Abstract
Diurnal enuresis can significantly affect a child’s biopsychosocial well-being; however, there is a lack of diagnostic and management algorithms on the diagnosis. The purpose of this literature review is to dissect the development of the evaluation and management of diurnal enuresis. A total of 44 articles published from January 1900 to December 2024 were chosen through literature searches in PubMed, Science Direct, Embase, and Google scholar. Search terms were “Diurnal Enuresis” or “Daytime Incontinence” as Mesh terms, and subsequent terms included “pediatrics”, “urinary bladder, overactive”, and “therapeutics”. Inclusion criteria included studies involving pediatric study subjects aged 5–18 years old with a specific diagnosis of diurnal enuresis, exclusion criteria were studies before 1900 and involving night-time wetting diagnoses. A consensus among the literature and the American Academy of Family Physicians recommends a stepwise diagnostic evaluation, including history taking followed by a focused physical exam, for diurnal enuresis has proven to be the most effective. Regarding treatment, biofeedback was shown to improve symptoms in 74% of cases in one study by Wiener, while pharmacological treatment via Mirabegron (beta 3 agonist) showed a 70% improvement in one study by Fryer, but older drugs such as oxybutynin (anticholinergics) are still preferred. A multidisciplinary approach with TENS therapy, behavioral modification, biofeedback, and pharmacology can enhance effectiveness, improve reliability, and provide more successful results while minimizing the impact of diurnal enuresis on a child’s well-being. Further research is needed to optimize pharmacologic management strategies and improve adherence to increase the likelihood of reaching treatment goals.
1. Introduction
Diurnal enuresis is defined as the involuntary voiding of urine during the daytime without injury to the nervous system or urinary tract [1]. To meet the diagnostic criteria, this voiding must occur at least twice per week for three months, though some sources define it as occurring once per month over a three-month period, indicating conflicting information [2]. Additionally, children must be at least five years old, the typical age by which bladder control has developed, to be diagnosed [1]. Kuwertz-Broking and von Gontard suggest that the term “diurnal enuresis” is outdated, proposing the use of separate diagnoses for daytime urinary incontinence and nocturnal enuresis, as they represent distinct elimination disorders. Diurnal enuresis is more common in females and can be classified as either primary or secondary [1]; primary enuresis refers to cases where the patient has never achieved a dry period, while secondary enuresis is defined by the onset of urinary incontinence after at least six months of dryness [1]. If urinary incontinence occurs less than once per month, it is considered a symptom rather than a disorder, with frequent wetting defined as at least four incidents per week [2]. Understanding these classifications and nuances is essential for accurate diagnosis and treatment.
The prevalence of diurnal enuresis varies slightly across age groups and populations, with most studies considering children at either age 5 or age 7 and above. Despite the variations, multiple studies consistently indicate a decline in incidence as children grow older. Kuwertz-Broking and von Gontard found that approximately 6% of 7-year-olds experience daytime incontinence, though prevalence estimates range from 1.8% to 9% in this age group [2]. Among children aged 11–13, the prevalence drops to 0.9%. A cross-sectional study conducted in Thailand found an overall enuresis prevalence of 4.2% in children aged 5–15, with rates declining from 10% at age 5 to 1.2% at age 12, and no enuresis observed in children aged 13–15 [3]. This study also reported no significant associations with parental education, birth order, socioeconomic status, diaper use, toilet training, or behavioral and school problems. In a Turkish study involving 2750 children aged 11–14, the prevalence of diurnal incontinence was 8.6%, with a higher rate observed in males. This study also found that 45.1% of children with enuresis had immediate family members with the condition, suggesting a possible genetic link [4]. Nieuwhof-Leppink et al. reported that 7–10% of children aged 5–13 are affected by daytime incontinence, while the prevalence of overactive bladder in children aged 5–10 is 5–12% [5]. These variations highlight the need for continued research to understand underlying factors and support targeted interventions.
