Abstract
Nonsteroidal anti-inflammatory drugs are the most commonly prescribed anti-inflammatory drugs worldwide. The most common side effects are gastrointestinal. Pantoprazole, a proton pump inhibitor (PPI), can be used to prevent these events from occurring. In this study, we attempt to develop and validate a novel method for determining and validating the fixed-dose combination of meloxicam and pantoprazole. A new method has been developed and validated to estimate pantoprazole and meloxicam in a fixed-dose combination using RP-HPLC. In order to separate the drugs, a mobile phase phosphate buffer/acetate was used (30:70, v/v), with a pH of 3.4 and a flow rate of 1.0 mL/min at 25 °C. The detection wavelength for the drugs was at a wavelength of 310 nm. The retention times for meloxicam and pantoprazole were 6 and 9 min, respectively. In concentrations ranging from 0.1 to 200 mg/L, the linearity of the detector was established. The r was 0.9998 for both drugs. Recovery rates ranged from 98 to 102% on average. According to the guidelines of the International Council on Harmonization, the results were satisfactory. Using the method presented herein, the pharmaceutical formulation of the combined meloxicam and pantoprazole can be routinely tested.
1. Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are among the most frequently used medications. NSAIDs are a drug class that the U.S. Food and Drug Administration (FDA) has approved for use as antipyretics, anti-inflammatory drugs, and analgesics. NSAIDs generally work by inhibiting two cyclooxygenase (COX) enzymes, which inhibits the production of prostaglandins (PGs). PGs play essential roles in various cellular processes, including gastrointestinal cytoprotection, hemostasis and thrombosis, inflammation, renal hemodynamics, cartilage turnover, and angiogenesis [1]. Around the world, 30 million people are estimated to take NSAIDs every day. Because of the COX pathway’s combined function in inflammation [2], it is a crucial choice in pain control. NSAIDs, either taken individually or in conjunction with other groups of medications, are used for symptomatic care in several cases, such as in short- and long-term pain states, several musculoskeletal troubles, dysmenorrhea, osteoarthritis, rheumatoid arthritis, pyrexia (fever), gout, migraines and, in some instances, cases of acute trauma. They are used as opioid-sparing agents. NSAIDs are classified according to their selectivity, based on the development of COX into cyclooxygenase -1 (COX-1) and cyclooxygenase isoenzymes into cyclooxygenase -2 (COX-2) [3].
COX-1 is considered a constitutive enzyme in almost all cells that controls the physiological development of prostanoids. In addition, COX-1 is encouraged in intercellular connections and in the planning of various homeostatic tasks by interplaying specific membrane receptors with G proteins (gastric, platelets, kidney) [3]. COX-2 is an inducible enzyme isoform in charge of developing specific prostanoid mediators that have a role in inflammation and pain transmission, in response to pro-inflammatory stimuli. COX-2 in its constitutive form is present in the Central Nervous System (CNS), kidney, prostate, testes, and vessels. Suppression of COX-2 via NSAIDs is the cause of the treatment outcomes, whereas COX-1 suppression will result in adverse drug reactions. Therefore, selective COX-2 Inhibitors (COXIBs) are selective for COX-2 inhibition. Meloxicam is a new NSAID that inhibits the inducible isoform of the COX-2 enzyme with a high selectivity [2,3]. Indeed, NSAIDs’ gastrointestinal (GI) side effects are well-known and include symptoms such as dyspepsia, heartburn, nausea, gastroduodenal ulcers, and ulcer complications (bleeding, perforation, and obstruction) [4]. At high doses, NSAID-Induced Gastropathy (NIG) happens. This happens when NSAIDs stop the production of prostaglandins, make the stomach move faster, and make the mucus more permeable. This causes neutrophil infiltration, the formation of free radicals, and, ultimately, mucosal lesions. Numerous factors, including the type and duration of the NSAID, and concurrent treatment, and patient factors, affect the relative risk of gastrointestinal complications. For example, NSAIDs in high doses regularly lead to a two-to-three-fold increase in the risk of upper gastrointestinal complications [5].
Among the common critical risk factors for developing NSAID-induced GI bleeding are being aged 65 years or above, having a previous experience of peptic ulcer disease, including gut bleeding accompanying NSAIDs, and the concurrent use of other treatments (i.e., systemic corticoids), oral anticoagulants, or other NSAIDs [6]. On the contrary, proton pump inhibitors (PPIs) are the most potent gastric-acid-suppressing agents in clinical use because they irreversibly inhibit proton pump (H+/K+ ATPase) function. There is now a substantial body of evidence demonstrating that PPIs outperform histamine H2 receptor antagonists and other drugs in treating acid-related disorders [7]. PPIs are considered in the treatment of esophagitis [8], Non-Erosive Reflux Disease (NERD) [9], Gastroesophageal Reflux Disease (GERD) [10], Peptic Ulcer Disease (PUD), NSAID-associated ulcers [11], Zollinger-Ellison Syndrome (ZES) [12], and functional dyspepsia [13]. They are also an essential part of Helicobacter pylori (H. pylori) eradication therapy [14].
