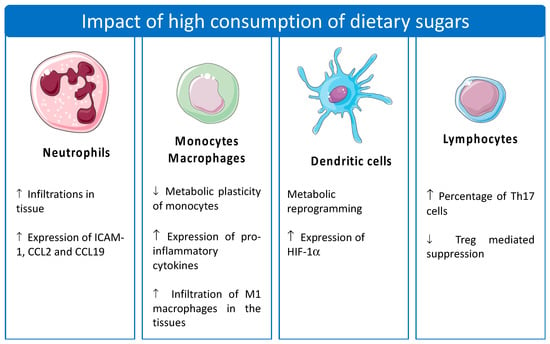
Figure 3.
Effect of the excessive consumption of dietary sugars on the immune cells. The effects of dietary sugars are different in each cell type. The figure was created using pictures from Servier Medical Art (smart.servier.com). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/, accessed in 10 January 2023).
The immunomodulation caused by the various dietary sugars can differ between the various immune cell populations [47]. Interestingly, immune cells are also able to change their metabolic demand, usually designated as metabolic switch. This can occur upon activation, and the carbon source that the cells use for their metabolism may influence these changes [34,48]. Usually, this metabolic switch occurs quickly upon activation, since these cells require fast ATP production to fulfill their energetic demands. Oxidative phosphorylation (OXPHOS) is reduced, while aerobic glycolysis is prioritized, in most immune cells [47,49], leading to a proinflammatory profile [48]. Menk et al. (2018) showed that the activation of naïve and memory CD4+ and CD8+ T cells by anti-CD3 and anti-CD28 for approximately 6 h led to an increase in glycolysis, measured by the extracellular acidification rate (ECAR), while OXPHOS was reduced, as measured by the oxygen consumption rate (OCR) [50]. Furthermore, Lee et al. (2019) studied the metabolism of classical monocytes (CD14+ CD16−), a subset of human monocytes, and discovered that LPS stimulation leads to an increase in the glycolysis rate of this subset of monocytes [51]. Furthermore, the authors also identified the glycolytic metabolism as a key pathway in the regulation of p38-MAPK, which is important for the activation and the adhesion function of the classical monocytes [51].
The intake of high-sugar-containing diets can enhance these important metabolic alterations, which are deleterious to human health and are some of the underlying causes of insulin resistance and T2DM development.

Table 1.
Impacts of high doses of dietary sugars on immune cell populations.
Table 1.
Impacts of high doses of dietary sugars on immune cell populations.
| Dietary Sugar | Experimental Model | Concentrations of Sugar | Treatment Duration | Impact in Immune Populations | Reference |
|---|---|---|---|---|---|
| Fructose | in vivo—IDH2 KO and C57BL/6J mice | 34% fructose in H2O | 6 weeks | ↑ Neutrophil infiltration into the liver of IDH2 mice | [52] |
| in vivo—male C57BL/6J mice | 15% fructose in H2O | 10 days | ↑ Neutrophil trafficking to limbal region | [53] | |
| in vivo—C57BL/6J mice | 30% fructose in H2O | 8 weeks | ↑ Neutrophil infiltration into the liver ↑ Expression of ICAM-1, CCL2 and CCL19 | [44] | |
| in vivo—male Swiss mice | 20% fructose in H2O | 6–10 weeks | ↑ Infiltration of M1 macrophages into AT ↓ Infiltration of M2 macrophages into AT | [54] | |
| in vitro—primary human DCs | 5 to 25 mM fructose in culture medium | 24–72 h | ↑ Pro-inflammatory cytokine production and activation markers 🞄 Metabolic reprogramming in DCs | [34] | |
| in vivo—male Sprague Dawley rats | 60% fructose in chow | 5 weeks | ↓ Immune suppressive function of Treg cells without changes in the percentage of the population | [55] | |
| in vitro—primary human monocytes | 11.1 mM fructose in culture medium | Different time-points | ↓ Metabolic plasticity of monocytes ↑ Expression of IL-1β, IL-6, IL-10, and TNF-α | [47] | |
| in vivo—Dahl salt-sensitive and Dahl salt-resistant rats | 20% fructose in H2O | 4 weeks | ↑ % of Th17 cells | [56] | |
| Fructose and sucrose | in vivo—male BALB/c and LysM-eGFP mice | 20% fructose20% sucrose (control),both in the chow | 12–14 weeks | ↑ Leukocyte count in circulation and infiltrated neutrophils in the liver ↓ Neutrophil infiltration into AT | [26] |
| Galactose | in vitro—human monocytes | 11.1 mM galactose in culture medium | Different time-points | ↓ Metabolic plasticity of monocytes | [47] |
| in vitro—THP-1 cells | 2 g/L of RPMI | 5 days until experiments | ↓ TNF-α expression and preference for OXPHOS | [57] | |
| in vitro—bone marrow DCs from male C57BL/6J mice | 10 mM of galactose in culture medium | 24–72 h | 🞄 Induces the maintenance of activating markers on DCs for 72h | [58] | |
| Glucose | in vitro—human DCs | Range from 5 to 25 mM glucose in culture medium | 24–72 h | ↑ Expression of HIF-1α | [34] |
| in vitro—human and male C57BL/6J mice CD8+ T cells | 25 mM glucose | 3 days | ↑ Glycolysis and cytotoxic capacity of CTLs | [59] | |
| Lactose | in vitro—human T cells | 30 mM of lactose30 mM of sucrose in culture medium | 3 days | ↓ Treg immunosuppression capacity | [46] |