Analyzing diurnal enuresis is crucial for understanding and managing daytime urinary incontinence in children, a condition often overlooked. Recent studies emphasize the importance of early recognition and intervention, as it can significantly impact a child’s psychological and social well-being. The survey conducted by Dutch general practitioners highlighted that many healthcare providers struggle with proper diagnosis and treatment of daytime incontinence, indicating the need for more robust clinical protocols [6]. Additionally, the article “Daytime Urinary Incontinence” provides a comprehensive review, distinguishing between nocturnal and diurnal enuresis and noting the diverse etiologies, including bladder dysfunction and psychological factors, that must be considered in the diagnosis [1]. Furthermore, Robson et al. underscores the importance of a thorough history, physical examination, and noninvasive tests such as urinalysis and ultrasonography in determining the causes of diurnal enuresis [7]. Addressing daytime urinary incontinence early can lead to improved outcomes, with interventions focusing on both physical and behavioral therapies to manage the condition effectively, thus improving quality of life for affected children and their families.
2. Materials and Methods
Articles published from January 1900 to December 2024 were chosen through literature searches in the PubMed, Science Direct, Embase, and Google scholar were used. Search terms were “Diurnal Enuresis” or “Daytime Incontinence” as Mesh terms and subsequent terms included “pediatrics”, “urinary bladder, overactive”, and “therapeutics” to name a few. Inclusion criteria included studies involving pediatric study subjects starting at 5 years old with a specific diagnosis of diurnal enuresis, exclusion criteria were any studies before 1900 and studies involving night-time wetting diagnoses. This investigation was done asynchronously among the student authors and took place during December 2024 and February 2025. It was noted that most studies and trials regarding the diagnosis and evaluation of diurnal enuresis originated outside of the United States. GenAI was not used for data collection, analysis, or any other purposes in this review.
3. Literature Review
3.1. Etiology and Pathophysiology of Diurnal Enuresis
Diurnal enuresis seems to be a multi-factorial disorder, etiologies comprising the neurological, bowel, psychological, and behavioral systems. Linderholm postulates that this “infant bladder behavior” is due to the child’s “basic deficiency in its ability to inhibit the rhythmic uninhibited contractions” which develops as part of the neuromuscular system [8]. Looking at the treatment options offered in one study by Schulman et al. at Children’s Hospital of Philadelphia [9], it can be gathered that Linderholm’s hypothesis holds true [8], as urge incontinence, or the inability to counteract uninhibited contractions of the bladder, was the most common urodynamic finding. Hellerstein also noted that detrusor instability, or uninhibited contractions of the bladder, was the predominant diagnosis in 175 of 226 children in their study [10]. Enuresis is also known to have a familial component, Gomez Rincon says, with children who have “one affected parent face a 44% likelihood of developing enuresis, and those with 2 affected parents have a 77% likelihood.” [11]. Twin studies and familial clustering suggest polygenic heritability of bladder control mechanisms involving autonomic and cortical inhibitory pathways. Other notable causes of enuresis that Gomez Rincon lists include, small bladder capacity, fecal incontinence or constipation, neurogenic bladder, significant life stressors, diabetes, and urethral obstruction [11].
At birth, bladder function and voiding are uncontrolled, and children mature toward bladder control in a stepwise process by age 4, Gomez Rincon explains [11]. As cortical and pontine centers mature, inhibitory control over the detrusor muscle develops. Delays in this neurodevelopmental integration or immaturity of micturition reflex circuits can predispose to diurnal enuresis. The first step is to become aware of the bladder filling, which then allows the development of voluntary suppression of detrusor contractions. Gomez Rincon notes that “detrusor overactivity is most commonly associated with daytime incontinence” [11], and many others agree with this statement as aforementioned. In enuresis, children do not have the ability to suppress detrusor contractions, leading to uninhibited contractions of the bladder and eventually unwanted micturition.