Fixed-dose combination products, also referred to as fixed-dose combinations (FDCs), are a single dosage set of two or more medications [15]. The FDA specifies a combination product as an output consisting of any mixture of a medicine and an apparatus, a biological product and an apparatus, a drug and a biological product, or a drug, an apparatus, and a biological product. FDCs or combination products with a fixed ratio shall be suitable only if each ingredient’s dose meets the criteria of the specified inhabitance group and if the collection has an established feature over single compounds given individually, in terms of therapeutic effect, safety, or compliance [16]. From 1990 until this day, many FDCs have risen with the inclusion of new products approved by the FDA.
NSAIDs with a gastroprotective agent, especially PPIs, have been linked to poor adherence. Non-compliance with gastroprotection, for example, is 61% when the third NSAID prescription is written. Upper Gastrointestinal Adverse Events (GI AEs) are 2.5-fold more common in patients with less than 80% adherence. The risk of NSAID-related GI complications rises linearly as adherence to the gastroprotection falls [16,17]. In this scenario, combining an NSAID and a gastroprotective agent in a single capsule should aid compliance and thus gastroprotection. Meloxicam is an enol-carboxamide NSAID similar to piroxicam. Moreover, it has a chemical structure shown in Figure 1A.

Figure 1.
(A) Chemical structure of meloxicam. (B) Chemical structure of pantoprazole.
Meloxicam is a solid, pastel-colored, crystalline powder. It is very slightly soluble in methanol and practically insoluble in water, with higher solubility observed in strong acids and bases. Pantoprazole is a substituted benzimidazole. Its empirical formula is C16H14F2N3NaO4S · 1.5 H2O, with a molecular weight of 405.4 g/mol. When reviewing the literature, one can discover various methods for analyzing meloxicam alone or in combination with other drugs [18,19,20,21,22]. The structural formula appears in Figure 1B.
Pantoprazole (a PPI) is a racemic crystalline powder that ranges from white to off-white. Pantoprazole’s acidic and basic properties are both weak. It is freely soluble in water, very slightly soluble in phosphate buffer at pH 7.4, and practically insoluble in n-hexane. However, numerous methods for analyzing pantoprazole alone and in combination with other medications have been reported [23,24,25,26]. With lansoprazole as an internal standard, a modified HPLC method for measuring pantoprazole sodium in pharmaceutical dosage forms was reported [23]. Another study used validated HPLC and high-performance thin-layer chromatography (HPTLC) methods to estimate pantoprazole and domperidone at the same time [24]. In a subsequent study, lansoprazole, omeprazole, and pantoprazole sodium sesquihydrate were determined using the Reversed Phase-High Performance Liquid Chromatographic Method (RP-HPLC) in the presence of their acid-induced degradation products [25]. An RP-HPLC method for the simultaneous separation and quantification of pantoprazole and its five major impurities in pharmaceutical formulations, on the other hand, was developed and validated [26].
HPLC is a beneficial analytical method for analyzing single or combined drugs [27,28,29,30,31]. Several studies on the validation and combination of other NSAIDs and PPIs have been published [32,33,34]. However, no study has validated or developed a method for analyzing pantoprazole and meloxicam in a combined formulation, to the best of our knowledge. The methodology for the validation and determination of meloxicam and pantoprazole is at the heart of this study.
The study’s novelty stems from the fact that it aims to establish and validate a novel, simple, accurate, precise, and cost-effective RP-HPLC method to determine and validate meloxicam and pantoprazole in a fixed-dose combination.
2. Materials and Methods
2.1. Chemicals and Reagents
The following reagents and chemicals were used for formulation. Active ingredients were from Dar Al-Dawa (Dar Al-Dawa, Amman, Jordan)and Hikma Pharmaceuticals (Hikma Pharmaceuticals, Amman, Jordan): meloxicam (15 mg), batch number 117,009 (Dar AlDawa), and pantoprazole (10 mg), batch number 20190126029 (Hikma Pharmaceuticals).
Inactive ingredients: Microcrystalline Cellulose (CAS: 9004-34-6), Hydroxypropyl Cellulose (CAS: 9004-64-2), Croscarmellose Sodium (CAS: 74811-65-7), Magnesium Stearate (CAS: 557-04-0), Hypromellose 2910 (CAS: 9004-65-3), Titanium Dioxide (CAS: 13463-67-7), Iron oxide red (E172, CAS: 1309-37-1), Macrogol 8000 (CAS: 25322-68-3), and Butyl Alcohol (CAS: 71-36-3).
The following reagents and chemicals were used: orthophosphoric acid, AR grade; potassium hydrogen phosphate, AR grade; acetonitrile, HPLC grade; water, Milli-Q grade; methanol, HPLC grade; 1- hexane sulfonic acid sodium salt; and triethylamine.