| in vivo—female BALB/c mice | 100 mg/kg of body weight | Different time-points | 🞄 Neutrophil and macrophage modulation in early acute pancreatitis 🞄 Possible interaction with lactose-Galectin 3 | [45] | |
| Sucrose | in vivo—male Wistar rats | 5% (w/v) in H2O | 12 weeks | ↑ Circulating neutrophils, lymphocytes, and basophiles | [11] |
| in vivo—male Sprague Dawley | 700 g/kg of chow | 5 weeks | ↑ CD68+ macrophage infiltration into tPVAT and aPVAT ↑ Expression of MCP-1 in both tissues | [57] |
↑—increase in; ↓—decrease in; IDH2 KO—isocitrate dehydrogenase 2 knock out mice; ICAM-1—intercellular adhesion molecule 1; CCL2—C-C motif chemokine ligand 2; CCL19—C-C motif chemokine ligand 19; AT—adipose tissue; DCs—dendritic cells; Treg—regulatory T cells; IL-1β—interleukin-1β; IL-6—interleukin-6; IL-10—interleukin-10; TNF-α—tumor necrosis factor-α; Th17—T helper 17 cells; OXPHOS—oxidative phosphorylation; HIF-1α—hypoxia-inducible factor-1α; CTLs—cytotoxic T lymphocytes; tPVAT—thoracic perivascular adipose tissue; aPVAT—abdominal perivascular adipose tissue; MCP-1—monocyte chemoattractant protein 1.
4.1. Monocytes/Macrophages
Macrophage infiltration may be a key mechanism leading to the pro-inflammatory status and subsequent onset of insulin resistance, especially in AT [54]. Once infiltrated into the tissues, monocytes differentiate into macrophages and usually become polarized into either pro-inflammatory M1 macrophages or anti-inflammatory M2 macrophages according to the microenvironment (Table 1). The chronic intake of a high-fructose diet (6–10 weeks; 20% fructose solution) was shown to favor the M1 macrophage subtypes, with a reduction in the number of M2 subtypes in epidydimal AT in mice. Moreover, the high recruitment of monocytes from the blood to AT (Ly6C+) was observed in mice after 10 weeks on a high-fructose diet. In addition, polarization into Ly6Chigh and Ly6Cmiddle M1 macrophages with a high pro-inflammatory capacity was described, with a parallel reduction in the Ly6C− M2 resident cells [54]. Jones et al. (2021) investigated the impacts of glucose (11.1 mM), fructose (11.1 mM), and galactose (11.1 mM), respectively, on the energy metabolism of circulating human monocytes. At the concentration of 11.1 mM, glucose drove the monocyte metabolism towards glycolysis, while fructose and galactose showed the opposite effect, with OXPHOS being prioritized in the basal state (Table 1). After stimulating the treated monocytes with LPS, the glucose-treated monocytes increased their glycolysis even further in contrast to the fructose- and galactose-treated monocytes, which increased in their OXPHOS capacity. However, inhibiting the hexokinase using 2-deoxyglucose, the authors noticed that the glucose-treated cells showed a reduction in ECAR followed by an increase in OCR, while the fructose- and galactose-treated monocytes indicated reductions in both ECAR and OCR [47]. Moreover, treating the fructose- and glucose-cultured monocytes with oligomycin led to a decrease in OCR in both cultures, although the fructose-treated monocytes also presented reduction in ECAR [47]. This study demonstrated the impaired metabolic flexibility in switching between OXPHOS and glycolysis for energy production by fructose, in contrast to glucose, in which case the cells can easily change their energy metabolism. Additionally, the treatment of the LPS-stimulated monocytes with fructose demonstrated a reduction in the cell viability when exposed to mitochondrial complex inhibitors, such as rotenone (complex I), antimycin A (complex III), and oligomycin (complex V), demonstrating that fructose is not a good substrate for the glycolytic pathway in human monocytes, leading to their functional impairment [47]. Furthermore, fructose had an impact in the secretory pattern of the monocytes, leading the increased secretion of IL-1β, IL-6, IL-10, and TNF-α [47]. In contrast to dendritic cells (DCs), fructose did not change the levels of surface activation marker expression in the monocytes, (e.g., HLA-DR, CD80, CD86) [47]. In addition, sucrose-enriched diets also appear to have a specific impact on macrophages, especially by increasing their proliferation in the peripheral tissues, as observed in the perivascular AT [57] and liver [60]. In accordance with the findings described by Jones et al. (2021), Millet et al. (2016) indicated that galactose (2 g/L of medium) is able to direct the monocyte metabolism towards OXPHOS, followed by a reduction in the TNF-α levels, compared to glucose at the same concentration (Table 1) [61]. Similar to galactose, the treatment of mice with lactose induced a reduction in the percentage of macrophages, but it also increased their production of IL-10 through a galectin-3–lactose interaction [45].