Psychological factors significantly influence bladder function in children, with conditions such as anxiety, stress, and depression potentially contributing to urinary incontinence. A study by Amin et al. found that children with complex urologic disorders often experience psychological and cognitive challenges, which can adversely affect treatment outcomes for urinary incontinence [12]. Additionally, research by Koff et al. highlights a high level of comorbidity between psychological distress, anxiety, and depression in patients with neurogenic lower urinary tract dysfunction [13], underscoring the need for integrated care approaches. Furthermore, a study by Neri et al. suggests that incontinence and psychological problems in children may share a common central nervous pathway [14], indicating that addressing psychological factors could be crucial in managing urinary incontinence. Recognizing and addressing these psychological factors is essential for developing effective treatment strategies and improving the quality of life for affected children.
3.2. Complications and Comorbidities of Diurnal Enuresis
Constipation is a common comorbidity seen in pediatric patients with diurnal enuresis, and it contributes to a diagnosis of non-monosymptomatic diurnal enuresis, along with fecal incontinence [2]. Others, such as Rodríguez-Ruiz et al., consider constipation to be a significant risk factors on non-monosymptomatic diurnal enuresis [15]; nevertheless, constipation is strongly associated with all forms of enuresis, as well as diurnal enuresis [16]. Von Gontard does suggest that any form fecal incontinence or constipation should be treated prior to daytime urinary incontinence or enuresis treatment [2], which one could postulate from this suggestion that bowel dysfunction is in fact a risk factor, if it is suggested to be corrected prior to any voiding dysfunction.
Complications of diurnal enuresis impact children’s self-perception and interpersonal relationships. Children are found to have increased anxiety in disclosing their condition to both teachers and friends due to embarrassment and fear of gossip. When choosing not to disclose their symptoms, students feel they are living a facade leading to feelings of isolation. In response, some choose to avoid making new friends or participating in school activities. As the management of diurnal enuresis requires frequent toileting trips, students report interruptions in learning and test taking. This results in more long-term consequences, including test anxiety and diminished confidence in pursuing higher education due to inability to sit for long standardized exams [17]. Research on parental impact is limited, mostly focusing on nocturnal enuresis. Studies suggest strained mother-child relationships due to harsh language or shame used during nighttime accidents. This was found to shift the mother-child relationship from secure to insecure attachment [18]. Further research is needed to explore parental challenges specific to diurnal enuresis. A summary of the diagnostic criteria, etiology, epidemiology, and complications of diurnal enuresis can be found in Figure 1 below.
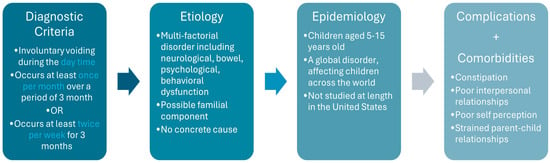
Figure 1.
Summary of Diurnal Enuresis.
3.3. Diagnostic Approaches to Daytime Wetting in Pediatric Patients
The American Academy of Family Physicians (AAFP) has published evidence rating recommendations for clinical practice of nocturnal enuresis but not diurnal enuresis [19]. The AAFP guidelines for evaluating diurnal enuresis begins with an enuresis-specific history and a physical examination of the rectum, genitalia, abdomen, spine, nose, ears, throat, abdomen, and a focused neurologic examination [19]. Additionally, the guidelines emphasize identifying any red flag symptoms and performing a urinalysis [19]. If red flags are present or the patient does not respond to initial treatment, secondary causes of incontinence are suspected or further imaging studies—such as renal and bladder ultrasound, voiding cystourethrography, MRI of the lumbosacral spine, and urodynamic studies—are recommended [20]. Based on imaging or urodynamic findings, the AAFP suggests referral to a subspecialist if an underlying medical condition is identified or recommends subspecialist management with biofeedback and a voiding regimen if dysfunctional voiding is suspected [19].
The AAFP recommends treatments with biofeedback and medications such as Oxybutynin and Desmopressin [19]. Additionally, these guidelines align with those from Italy regarding the general pediatrician’s workup. Italian guidelines differentiate between diurnal incontinence with urgency and without urgency [20]. For incontinence with urgency, the recommendations begin with oxybutynin, followed by TENS therapy or botulinum toxin A treatment; for incontinence without urgency, secondary medical conditions are identified and treatment options such as biofeedback, alpha-blocker medications, urotherapy, intermittent catheterization, or surgery are recommended based on the specific condition [20].