The following active ingredients were from Dar AL Dawa and Hikma Pharmaceuticals: film-coated meloxicam (15 mg), tablet batch number 117,009, and film-coated pantoprazole (10 mg), tablet batch number 20190126029, Dar AL Dawa and Hikma Pharmaceuticals, Amman, Jordan.
2.2. Formulation of Tablets
First, all ingredients were sieved in a sieve with a mesh size of 36, except Mg-stearate, which was sieved in a sieve with a mesh size of 60. Then, the drum mixer added both active ingredients (pantoprazole and meloxicam) and mixed the inactive ingredients afterward. The mixture was compressed using a single punch machine (cadmic) to yield a 50 mg tablet. Then, coating was used to protect it. The coating used was cellulose acetate phthalate (CAP) because pantoprazole is acid-sensitive. The coating material was prepared by diluting 10 g of CAP in 90 mL of acetone.
Finally, GMP tests on the tablets were conducted and obtained the following results:
The disintegration test result was no disintegration in buffer up to 2 h, disintegration in water after 4.55 min.
The Friability result was 0.562%. For most products, a maximum weight loss of no more than 1.0 percent (obtained from a single test or the mean of three tests) is considered acceptable.
The hardness result was between 23–38 N. Although there is no definite number for the tablet’s hardness, a recommended range between 15 and 14 newtons is most likely adequate for handling,.
According to the above results, the formulation was suitable to start validation. Therefore, 140 sustained-release enteric-coated tablets were prepared.
2.3. Instruments
Chromatographic separation was accomplished using an ACE C18 250 mm × 4.6 mm (particle size: 5 μm) column. Analysis was achieved on an HPLC (Finnigan Surveyor) (Thermo Electron Corporation, San Jose, CA, USA), equipped with the detector (UV-VIS plus Detector), the pump (solvent delivery systems pump) (LC Pump plus), and the auto-sampler (Auto-sampler Plus).
2.4. Preparation of Phosphate Buffer: Acetonitrile (30:70 v/v) Mobile Phase
A volume of 700 mL of acetonitrile was mixed with a 300 mL buffer solution, and orthophosphoric acid was used to adjust the pH to 3.4. The mobile phase was degassed by sonication after being filtered through a 0.45 μm membrane filter.
2.5. Preparation of Stock Solution and Working Solution
Meloxicam and pantoprazole stock solution (1000 mg/L) was prepared by weighing and transferring 100 mg of each active ingredient into a 100 mL volumetric flask. Then, diluent up to 100 mL was used to prepare the stock solutions. As shown in Table 1, the working solutions were prepared.

Table 1.
Preparation of working solutions of meloxicam and pantoprazole (mg/L).
2.6. Preparation of Buffer
The buffer solution was made by dissolving about 6.8 g of potassium dihydrogen phosphate in 1000 mL of HPLC-grade water. Next, 0.2 g of hexane sulfonic acid sodium salt and 1 mL of triethanolamine were added; the solution was adjusted to pH 3.4 with orthophosphoric acid.
2.7. Wavelength Selection
An Ultraviolet-visible (UV-VIS) scan with a 200–550 nm wavelength range was performed on each meloxicam and pantoprazole solution. The maximum absorbance of both drugs was in the range of 200–400 nm.
2.8. Chromatographic Conditions
The effect of various chromatographic conditions on the separation of meloxicam and pantoprazole was investigated, including the pH, ion pair, mobile phase composition, and column composition, to determine the most appropriate method for designing these drugs. Table 2 displays the best, final selected conditions of the proposed method.

Table 2.
Chromatographic conditions were used in the analysis.
Run time: 7.0 min. Retention time: meloxicam, 6 min; pantoprazole, 9 min, which indicate good peaks of symmetry.
2.9. Selectivity and Sensitivity Test Preparation
The test was carried out by dissolving foreign and local drugs in a mobile phase solution acting as a solvent, and injecting them into the system (test formulation). In contrast, the sample solution was carried out by dissolving raw material in a mobile phase with placebo content and injecting it into the system (reference formulation).
2.10. Linearity Sample Test Preparation
Seven standard samples of the standard-sample concentration of meloxicam and pantoprazole were prepared to evaluate linearity. Table 1 shows how the various stocks were prepared. Each sample was subjected to a triple injection study, followed by linear analysis of the average peak areas versus the level of the concentration studied.
2.11. Preparations for System Precision Test of Samples
Meloxicam and pantoprazole were weighed to make homogeneous solutions, then dissolved in 100 mL of mobile phase solution acting as a solvent before being injected repeatedly (i.e., ten injections).
2.12. Preparations for Method Precision Test of Samples
Six sample solutions of the same homogeneous solution were prepared and injected three times each to measure the Relative Standard Deviation (RSD) and assay percents.
2.13. Preparations for Intermediate Precision Tests for Samples
Each sample was injected three times at different times to assess process precision, and the analyst’s RSD percent and assay percent were computed for the same six samples.