4.2. Dendritic Cells
Dendritic cells are important antigen-presenting cells (APCs) that allow for an adaptative immune response but also promote tolerance through the degradation of self-antigen-reactive thymocytes [34,62]. Jaiswal et al. (2019) tested concentrations ranging from 5 to 25 mM of fructose and glucose in vitro using human DCs collected from healthy donors for 24–72 h. The results indicated that 15 mM of fructose was able to induce an increase in the expression of key activation markers in the DCs, such as CD86, a co-stimulatory molecule (Table 1). This, in turn, was followed by an increase in proinflammatory cytokine secretion, including IL-1β, IL-6, and TNF-α, by the DCs. Additionally, the treatment with 15 mM of glucose also induced an increase in the expression of the TNF-α levels after 72 h. Moreover, a co-culture of fructose-exposed DCs with CD3+ T cells induced an increase in IFN-γ secretion by the T cells when compared to the control (5 mM of glucose), a possible consequence of the increased TNF-α release by the DCs when exposed to the 15 mM of fructose. Interestingly, despite the indirect effect through the DCs, the authors did not observe a direct effect of fructose on cytokine secretion in the cultured T cells. Furthermore, during the treatment of the DCs, the authors analyzed their metabolic profile, identifying a process of metabolic reprogramming through a reduction in OCR and an increase in ECAR when higher concentrations of fructose were used [34]. The reduction in OCR was supported by a reduction in the ROS production of the fructose-treated cells when compared with the glucose-treated and control DCs after 24 h of treatment [34]. After 72 h of treatment, the authors found a similar result for the fructose-treated DCs, although phospho-p70S6 kinase and hypoxia-inducible factor (HIF)-1α had higher expressions than those observed in the glucose-treated and control DCs [34]. The data suggest that cytokine secretion in fructose-treated DCs is independent of the AKT–mTOR axis [34]. The metabolic switch of the DCs culminated in their chronic activation, promoting a pro-inflammatory profile through the accumulation of advanced glycation end products, especially in the fructose-treated DCs, with the consequent enhanced activation of NF-κB [34].
On the other hand, glucose also seems to play an important role in the metabolic function of DCs and their interaction with CD8+ T cells (Table 1). Lawless et al. (2017) showed that the activation of DCs with LPS for 24 h led to an increased expression of co-stimulatory molecules, such as CD80 and CD86, with a decline in their expression after 48 h and 72 h when treated with 10 mM of glucose, although, in the presence of 10 mM of galactose, the DCs maintained the expression of co-stimulatory molecules for approximately 72 h after stimulation. This co-stimulatory expression was corroborated by co-culturing the DCs with CD8+ T cells for 72 h in glucose and galactose at 10 mM (Table 1). As expected, the galactose-treated DCs retained the capacity to induce the clonal expansion of the CD8+ T cells for 72 h, with the further increased expression of IFNγ production by the T cells, in contrast to the glucose-treated DCs, which started to decline in their capacity to induce the CD8+ T cells after 48 h of co-culture [58]. The authors also postulated that a reduction in the glucose concentration in the medium (10 mM to 2 mM) was followed by a reduction in the expression of HIF-1α in the DCs after LPS activation [58]. Interestingly, galactose was also capable of inducing a similar effect [58]. However, under 2 mM of glucose, the expression of HIF-1α was limited by the inactivation of mammalian target of rapamycin complex 1 (mTORC1) signaling, consequently inducing the activation of 5′AMP-activated protein kinase (AMPK), while under the 10 mM galactose treatment, it was enough to maintain low levels of glycolysis and OXPHOS for ATP synthesis, maintaining mTORC1 activation, and the reduction in HIF-1α was described as mTORC1-independent [58]. These data provide a good example illustrating how different dietary sugars may act in different ways, at least in vitro (Table 1). However, much research is needed in the future to understand the impacts of these sugars on more complex organisms, including the human physiology.