3.4. Non-Pharmacological Management Options for Diurnal Enuresis
Initial treatment for diurnal enuresis emphasizes conservative options, including behavioral modifications and biofeedback. Wiener found that behavioral strategies such as timed voiding, fluid intake adjustments, and voiding diaries improve symptoms in approximately 74% of cases after one year [21]. However, a downfall can be argued as these methods are labor intensive and require a high level of motivation in both parents and children [22]. Biofeedback enhances treatment by using electrode pads to measure pelvic floor muscle function, converting data into an interactive game where children control animations through muscle contractions. Symptom improvement is observed in at least half of patients, particularly those over nine years old [23]. However, biofeedback’s invasiveness, requiring perineal electrodes or anal plugs, remains a drawback [21]. The absence of standardized guidelines contributes to variability in treatment, often leading to overuse of anticholinergics as a first-line option [22]. Tailoring treatment based on individual diagnostic factors such as bladder capacity and voiding patterns may optimize outcomes and reduce unnecessary pharmacologic use [24]. Further research and consensus are needed to develop clearer guidelines that balance efficacy, feasibility, and patient-centered care in the treatment of diurnal enuresis.
Osteopathic manipulative treatment can be an effective approach for patients with various medical conditions. A randomized, prospective clinical trial by Ibrahim Elgohary et al. evaluated the effectiveness of combining osteopathic techniques with biofeedback and behavioral modification to treat functional daytime urinary incontinence (FDTUI) [25]. The study randomly assigned 117 children, aged 6 to 16 years, into three groups: Group A received biofeedback, osteopathic techniques, and behavioral training; Group B received biofeedback and behavioral training; and Group C received osteopathic techniques and behavioral training [25]. The study implemented interventions for 10 weeks, followed by an 8-week follow-up period [25]. Urinary incontinence severity was assessed using a 24 h pad test, lower urinary tract symptoms (LUTS) were evaluated with the Pediatric Lower Urinary Tract Scoring System (PLUTSS) questionnaire, and parameters such as voiding frequency, voiding volume, and leakage episodes were recorded in a bladder diary [25]. Visceral mobility was improved using visceral, articular, myofascial, and high-velocity low-amplitude techniques, which included stretching the greater omentum, inhibiting psoas muscle hypertonicity, massaging the obturator foramen, and treating the T10-L2 spinal region and sacroiliac joint [25]. Superficial electrodes and EMG-guided biofeedback on pelvic floor and hip flexor muscles were used for biofeedback treatment, and patients and their parents received education on anatomy, bladder diaries, and therapies for behavioral training adjustments [25]. While all three groups showed decreases in PLUTSS and pad test scores after the intervention, Group A, which received biofeedback with osteopathic techniques and behavioral training, experienced the greatest improvement [25]. The combined treatment approach of biofeedback, osteopathic techniques, and behavioral modifications was the most effective in reducing urinary incontinence symptoms, suggesting that biofeedback may have the strongest impact on bladder control [25]. Although these findings support the use of OMT, it is only in the context as an adjunctive approach, not a standard or guideline-endorsed therapy. Evidence is limited to one randomized controlled trial suggesting potential benefit, and this is not enough to confirm that OMT should be considered standard of care for management of diurnal enuresis. Further randomized controlled studies would be required to assess the efficacy of OMT monotherapy versus OMT as continued adjunctive therapy. However, these findings do support a holistic, integrative approach to managing FDTUI and highlight the need for further research on combination therapies, including osteopathic techniques, to optimize treatment strategies.