2.14. Preparations for Accuracy Test for Samples
Three samples at three concentrations were prepared by being dissolved in mobile phase solution (solvent) and diluted to 100 mL by the mobile phase solution, as in the sample solution preparation. The injection was performed in triplicate for each concentration level, just like the standard sample solution, which was also made in the same manner.
2.15. Recovery Test
Triplicate pantoprazole and meloxicam samples were prepared. The total peak areas obtained from injections of the prepared standards are compared to the total peak areas of comparable mobile phase standards, prepared with a concentration of analytes from analysts, ensuring 100 percent recovery. The analyte recovery extents should be precise, consistent, and reproducible. Triplicates of each Quality Control (QC) level of meloxicam and pantoprazole, and triplicates of each QC level prepared in the mobile process, were used to perform the recovery.
2.16. Preparations for Robustness Test
2.16.1. Robustness of the Wavelength (±5 nm)
The sample solutions used in this test were prepared the same way as previous sample solution preparations. By adjusting the UV detection reading to 305 and 315 nm (310 ± 5 nm) and recording the absorbance for triple injections, the wavelength of the sample solution was changed.
2.16.2. Robustness of the Temperature (±5 °C)
The sample solution was prepared in the usual way, but with a temperature variation. The temperature was increased to a maximum of 30 °C, and the injection was performed in triplicate.
2.16.3. Robustness of Organic Modified Composition (±5%)
In this test, the organic phase was changed by adding or subtracting 5% acetonitrile to prepare the mobile phase (75:25), and another mobile phase solution with the same buffer concentration and pH value was prepared by increasing the buffer solution (65:35) and injecting it with the sample solution.
2.16.4. Robustness of Using Different Columns
The sample solution was prepared as usual, except for a column change. The analysis was performed on triple injections.
2.17. Assay Test
The assay test is a test that is performed to assess the existence and quantity of a drug, as in the following equation (Equation (1)):
2.18. Stability of Preparation for Analytical Solution Test
The standard solution’s stability was tested at room temperature when freshly prepared and after 24 h. The results were then compared to a 100% fresh standard solution, as per International Council on Harmonization (ICH) guidelines [35].
3. Results and Discussion
3.1. Identification and Compatibility
Identification aims to ensure the tested compounds’ specific identity and check whether they interact with the components themselves. Identification in chromatography also evaluates each drug’s result under the previously mentioned chromatographic conditions, suggesting that the drugs’ peaks did not interfere with any other unidentified peaks.
All methods yielded asymmetrical peaks, but overlapping and irregular chromatograms for the drugs, separately and in the mixture of the solution, were excluded. However, the ACN:buffer method (70:30), with a pH of 3.4, was the best for this group of drugs in terms of peak symmetry, resolution, and retention time, when analyzed by the HPLC system.
Meloxicam and pantoprazole had the best absorbance profiles, ranging between 200 and 400 nm. The wavelength of 310 nm was chosen for the simultaneous assaying of both drugs using HPLC because it has the best absorptivity, as shown in Figure 2A,B.


Figure 2.
(A) The absorbance profile of meloxicam in the 200–400 nm range. (B) The absorbance profile of pantoprazole in the range of 200–400 nm.
The best chromatographic conditions for simultaneously measuring meloxicam and pantoprazole were based on each drug’s resolution and retention time. The mixture of the mobile phase at a pH of 3.4, as appears in Table 2, provided the best resolution.
All of the excipients used in the formula were compatible with both drugs, as is apparent in FTIR and DSC studies.
3.2. FTIR of Pantoprazole and Meloxicam
Tests using Fourier Transform Infrared Spectroscopy (FTIR) for the formerly mentioned drugs match with their fingerprints and agree with what is seen in the literature [36,37]. The pantoprazole FTIR spectra revealed absorption bands in the range of 3000 to 3500 cm−1, with significant O-H and C-H absorption bands; the range of 1800 to 1500 cm−1 exhibits C=C and C=N absorption bands. On the other hand, bands can be seen at about 3290 cm−1 (the stretching vibration of an amide group (N–H)), 2917 cm−1 (the stretching vibration of an alkyl group), 1622 cm−1 (the stretching mode of an amide group), as can a sharp band at 1522 cm−1 (C=C aromatic stretching vibration). The meloxicam’s characteristic bands are at 1384 and 1172 cm−1 (for two sulphonyl groups (S=O stretching vibration)) [38]. The FTIR of pantoprazole and meloxicam appear in Figure 3A,B, respectively.

Figure 3.
(A) FTIR spectra of pantoprazole. (B) FTIR spectra of meloxicam.