4.3. Lymphocytes
Lymphocytes, which comprise T, B, and NK populations, are responsible for the adaptative immune response and play important roles in tissues inflammation. In fact, T cells play an important role in AT inflammation and insulin resistance during the onset of obesity [63,64]. There are few studies that have described the impact of high-sugar diet consumption on humans (Table 1), although it has been suggested that high-sugar diets have impacts on the T cell metabolism and function [47,55]. In fact, it was demonstrated that human-activated CD8+ T cells (CTLs) cultured with high glucose (25 mM, mimicking a hyperglycemic condition) led to a higher absorption of glucose, in contrast to CTLs treated with 5.6 mM of glucose, resulting in a higher glycolytic rate in the case of the high-glucose-treated CTLs [59]. Considering the importance of the glycolytic pathway in the regulation of the effector killing function of these cells, Zhu et al. (2021) postulated that a hyperglycemic microenvironment would enhance the cytotoxicity capacity of the CTLs after 3 and 6 days following in vitro activation [59]. Additionally, the authors evaluated the cytotoxic capacity of incubated CTLs from diabetic mice, compared with CTLs from healthy mice, and concluded that the CTLs collected from the diabetic mice showed a faster killing kinetic in contrast to the control [59]. However, patients with type 2 diabetes have an increased percentage of the CCR7- CD45RA+ CD8+ T cell subset compared to healthy people. This subset of CD8+ T cells represents a decline in immune function, also called immunosenescence [65]. Furthermore, high glucose intake could be an important factor in the exacerbation of autoimmune disease, especially through the mediation of CD4+ T helper (Th) 17 [66]. In line with this, high fructose intake (20% solution) also showed a Th17-mediated inflammation response through the production of IL-17A in Dahl salt-sensitive rats, representing a model of hypertension (Table 1) [56].
In 2013, Leibowitz et al. indicated that fructose-enriched diets, administered for 5 weeks, induced functional alterations in the T cells of Sprague Dawley rats, particularly in Treg [55]. Furthermore, after feeding the animals on a high-fructose diet (60% fructose) for 5 weeks, the authors identified the dysfunction of IL-10 production by Treg when compared to the control chow diet, even in the absence of alterations in the percentage of the Treg population [55]. On the other hand, results reported by Jaiswal et al. (2019) indicated that 15 mM of fructose did not change or have a direct effect on cultured T lymphocyte populations [34].
Lactose, in circulation, can have deleterious effects on immune cells, leading to inflammation (Table 1) [46]. Lactose has the potential to bind to Gal-9, reducing its ability to bind its receptor, TIM-3, on the surfaces of different immune cells, including the macrophages and T cell populations. Gal-9/TIM-3 signaling plays an important role in Treg cell differentiation and effector T cell exhaustion [46]. Moreover, this pathway is important for the regulation and resolution of inflammation through the regulation of the Th1 and Th17 immune responses [46]. Moreover, Paasela et al. (2014) incubated enriched Treg cells from healthy donors with effector T cells (Teff) for 3 days. They reported a decrease in IFN-γ and IL-17 secretion by Teff. However, after adding 30 mM of lactose, the authors reported a reduction in Treg-mediated suppression and a consequent increase in IFN-γ and IL-17 secretion [46]. Furthermore, the authors observed an increase in the number of CD4+ TIM-3+ cells producing IL-17 after incubation with lactose, even when co-cultured with Treg [46].
Naïve T lymphocytes and Treg cells preferentially utilize fatty acid substrates during their metabolism, with a low activity of mTOR. On the other hand, after activation, T cells rely mainly on aerobic glycolysis, displaying higher mTOR activity, and in the case of Treg, this can induce the downregulation of transcription factor Foxp3, promoting a reduction in the cells’ proliferation [67]. Treg cells are important, as they mediate the homeostasis of the organism and counterbalance the Teff cells [67].
5. Concluding Remarks
The propagation of the Western diet has become a worldwide burden, together with the excessive consumption of free/added sugars. These can trigger chronic low-grade inflammation not only by alterations in the AT metabolism and other insulin-sensitive organs but also by the induction of critical changes in the immune system. These alterations can lead to the loss of function of important immune cell populations. Importantly, many of these alterations have key impacts on the plasticity of immune cells, reducing their capacity to adjust to the surrounding environment. However, more research needs to be performed in order to better understand the deeper impacts of the free sugars, monosaccharides, and disaccharides on human cells, particularly their interactions with AT. Many studies have been performed to date, mostly in vitro and on rodents. However, the experimental conditions were different across these studies, including the way in which sugar was incorporated into the diet, the gender studied, and the type of model used, along with the periodic caloric intake.
More research is still needed in this field, using clinical trials to effectively understand the impact of chronic exposure to high levels of sugar on the metabolism, with the impact on immunomodulation being especially important.