The clinical trial by Ibrahim Elgohary et al. treated the sacroiliac joint, psoas muscle, lower thoracic and upper lumbar vertebrae, and obturator foramen with OMT, as these are the viscero-somatic reflexes that help guide treatment for urinary incontinence [25]. Viscero-somatic reflexes are interactions between the spinal cord, soft tissues, and viscera that manifest as somatic findings; osteopathic physicians can treat these reflexes to address underlying visceral dysfunctions in the body [26]. Since the early 1900s, osteopathic physicians have studied and treated viscero-somatic reflexes, which are commonly referred to by allopathic physicians as central sensitization. Central sensitization occurs when the central nervous system undergoes functional, structural, and chemical changes, altering its response to sensory stimuli and pain [27]. When these changes occur, physicians can diagnose and treat dysfunctions that present as somatic findings, ultimately addressing underlying visceral dysfunctions. Osteopathic techniques can help restore function in the body and serve as a valuable adjunct therapy in managing diurnal enuresis in children.
3.5. Pharmacological Management Options for Diurnal Enuresis
Pharmacologic treatment is often necessary for children with severe overactive bladder (OAB) who do not achieve dryness with behavioral and non-pharmacologic intervention [28]. A typical micturition cycle relies on the coordinated activity of the sympathetic and parasympathetic nervous systems. The sensation of urinary urgency arises from a decrease in sympathetic tone or an increase in parasympathetic activity. By targeting these pathways, pharmacologic agents can modulate bladder function to improve urinary continence [29].
The primary pharmacologic approach involves the use of muscarinic receptor blockers or anticholinergic drugs, which inhibit parasympathetic activity to reduce detrusor muscle overactivity. Additionally, beta-3 adrenergic agonists can enhance bladder storage by acting on the predominant beta-3 receptors in the bladder’s smooth muscle. Although alpha-adrenergic agonists theoretically support bladder storage by activating the internal sphincter, they are rarely used in clinical practice due to their widespread systemic vascular effects [29]. The most common side effects associated with antimuscarinic drugs include xerostomia, dry eyes, dry skin, constipation, dizziness, insomnia, and blurred vision [29]. These adverse effects are a common reason for discontinuing treatment. It is important to emphasize that conservative treatment options should be attempted first and reinforced at every physician’s visit. Additionally, finding the ideal medication and dosage may require several trials to maximize therapeutic benefit while minimizing side effects in the long-term management of diurnal enuresis.
3.5.1. Oxybutynin
Oxybutynin is an anticholinergic agent that relaxes the bladder muscle, reducing urinary urgency and frequency. It is commonly prescribed in pediatric patients, available in both immediate-release (IR) and extended-release (ER) formulations. Studies have demonstrated that ER oxybutynin is more effective than the IR form for managing diurnal enuresis [30]. However, adherence can be a challenge due to side effects such as dry mouth and constipation. Additionally, this medication must be swallowed intact, posing an obstacle for some children who cannot swallow pills whole. While intravesical instillation allows for higher dosing with fewer systemic effects, this method is impractical for most pediatric patients who do not use intermittent catheters and cannot sit still long enough for voluntary catheter placement. Furthermore, a significant proportion of children do not achieve sufficient symptom relief with this medication, leading to high rates of treatment discontinuation.
3.5.2. Tolterodine
Tolterodine is another anticholinergic medication used to treat detrusor overactivity and OAB [31]. A study by Reinberg et al. compared different formulations of tolterodine and oxybutynin, concluding that ER oxybutynin chloride was more effective than long-acting tolterodine for managing diurnal urinary incontinence [32]. As a result, oxybutynin is generally recommended as the first-line treatment, with tolterodine reserved for cases where oxybutynin is inadequate or poorly tolerated [32].
3.5.3. Terodiline
Terodiline is an anticholinergic agent with additional calcium antagonist properties, which contribute to its ability to reduce abnormal bladder contractions [33]. A double-blind study with 43 children demonstrated that a 25 mg dose of terodiline was well tolerated and effective for treating diurnal enuresis, with a low incidence of adverse effects [34].
3.5.4. Fesoterodine
Fesoterodine is a long-acting antimuscarinic agent that can be given to children weighing over 25 kg [29]. However, limited data exists regarding its efficacy specifically for diurnal enuresis in pediatric patients. Further research is needed to determine its effectiveness and safety in this population. The primary side effect observed was a slight increase in heart rate [35].