3.3. DSC of Pantoprazole and Meloxicam
DSC was performed on both drugs alone and in combination to confirm their identity and look for potential interactions. Figure 4A,B show the DSC thermogram for pantoprazole and meloxicam alone, respectively. DSC for pantoprazole revealed an endothermic peak in the 140–160 °C range, corresponding to the melting and dehydration of pantoprazole (Figure 4A). The DSC thermograph of pure meloxicam revealed a strong endotherm near about 260 °C, suggesting its melting point temperature (Figure 4B). These figures confirm the identity of the compounds before combining them. In the DSC analysis, however, the combination of both drugs retained the characteristic peaks of the DSC thermogram for both drugs, indicating little or no interaction (Figure 4C).


Figure 4.
(A) DSC thermogram of pantoprazole. (B) DSC thermogram of meloxicam. (C) DSC thermogram of a combination between meloxicam and pantoprazole retained the characteristic peaks of the DSC thermogram for both drugs, indicating little or no interaction.
3.4. Selectivity
The method’s selectivity must be investigated to evaluate the analytical procedure’s ability to measure precisely, particularly in the presence of the active components, placebo, and another ingredient. Using the devised method’s parameters, a reference, sample, solvent, and placebo solution were injected into the column. It was discovered that the analyte, the solvent, and the placebo have no interaction.
3.5. Linearity and Range
Several concentrations of standard samples of the target chemicals were generated. After that, each concentration point was analyzed twice, and a linearity test was performed on the average peak areas versus the concentrations of the examined levels.
The linear equation of pantoprazole is y = 25,828 x + 8381.6. It was found that the linearity range was in the interval of 0.1–200 mg/L. The correlation coefficient (r) for pantoprazole was 0.9998, indicating strong linearity within the specified limit of the linearity validation method
An excellent linear relationship (r = 0.9998) was obtained between the concentrations of meloxicam and the corresponding average area, with a calibration curve equation of y = 12,786 x + 3593.6. The r-value for meloxicam indicated strong linearity within the specified limit of the linearity validation method.
3.6. System Precision
System precision aims to determine how beneficial each test result is when the process is repeated for several injections (ten injections) of the same homogeneous sample. The RSD percent values were less than 2%, indicating a suitable device; additionally, as shown in Table 3, the first and last retention periods do not overlap, indicating a good resolution.

Table 3.
System parameters for simultaneous measurements of diluent-containing meloxicam and pantoprazole.
As illustrated in Figure 5, the chromatogram clearly separates meloxicam and pantoprazole, with no overlap between the data-derived resolution peaks, indicating a precise method.
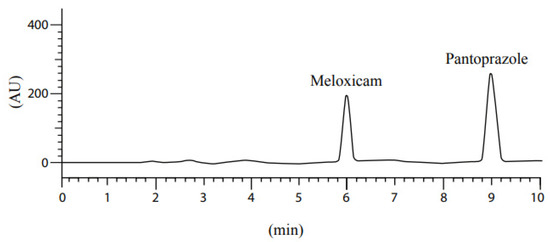
Figure 5.
The resolution pattern of meloxicam and pantoprazole mixture.
3.7. Method Precision
Using both drugs, the method’s accuracy was tested six times. The RSD values for samples in dilution were less than 2%, indicating that the procedure was precise. Furthermore, the recovered concentrations of both samples were found to be in the interval of 98–102%.
3.8. Intermediate Precision
Intermediate precision was obtained by running composite samples on two different days using different equipment. The six sample preparations were analyzed on the first day, and the data (assay percent, RSD percent) were obtained. On the second day, the analysis was repeated using different analysts with the same chromatographic conditions and concentrations. The assay value obtained was 98–102%, as shown in Table 4, Table 5, Table 6 and Table 7.

Table 4.
Intermediate precision (Intra-day) of pantoprazole.

Table 5.
Intermediate precision (Intra-day) of meloxicam.

Table 6.
Intermediate precision (Inter-day) of pantoprazole.

Table 7.
Intermediate precision (Inter-day) of meloxicam.
3.9. Recovery “Accuracy”
In order to estimate the recovery accuracy, samples at three different concentration levels were analyzed. The injection was performed in triplicate at each concentration level compared to the standard sample. The results are shown in Table 8. The percent-of-recovery equation was calculated according to the following equation (Equation (2)):

Table 8.
Recovery of pantoprazole and meloxicam.
According to ICH guidelines [35], the acceptable recovery limits are 98–102%.
Concentrations levels were 70, 100, and 130 mg/L for pantoprazole. However, the limits for meloxicam were 100.5, 150, and 190.5 mg/L.
Besides the excellent separation seen in the peaks, Figure 6 shows the relationship between concentration and peak area (AUC); as the concentration increases, the AUC increases, indicating the validity of the accuracy test’s results.
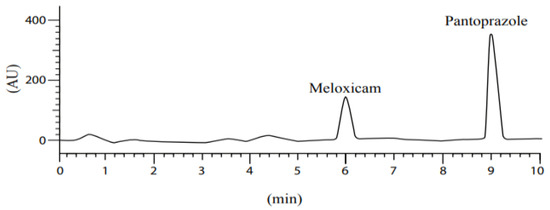
Figure 6.