Author Contributions
Conceptualization, P.B. and E.C.; writing—original draft preparation, P.B.; writing—review and editing, P.B. and E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by the European Regional Development Fund (ERDF), through the Centro 2020 Regional Operational Programme under the project Healthy Aging 2020—CENTRO-01-0145-FEDER-000012 and through the COMPETE 2020—Operational Programme for Competitiveness and Internationalisation and Portuguese national funds via FCT—Fundação para a Ciência e a Tecnologia, under projects POCI-01-0145-FEDER-007440, UIDB/04539/2020, UIDP/04539/2020, and LA/P/0058/2020, and Pedro Barbosa’s PhD grant—SFRH/BD/143849/2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kopp, W. How Western Diet and Lifestyle Drive the Pandemic of Obesity and Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [PubMed]
- Amano, T.; Watanabe, D.; Otsuka, J.; Okamoto, Y.; Takada, S.; Fujii, N.; Kenny, G.P.; Enoki, Y.; Maejima, D. Comparison of hydration efficacy of carbohydrate-electrolytes beverages consisting of isomaltulose and sucrose in healthy young adults: A randomized crossover trial. Physiol. Behav. 2022, 249, 113770. [Google Scholar] [CrossRef] [PubMed]
- Arias-Chávez, D.J.; Mailloux-Salinas, P.; Altamirano, J.; Huang, F.; Gómez-Viquez, N.L.; Bravo, G. Consumption of combined fructose and sucrose diet exacerbates oxidative stress, hypertrophy and CaMKIIδ oxidation in hearts from rats with metabolic syndrome. Mol. Cell. Biochem. 2022, 477, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, P.J.; Bajka, B.H.; Edwards, C.H.; Warren, F.J.; Ellis, P.R. Enzyme kinetic approach for mechanistic insight and predictions of in vivo starch digestibility and the glycaemic index of foods. Trends Food Sci. Technol. 2022, 120, 254–264. [Google Scholar] [CrossRef]
- Omar, N.A.M.; Frank, J.; Kruger, J.; Bello, F.D.; Medana, C.; Collino, M.; Zamaratskaia, G.; Michaelsson, K.; Wolk, A.; Landberg, R. Effects of High Intakes of Fructose and Galactose, with or without Added Fructooligosaccharides, on Metabolic Factors, Inflammation, and Gut Integrity in a Rat Model. Mol. Nutr. Food Res. 2021, 65, e2001133. [Google Scholar] [CrossRef] [PubMed]
- Drake, I.; Sonestedt, E.; Ericson, U.; Wallström, P.; Orho-Melander, M. A Western dietary pattern is prospectively associated with cardio-metabolic traits and incidence of the metabolic syndrome. Br. J. Nutr. 2018, 119, 1168–1176. [Google Scholar] [CrossRef]
- Vasbinder, A.; Anderson, E.; Shadid, H.; Berlin, H.; Pan, M.; Azam, T.U.; Khaleel, I.; Padalia, K.; Meloche, C.; O’Hayer, P.; et al. Inflammation, Hyperglycemia, and Adverse Outcomes in Individuals With Diabetes Mellitus Hospitalized for COVID-19. Diabetes Care 2022, 45, 692–700. [Google Scholar] [CrossRef]
- Pepin, A.; Stanhope, K.L.; Imbeault, P. Are Fruit Juices Healthier Than Sugar-Sweetened Beverages? A Review. Nutrients 2019, 11, 1006. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Karin, M. “Sweet death”: Fructose as a metabolic toxin that targets the gut-liver axis. Cell Metab. 2021, 33, 2316–2328. [Google Scholar] [CrossRef]
- McCain, H.; Kaliappan, S.; Drake, M. Invited review: Sugar reduction in dairy products. J. Dairy Sci. 2018, 101, 8619–8640. [Google Scholar] [CrossRef]
- Patkar, O.L.; Mohamed, A.Z.; Narayanan, A.; Mardon, K.; Cowin, G.; Bhalla, R.; Stimson, D.H.R.; Kassiou, M.; Beecher, K.; Belmer, A.; et al. A binge high sucrose diet provokes systemic and cerebral inflammation in rats without inducing obesity. Sci. Rep. 2021, 11, 11252. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Hu, F.B. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010, 121, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Shendurse, A.M.; Khedkar, C.D. Lactose. Encycl. Food Health 2016, 509–516. [Google Scholar] [CrossRef]
- Meyers, A.M.; Mourra, D.; Beeler, J.A. High fructose corn syrup induces metabolic dysregulation and altered dopamine signaling in the absence of obesity. PLoS ONE 2017, 12, e0190206. [Google Scholar] [CrossRef]
- Taskinen, M.-R.; Packard, C.J.; Borén, J. Dietary Fructose and the Metabolic Syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef]