3.5.5. Solifenacin
Solifenacin is a competitive M3 receptor antagonist with a long half-life (45–68 h) and is commonly used to manage OAB and urgency incontinence [36]. In a study by Hu and Zhang, Solifenacin combined with biofeedback produced encouraging results in children with OAB, with over 50% of participants experiencing symptom improvement within two weeks [37]. However, most studies on Solifenacin focus on its use in OAB rather than diurnal enuresis, and research predominantly involves adult populations, necessitating the need for pediatric-specific studies.
3.5.6. Propiverine
Propiverine is an antimuscarinic, L-Type calcium channel blocker and alpha 1 adrenoceptor antagonist medication that can be used to treat OAB [38]. A randomized, double-blind, placebo-controlled clinical trial found that Propiverine was significantly more effective than placebo in reducing urinary incontinence in children with OAB. Additionally, it was well tolerated, with no notable side effects reported [39].
3.5.7. Imipramine
Imipramine is a tertiary amine tricyclic antidepressant that exerts its effects by inhibiting the reuptake of norepinephrine and serotonin, with a stronger affinity for serotonin. Its anticholinergic properties contribute to its effectiveness in managing enuresis [40]. Franco et al. found that two-thirds of children with refractory daytime incontinence responded to imipramine treatment. However, they emphasized that the primary management approach remains a structured voiding regimen, with pharmacologic intervention serving as an adjunct for difficult cases [41].
3.5.8. Mirabegron
Mirabegron is a beta-3 adrenoceptor agonist that enhances bladder storage by relaxing the detrusor muscle [42]. Fryer et al. found that mirabegron improved symptoms in 70% of pediatric patients with refractory OAB [43]. In a comparative study assessing the efficacy of mirabegron versus Solifenacin in children with idiopathic OAB, researchers found that mirabegron was equally effective, with minimal adverse effects reported. Given its favorable tolerability profile, mirabegron represents a promising alternative to antimuscarinic agents, particularly for children who experience intolerable side effects from traditional treatments [44].
Antimuscarinic agents, particularly oxybutynin and tolterodine, remain the primary pharmacologic options for managing pediatric diurnal enuresis. Despite their efficacy, these medications are often discontinued due to side effects such as dry mouth and constipation. Emerging therapies, including newer antimuscarinics, such as Solifenacin, may offer improved safety and tolerability. Additionally, beta-3 adrenergic agonists such as mirabegron have demonstrated effectiveness with fewer adverse effects, positioning them as viable alternatives. A summary of the aforementioned mechanisms of actions, efficacy, and side effects can be seen in Table 1 below.

Table 1.
Summary of Pharmacological Management.
4. Conclusions
Diurnal enuresis is the involuntary voiding of urine during waking hours without any obvious injury to the nervous system or urinary tract [1]. Diagnosis requires voiding occurring at least twice per week for a period of three months in a child that is at least five years old, although some sources say that it should occur once per month for a period of three months [2].
The literature has proven that a stepwise diagnostic process for the evaluation of diurnal enuresis has proven to be the most effective. The AAFP recommends history-taking to be the first step, with evaluation of any medical diagnosis, followed by a focused and pertinent physical exam. Further evaluation of anatomical structures by way of diagnostic imaging or urodynamic studies can be done in the setting of first line treatment failure or alarm symptoms. Depending on the compiled findings of these studies, referral to a subspecialist for treatment of an underlying condition or management with biofeedback and voiding regimen is recommended [19].
Effective management options come two-fold—suggested first-line therapies are conservative and include biofeedback therapy and behavioral modifications, while second- and third-line therapies include pharmacological management. Osteopathic manipulative treatment is not necessarily widely recommended, but it has proven to be an effective method in the management of FDTUI in one random controlled study by improving visceral mobility, inhibition of muscle hypertonicity, and overall improving autonomics to the bladder for mitigation of symptoms [25]. It would be remiss to not mention this ground-breaking discovery in the hopes to one day apply osteopathic techniques to diurnal enuresis. While behavioral strategies are shown to improve symptoms in 74% of cases in one study, it is often insufficient to provide noticeable or long-lasting relief from symptoms leading to the necessary use of pharmacological intervention [21].