Accuracy results of meloxicam and pantoprazole.
3.10. Robustness
Robustness is a test applied to find out the ability of the analytical procedure to withstand minor changes and how much these changes reflect in the results obtained [39].Consequently, to check if an analytical method is robust, several parameters in the analytical procedure were changed to examine the results obtained.
Robustness was performed using solutions prepared similarly to the system or method precisions tests in terms of the concentration and the number of replicates (typically 3), and was evaluated based on system suitability parameters or recovered amounts, compared to the original method’s data (Table 9).

Table 9.
Robustness of pantoprazole and meloxicam.
The following changes were completed separately:
Detector wavelength (±5 nm); mobile phase composition (±5%); acetonitrile volume; temperature + 5 °C.
3.11. Assay Test
The assay test results calculated based on Equation (1) appear in Supplementary Table S1A,B, both for meloxicam and pantoprazole, respectively. The results for meloxicam show about 100% assay results with a 1% RSD value for the analyzed samples (Supplementary Table S1A). On the other hand, a similar analysis of pantoprazole also showed about 100% assay results with a 2% RSD value for the analyzed samples (Supplementary Table S1B).
4. Dissolution
The dissolution was completed using the parameter described in the Methods section above. The dissolution of meloxicam results in an accuracy percent ranging from 99–102, whereas the results for pantoprazole were 98–108. The results are shown in Supplementary Table S2A,B.
Stability of Drugs in Analytical Solution
It is critical to understand at what concentration the drug of interest is stable. The solution’s stability should be evaluated by keeping it at room temperature for 24 h at a known concentration and comparing it to a new, fresh reference solution. The 100 percent solution is compared to the standard solution. Each sample contains 75 mg/L of pantoprazole and 50 mg/L of meloxicam. The stability results within the stated limit of 98−102% for fresh and 24 h samples are listed in Supplementary Table S3A,B.
5. Conclusions
FDCs are important throughout a wide range of illnesses. Their importance comes from improving disease management, reducing side effects, and improving compliance rates.
The proposed HPLC analytical method provides a simple, accurate, specific, and precise quantitative method for the simultaneous analysis of meloxicam and pantoprazole in a fixed-dose combination. According to ICH guidelines regarding linearity, accuracy, precision, and reproducibility, the method was validated. The proposed method can be used for the routine analysis and quality control assays of meloxicam and pantoprazole in a fixed-dose combination. This method is recommended for future bioanalytical analyses because it can be easily modified to estimate meloxicam and pantoprazole in various biological samples.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/analytica3020012/s1, Table S1A: Assay test details of meloxicam; Table S1B: Assay test details of pantoprazole; Table S2A: Dissolution of meloxicam; Table S2B: Dissolution of Pantoprazole; Table S3A: Stability of meloxicam; Table S3B: Stability of pantoprazole.
Author Contributions
Conceptualization, R.A., M.H., Z.Z. and W.A.D.; Data curation, M.H., O.A.M. and W.A.D.; Formal analysis, M.H. and W.A.D.; Funding acquisition, M.H. and W.A.D.; Investigation, R.A., M.H., Z.Z. and W.A.D.; Methodology, M.H., Z.Z. and W.A.D.; Project administration, M.H., O.A.M. and W.A.D.; Resources, M.H. and W.A.D.; Software, M.H., O.A.M. and W.A.D.; Supervision, M.H. and W.A.D.; Validation, M.H. and W.A.D.; Visualization, M.H. and W.A.D.; Writing—original draft, M.H. and W.A.D.; Writing—review and editing, M.H. and W.A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no financial or non-financial competing interests related to this manuscript.