- Krycer, J.R.; Quek, L.-E.; Francis, D.; Zadoorian, A.; Weiss, F.C.; Cooke, K.C.; Nelson, M.E.; Diaz-Vegas, A.; Humphrey, S.J.; Scalzo, R.; et al. Insulin signaling requires glucose to promote lipid anabolism in adipocytes. J. Biol. Chem. 2020, 295, 13250–13266. [Google Scholar] [CrossRef]
- Blanco, A.; Blanco, G. Carbohydrates. In Medical Biochemistry; Elsevier: Amsterdam, Netherlands, 2022; pp. 77–103. [Google Scholar] [CrossRef]
- Olofsson, C.; Eriksson, M.; Helin, A.-C.B.; Anderstam, B.; Orsini, N.; Stenvinkel, P.; Ekberg, N.R. Effects of Acute Fructose Loading on Markers of Inflammation—A Pilot Study. Nutrients 2021, 13, 3110. [Google Scholar] [CrossRef]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef]
- Legeza, B.; Marcolongo, P.; Gamberucci, A.; Varga, V.; Bánhegyi, G.; Benedetti, A.; Odermatt, A. Fructose, Glucocorticoids and Adipose Tissue: Implications for the Metabolic Syndrome. Nutrients 2017, 9, 426. [Google Scholar] [CrossRef]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef]
- Varma, V.; Boros, L.G.; Nolen, G.T.; Chang, C.-W.; Wabitsch, M.; Beger, R.D.; Kaput, J. Metabolic fate of fructose in human adipocytes: A targeted 13C tracer fate association study. Metabolomics 2015, 11, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.; Boros, L.G.; Nolen, G.T.; Chang, C.-W.; Wabitsch, M.; Beger, R.D.; Kaput, J. Fructose Alters Intermediary Metabolism of Glucose in Human Adipocytes and Diverts Glucose to Serine Oxidation in the One–Carbon Cycle Energy Producing Pathway. Metabolites 2015, 5, 364–385. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, P.M.; Markert, E.K.; Gounder, M.; Lin, H.; Dvorzhinski, D.; Dolfi, S.C.; Chan, L.L.Y.; Qiu, J.; DiPaola, R.S.; Hirshfield, K.M.; et al. Contribution of serine, folate and glycine metabolism to the ATP, NADPH and purine requirements of cancer cells. Cell Death Dis. 2013, 4, e877. [Google Scholar] [CrossRef] [PubMed]
- Pektas, M.B.; Koca, H.B.; Sadi, G.; Akar, F. Dietary Fructose Activates Insulin Signaling and Inflammation in Adipose Tissue: Modulatory Role of Resveratrol. BioMed Res. Int. 2016, 2016, 8014252. [Google Scholar] [CrossRef]
- Rodrigues, D.F.; Henriques, M.C.D.C.; Oliveira, M.C.; Menezes-Garcia, Z.; Marques, P.E.; Souza, D.D.G.; Menezes, G.B.; Teixeira, M.M.; Ferreira, A.V.M. Acute intake of a high-fructose diet alters the balance of adipokine concentrations and induces neutrophil influx in the liver. J. Nutr. Biochem. 2014, 25, 388–394. [Google Scholar] [CrossRef]
- Zhang, D.-M.; Jiao, R.-Q.; Kong, L.-D. High Dietary Fructose: Direct or Indirect Dangerous Factors Disturbing Tissue and Organ Functions. Nutrients 2017, 9, 335. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Mehta, V.; Onkaramurthy, N.; O’Keefe, J.H. Fructose-induced inflammation and increased cortisol: A new mechanism for how sugar induces visceral adiposity. Prog. Cardiovasc. Dis. 2018, 61, 3–9. [Google Scholar] [CrossRef]
- Marek, G.; Pannu, V.; Shanmugham, P.; Pancione, B.; Mascia, D.; Crosson, S.; Ishimoto, T.; Sautin, Y.Y. Adiponectin Resistance and Proinflammatory Changes in the Visceral Adipose Tissue Induced by Fructose Consumption via Ketohexokinase-Dependent Pathway. Diabetes 2015, 64, 508–518. [Google Scholar] [CrossRef]
- Kovačević, S.; Brkljačić, J.; Milutinović, D.V.; Gligorovska, L.; Bursać, B.; Elaković, I.; Djordjevic, A. Fructose Induces Visceral Adipose Tissue Inflammation and Insulin Resistance Even Without Development of Obesity in Adult Female but Not in Male Rats. Front. Nutr. 2021, 8, 749328. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, W.; Song, G.; Pang, S.; Peng, Z.; Li, Y.; Wang, P. High-Fructose Diet Increases Inflammatory Cytokines and Alters Gut Microbiota Composition in Rats. Mediat. Inflamm. 2020, 2020, 6672636. [Google Scholar] [CrossRef]
- Alkhouri, N.; Dixon, L.J.; Feldstein, A.E. Lipotoxicity in nonalcoholic fatty liver disease: Not all lipids are created equal. Expert Rev. Gastroenterol. Hepatol. 2009, 3, 445–451. [Google Scholar] [CrossRef]