References
- Gunaydin, C.; Bilge, S.S. Effects of Nonsteroidal Anti-Inflammatory Drugs at the Molecular Level. Eurasian, J. Med. 2018, 50, 116. [Google Scholar] [CrossRef] [PubMed]
- Osafo, N.; Agyare, C.; Obiri, D. Mechanism of action of nonsteroidal anti-inflammatory drugs. Anti-Inflamm. Drugs 2017, 18, 120–121. [Google Scholar]
- Perrone, G.M.; Scilimati, A.; Simone, L.; Vitale, P. Selective COX-1 Inhibition: A Therapeutic Target to be Reconsidered. Curr. Med. Chem. 2010, 17, 3769–3805. [Google Scholar] [CrossRef] [PubMed]
- Singh, G. NSAID induced gastrointestinal complications: The ARAMIS perspective–1997. Arthritis, Rheumatism, and Aging Medical Information System. J. Rheumatol. Suppl. 1998, 25, 8–16. [Google Scholar]
- Castellsague, J.; Riera-Guardia, N.; Calingaert, B.; Varas-Lorenzo, C.; Fourrier-Reglat, A.; Nicotra, F.; Sturkenboom, M.; Perez-Gutthann, S. Individual NSAIDs and Upper Gastrointestinal Complications. Drug Saf. 2012, 35, 1127–1146. [Google Scholar] [CrossRef]
- Chi, T.-Y.; Zhu, H.-M.; Zhang, M. Risk factors associated with nonsteroidal anti-inflammatory drugs (NSAIDs)-induced gastrointestinal bleeding resulting on people over 60 years old in Beijing. Medicine 2018, 97, 18. [Google Scholar] [CrossRef]
- Richardson, P.; Hawkey, C.J.; Stack, W.A. Proton Pump Inhibitors. Drugs 1998, 65, 30–335, Erratum in Drugs 2012, 56, 30–335. [Google Scholar] [CrossRef]
- Dellon, E.S.; Speck, O.; Woodward, K.; Covey, S.; Rusin, S.; Gebhart, J.H.; Chen, X.; Woosley, J.T.; Shaheen, N.J. Markers of eosinophilic inflammation for diagnosis of eosinophilic esophagitis and proton pump inhibitor-responsive esophageal eosinophilia: A prospective study. Clin. Gastroenterol. Hepatol. 2014, 12, 2015–2022. [Google Scholar] [CrossRef]
- Long, J.D.; Orlando, R.C. Nonerosive reflux disease: A pathophysiologic perspective. Curr. Gastroenterol. Rep. 2008, 10, 200–207. [Google Scholar] [CrossRef]
- Sandhu, D.S.; Fass, R. Current Trends in the Management of Gastroesophageal Reflux Disease. Gut Liver 2018, 12, 7–16. [Google Scholar] [CrossRef]
- Khan, M.A.; Howden, C.W. The Role of Proton Pump Inhibitors in the Management of Upper Gastrointestinal Disorders. Gastroenterol. Hepatol. 2018, 14, 169. [Google Scholar]
- McCarthy, D.M.; Olinger, E.J.; May, R.J.; Long, B.W.; Gardner, J.D. H2-Histamine receptor blocking agents in the Zollinger-Ellison syndrome. Experience in seven cases and implications for long-term therapy. Ann. Intern. Med. 1977, 87, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez, M.I.; Yuan, Y.; Hassan, A.; Bercik, P.; Moayyedi, P.; Group CUG and PD. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst. Rev. 2017, 2017, 1–75. [Google Scholar]
- Smoot, D.T. How does Helicobacter pylori cause mucosal damage? Direct mechanisms. Gastroenterology 1997, 113, S31–S34. [Google Scholar] [CrossRef]
- Sreedhar, D.; Subramanian, G.; Udupa, N. Combination drugs: Are they rational? Curr. Sci. 2006, 91, 343–354. [Google Scholar]
- World Health Organization. Guidelines for Registration of Fixed-Dose Combination Medicinal Products; WHO Technical Report Series; No. 929; WHO Headquarters: Geneva, Switzerland, 2005; pp. 95–141. [Google Scholar]
- Walton, S.; Mclaughlin, T.; Kruzikas, D. Gastroenterology undefined, and undefined. Impact of adherence to concomitant gastroprotective therapy on nonsteroidal-related gastroduodenal ulcer complications. Clin. Gastroenterol. Hepatol. 2006, 4, 1337–1345. [Google Scholar]
- Cox, S.; Hayes, J.; Yarbrough, J.; Veiga-Parga, T.; Greenacre, C. High-Performance Liquid Chromatography Determination of Meloxicam and Piroxicam with Ultraviolet Detection. Chromatogr. Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Zhang, H.; Choi, H.K. Analysis of meloxicam by high-performance liquid chromatography with cloud-point extraction. Anal. Bioanal. Chem. 2008, 392, 947–953. [Google Scholar] [CrossRef]
- Bae, J.W.; Kim, M.J.; Jang, C.G.; Lee, S.Y. Determination of meloxicam in human plasma using a HPLC method with UV detection and its application to a pharmacokinetic study. J. Chromatography. B Anal. Technol. Biomed. Life Sci. 2007, 859, 69–73. [Google Scholar] [CrossRef]
- Miyamoto, A.; Aoyama, T.; Matsumoto, Y. The Measurement of Meloxicam and Meloxicam Metabolites in Rat Plasma Using a High-Performance Liquid Chromatography-Ultraviolet Spectrophotometry Method. Chem. Pharm. Bull. 2017, 65, 121–126. [Google Scholar] [CrossRef][Green Version]