- Della Corte, K.W.; Perrar, I.; Penczynski, K.J.; Schwingshackl, L.; Herder, C.; Buyken, A.E. Effect of dietary sugar intake on biomarkers of subclinical inflammation: A systematic review and meta-analysis of intervention studies. Nutrients 2018, 10, 606. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Agrawal, S.; Agrawal, A. High fructose-induced metabolic changes enhance inflammation in human dendritic cells. Clin. Exp. Immunol. 2019, 197, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, G.; Burton, K.J.; Rosikiewicz, M.; Freiburghaus, C.; von Ah, U.; Münger, L.H.; Pralong, F.P.; Vionnet, N.; Greub, G.; Badertscher, R.; et al. Blood lactose after dairy product intake in healthy men. Br. J. Nutr. 2017, 118, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Conte, F.; van Buuringen, N.; Voermans, N.C.; Lefeber, D.J. Galactose in human metabolism, glycosylation and congenital metabolic diseases: Time for a closer look. Biochim. et Biophys. Acta (BBA) Gen. Subj. 2021, 1865, 129898. [Google Scholar] [CrossRef] [PubMed]
- Krycer, J.R.; Elkington, S.D.; Diaz-Vegas, A.; Cooke, K.C.; Burchfield, J.G.; Fisher-Wellman, K.H.; Cooney, G.J.; Fazakerley, D.J.; James, D.E. Mitochondrial oxidants, but not respiration, are sensitive to glucose in adipocytes. J. Biol. Chem. 2020, 295, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.S.; Revathy, V.M.; Jaleel, A. Adipocytes utilize sucrose as an energy source—Effect of different carbohydrates on adipocyte differentiation. J. Cell. Physiol. 2020, 235, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Morabito, R.; Spinelli, S.; Trichilo, V.; Loddo, S.; Sarikas, A.; Dossena, S.; Marino, A. D-Galactose Decreases Anion Exchange Capability through Band 3 Protein in Human Erythrocytes. Antioxidants 2020, 9, 689. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Umbayev, B.; Askarova, S.; Almabayeva, A.; Saliev, T.; Masoud, A.-R.; Bulanin, D. Galactose-Induced Skin Aging: The Role of Oxidative Stress. Oxidative Med. Cell. Longev. 2020, 2020, 7145656. [Google Scholar] [CrossRef]
- Sasaoka, N.; Imamura, H.; Kakizuka, A. A Trace Amount of Galactose, a Major Component of Milk Sugar, Allows Maturation of Glycoproteins during Sugar Starvation. Iscience 2018, 10, 211–221. [Google Scholar] [CrossRef]
- Bouwman, L.M.S.; Fernández-Calleja, J.M.S.; van der Stelt, I.; Oosting, A.; Keijer, J.; van Schothorst, E.M. Replacing Part of Glucose with Galactose in the Postweaning Diet Protects Female But Not Male Mice from High-Fat Diet–Induced Adiposity in Later Life. J. Nutr. 2019, 149, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Kanuri, G.; Spruss, A.; Wagnerberger, S.; Bischoff, S.C.; Bergheim, I. Role of tumor necrosis factor α (TNFα) in the onset of fructose-induced nonalcoholic fatty liver disease in mice. J. Nutr. Biochem. 2011, 22, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-L.; Deng, Y.-Y.; Wang, R.; Wu, C.; Li, J.; Niu, W.; Yang, Q.; Bhatia, M.; Gudmundsson, G.H.; Agerberth, B.; et al. Lactose Induces Phenotypic and Functional Changes of Neutrophils and Macrophages to Alleviate Acute Pancreatitis in Mice. Front. Immunol. 2018, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Paasela, M.; Kolho, K.-L.; Vaarala, O.; Honkanen, J. Lactose inhibits regulatory T-cell-mediated suppression of effector T-cell interferon-γ and IL-17 production. Br. J. Nutr. 2014, 112, 1819–1825. [Google Scholar] [CrossRef]
- Jones, N.; Blagih, J.; Zani, F.; Rees, A.; Hill, D.G.; Jenkins, B.J.; Bull, C.J.; Moreira, D.; Bantan, A.I.M.; Cronin, J.G.; et al. Fructose reprogrammes glutamine-dependent oxidative metabolism to support LPS-induced inflammation. Nat. Commun. 2021, 12, 1209. [Google Scholar] [CrossRef]
- Braun, C.; Weichhart, T. mTOR-dependent immunometabolism as Achilles’ heel of anticancer therapy. Eur. J. Immunol. 2021, 51, 3161–3175. [Google Scholar] [CrossRef]
- Hortová-Kohoutková, M.; Lázničková, P.; Frič, J. How immune-cell fate and function are determined by metabolic pathway choice: The bioenergetics underlying the immune response. BioEssays 2021, 43, e2000067. [Google Scholar] [CrossRef]
- Menk, A.V.; Scharping, N.E.; Moreci, R.S.; Zeng, X.; Guy, C.; Salvatore, S.; Bae, H.; Xie, J.; Young, H.A.; Wendell, S.G.; et al. Early TCR Signaling Induces Rapid Aerobic Glycolysis Enabling Distinct Acute T Cell Effector Functions. Cell Rep. 2018, 22, 1509–1521. [Google Scholar] [CrossRef]