- Velpandian, T.; Jaiswal, J.; Bhardwaj, R.K.; Gupta, S.K. Development and validation of a new high-performance liquid chromatographic estimation method of meloxicam in biological samples. J. Chromatography. B Biomed. Sci. Appl. 2000, 738, 431–436. [Google Scholar] [CrossRef]
- Ashour, S.; Omar, S. A modified high-performance liquid chromatographic method for the analysis of pantoprazole sodium in pharmaceutical dosage forms using lansoprazole as internal standard. Arab. J. Chem. 2016, 9, S114–S119. [Google Scholar] [CrossRef]
- Patel, B.H.; Suhagia, B.N.; Patel, M.M.; Patel, J.R. Simultaneous estimation of pantoprazole and domperidone in pure powder and a pharmaceutical formulation by high-perfomance liquid chromatography and high-performance thin-layer chromatography methods. J. AOAC Int. 2007, 90, 142–146. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, Z.A.; Mohamed, A.O.; El-Bardicy, M.G.; El-Tarras, M.F. Reversed-phase high performance liquid chromatographic method for the determination of lansoprazole, omeprazole and pantoprazole sodium sesquihydrate in presence of their acid-induced degradation products. Chem. Pharm. Bull. 2006, 54, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Letica, J.; Marković, S.; Zirojević, J.; Nikolić, K.; Agbaba, D. High-performance liquid chromatographic determination of pantoprazole and its main impurities in pharmaceuticals. J. AOAC Int. 2010, 93, 1121–1128. [Google Scholar] [CrossRef]
- Habash, I.W.; Al-Shdefat, R.I.; Hailat, M.M.; Dayyih, W.A. A stability indicating rp-hplc method development for simultaneous estimation of alogliptin, pioglitazone, and metformin in pharmaceutical formulations. Acta Pol. Pharm. Drug Res. 2020, 77, 549–562. [Google Scholar] [CrossRef]
- Hailat, M.; Al-Ani, I.; Hamad, M.; Zakareia, Z.; Dayyih, W.A. Development and Validation of a Method for Quantification of Favipiravir as COVID-19 Management in Spiked Human Plasma. Molecules 2021, 26, 3789. [Google Scholar] [CrossRef]
- Alkather, Z.; Hailat, M.; Al-Shdefat, R.; Abu Dayyih, W. Development and Validation of HPLC Method for Five Gliptins in Pharmaceutical Dosage Forms in Finished Marketed Products. Curr. Pharm. Anal. 2020, 17, 1263–1271. [Google Scholar] [CrossRef]
- Al-Shdefat, R.; Hailat, M.; Kharshid, A.M.; Saadh, M.J.; Hamed, M.F.; Answer, M.K.; Abdel-Halim, H.; Dayyih, W.A. Evidence of human metabolites of omeprazole and its structure elucidation by using HPLC-MS. J. Mol. Struct. 2021, 1230, 129902. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Alfaraj, M.; Hammad, A.M.; Kasabri, V.; Shalabi, D.; Deeb, A.A.; Ibrahim, L.H.; Shnewer, K.; Yousef, I. Development of a Thymoquinone Polymeric Anticancer Nanomedicine through Optimization of Polymer Molecular Weight and Nanoparticle Architecture. Pharmaceutics 2020, 12, 811. [Google Scholar] [CrossRef]
- Jain, D.K.; Jain, N.; Charde, R. The RP-HPLC method for simultaneous estimation of esomeprazole and naproxen in binary combination. Pharm. Methods 2011, 2, 167–172. [Google Scholar] [CrossRef]
- Mohideen, S.; Shivakanth, M.; Sureshkumar, P.; Krishnan, S.N.; Surendranath, Y.; Satyanarayana, T. Development and validation of analytical method for naproxen and pantoprazole in capsule dosage form. Int. J. PharmTech Res. 2011, 3, 1169–1173. [Google Scholar]
- Kumar, R.S.; Sree, U.G.; Malleswara Babu, N.B. A RP-HPLC Method Development and Its Validation for the Simultaneous Estimation of Naproxen and Pantoprazole Sodium in Capsule Dosage Form. Asian J. Res. Chem 2013, 6, 155–168. [Google Scholar]
- ICH. ICH Official Website: ICH Quality Guidelines. Available online: https://www.ich.org/page/quality-guidelines (accessed on 16 May 2020).
- Nasef, A.M.; Gardouh, A.R.; Ghorab, M.M. Formulation and in-vitro evaluation of pantoprazole loaded pH-sensitive polymeric nanoparticles. Future J. Pharm. Sci. 2017, 3, 103–117. [Google Scholar] [CrossRef]
- Pathak, D.; Dahiya, S.; Pathak, K. Solid dispersion of meloxicam: Factorially designed dosage form for geriatric population. Acta Pharm. 2008, 58, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Abd Elbary, A.; Ali, A.A.; Aboud, H.M. Enhanced dissolution of meloxicam from orodispersible tablets prepared by different methods. Bull. Fac. Pharm. Cairo Univ. 2012, 50, 89–97. [Google Scholar] [CrossRef]
- Ahmad, R.; Hailat, M.; Jaber, M.; Alkhawaja, B.; Rasras, A.; Al-Shdefat, R.; Abu Dayyih, W. RP-HPLC Method Development for Simultaneous Estimation of Empagliflozin, Pioglitazone, and Metformin in Bulk and Tablet Dosage Forms. Acta Pol. Pharm. Drug Res. 2021, 78, 305–315. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).