- Lee, M.K.S.; Al-Sharea, A.; Shihata, W.A.; Veiga, C.B.; Cooney, O.D.; Fleetwood, A.J.; Flynn, M.C.; Claeson, E.; Palmer, C.S.; Lancaster, G.I.; et al. Glycolysis Is Required for LPS-Induced Activation and Adhesion of Human CD14+CD16− Monocytes. Front. Immunol. 2019, 10, 2054. [Google Scholar] [CrossRef]
- Pan, J.H.; Kim, H.-S.; Beane, K.E.; Montalbano, A.M.; Lee, J.H.; Kim, Y.J.; Kim, J.H.; Kong, B.C.; Kim, S.; Park, J.-W.; et al. IDH2 Deficiency Aggravates Fructose-Induced NAFLD by Modulating Hepatic Fatty Acid Metabolism and Activating Inflammatory Signaling in Female Mice. Nutrients 2018, 10, 679. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Jiao, X.; Sun, X.; Huang, Y.; Xu, P.; Xue, Y.; Fu, T.; Liu, J.; Li, Z. Short-Term High Fructose Intake Impairs Diurnal Oscillations in the Murine Cornea. Investig. Opthalmology Vis. Sci. 2021, 62, 22. [Google Scholar] [CrossRef] [PubMed]
- Gambaro, S.E.; Zubiría, M.G.; Portales, A.E.; Rey, M.A.; Rumbo, M.; Giovambattista, A. M1 macrophage subtypes activation and adipocyte dysfunction worsen during prolonged consumption of a fructose-rich diet. J. Nutr. Biochem. 2018, 61, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, A.; Rehman, A.; Paradis, P.; Schiffrin, E.L. Role of T Regulatory Lymphocytes in the Pathogenesis of High-Fructose Diet–Induced Metabolic Syndrome. Hypertension 2013, 61, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, N.; Kang, J.; Yoon, S.; Lee, H.-A.; Jung, H.; Kim, S.-H.; Kim, I. Activated pathogenic Th17 lymphocytes induce hypertension following high-fructose intake in Dahl salt-sensitive (SS) but not Dahl salt-resistant (SR) rats. Dis. Model. Mech. 2020, 13, dmm044107. [Google Scholar] [CrossRef] [PubMed]
- Sasoh, T.; Kugo, H.; Kondo, Y.; Miyamoto, K.; Minami, M.; Higashihara, M.; Kawamoto, H.; Takeshita, F.; Moriyama, T.; Zaima, N. Different effects of high-fat and high-sucrose diets on the physiology of perivascular adipose tissues of the thoracic and abdominal aorta. Adipocyte 2021, 10, 412–423. [Google Scholar] [CrossRef]
- Lawless, S.J.; Kedia-Mehta, N.; Walls, J.F.; McGarrigle, R.; Convery, O.; Sinclair, L.V.; Navarro, M.N.; Murray, J.; Finlay, D.K. Glucose represses dendritic cell-induced T cell responses. Nat. Commun. 2017, 8, 15620. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, W.; Zhou, X.; Zöphel, D.; Soriano-Baguet, L.; Dolgener, D.; Carlein, C.; Hof, C.; Zhao, R.; Ye, S.; et al. High Glucose Enhances Cytotoxic T Lymphocyte-Mediated Cytotoxicity. Front. Immunol. 2021, 12, 689337. [Google Scholar] [CrossRef]
- Castro, M.C.; Villagarcía, H.; Nazar, A.; Arbeláez, L.G.; Massa, M.L.; Del Zotto, H.; Ríos, J.L.; Schinella, G.; Francini, F. Cacao extract enriched in polyphenols prevents endocrine-metabolic disturbances in a rat model of prediabetes triggered by a sucrose rich diet. J. Ethnopharmacol. 2020, 247, 112263. [Google Scholar] [CrossRef]
- Millet, P.; Vachharajani, V.; McPhail, L.; Yoza, B.; McCall, C.E. GAPDH Binding to TNF-α mRNA Contributes to Posttranscriptional Repression in Monocytes: A Novel Mechanism of Communication between Inflammation and Metabolism. J. Immunol. 2016, 196, 2541–2551. [Google Scholar] [CrossRef]
- Hasegawa, H.; Matsumoto, T. Mechanisms of Tolerance Induction by Dendritic Cells In Vivo. Front. Immunol. 2018, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, J.; Kumar, J.M.; Arindkar, S.; Das, B.; Pramod, U.; Juyal, R.C.; Majumdar, S.S.; Nagarajan, P. Role of immunodeficient animal models in the development of fructose induced NAFLD. J. Nutr. Biochem. 2014, 25, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Lynch, L.A.; O’Connell, J.M.; Kwasnik, A.K.; Cawood, T.J.; O’Farrelly, C.; O’Shea, D. Are Natural Killer Cells Protecting the Metabolically Healthy Obese Patient? Obesity 2009, 17, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jin, W.; Wu, R.; Li, J.; Park, S.-A.; Tu, E.; Zanvit, P.; Xu, J.; Liu, O.; Cain, A.; et al. High Glucose Intake Exacerbates Autoimmunity through Reactive-Oxygen-Species-Mediated TGF-β Cytokine Activation. Immunity 2019, 51, 671–681.e5. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Chi, H. The interplay between regulatory T cells and metabolism in immune regulation. Oncoimmunology 2013, 2, e26586. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).