Anticholinergics are the primary drug of choice, as they work to inhibit the muscarinic receptors and in turn, inhibit the act of diuresis. While anticholinergics tend to have multiple setbacks, including the necessitation for children to swallow tablets, their major side effects, and adherence to regimen, they have proven to be the most effective treatment for diurnal enuresis when combined with the non-pharmacological options. Oxybutynin and tolterodine are older anticholinergics that have proved effective in the management of diurnal enuresis, with the former being the drug of choice. Additionally, Mirabegron, a beta 3 agonist, has proven to be both effective and less likely to cause bothersome side effects, with 70% improvement in one study [43]. However, it is still too new to say whether beta 3 agonists are preferred over antimuscarinics; therefore, anticholinergics remain the preferred method of pharmacological management of diurnal enuresis. Further research is needed to optimize pharmacologic management strategies, particularly in pediatric populations, to improve adherence and increase the likelihood of reaching treatment goals.
Diurnal enuresis can significantly affect a child’s social, physical, emotional, and psychological well-being. Current treatment options include TENS therapy, behavioral modifications, biofeedback, medication management, and osteopathic techniques. While these methods have shown some success individually in managing daytime urinary incontinence, combining them may lead to even better outcomes. Figure 2 below illustrates the overall management of diurnal enuresis. Ultimately, a multidisciplinary approach that integrates these treatments can enhance effectiveness, improve reliability, and provide more successful results while minimizing the impact of diurnal enuresis on a child’s overall well-being.
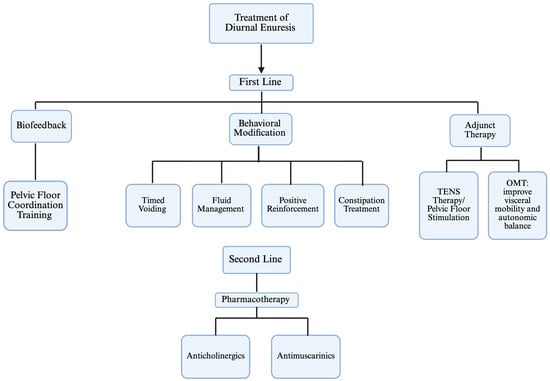
Figure 2.
Diurnal Enuresis Therapy Algorithm.
Author Contributions
Conceptualization: A.D., C.L., V.V., R.M., Z.F., H.F. and B.B.; Methodology, A.D., C.L., V.V., R.M., Z.F., H.F. and B.B.; Data Curation, A.D., C.L., V.V., R.M., Z.F., H.F. and B.B.; Writing—Original Draft Preparation, A.D., C.L., V.V., R.M., Z.F., H.F. and B.B.; Writing—Review and Editing, A.D., Supervision, H.F. and B.B.; Project Administration, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bereda, G. Enuresis: Nocturnal Enuresis and Diurnal Enuresis. Arch. Clin. Investig. 2022, 1, 1–3. [Google Scholar] [CrossRef] [PubMed]
- von Gontard, A.; Kuwertz-Bröking, E. The diagnosis and treatment of enuresis and functional daytime urinary incontinence. Dtsch. Aerzteblatt Online 2019, 116, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hansakunachai, T.; Ruangdaraganon, N.; Udomsubpayakul, U.; Sombuntham, T.; Kotchabhakdi, N. Epidemiology of Enuresis Among School-Age Children in Thailand. J. Dev. Behav. Pediatr. 2005, 26, 356. [Google Scholar] [CrossRef]
- Savaser, S.; Beji, N.K.; Aslan, E.; Gozen, D. The Prevalence of Diurnal Urinary Incontinence and Enuresis and Quality of Life: Sample of School. Urol. J. 2018, 15, 173–179. [Google Scholar] [PubMed]
- Nieuwhof-Leppink, A.J.; Schroeder, R.P.J.; van de Putte, E.M.; de Jong, T.P.V.M.; Schappin, R. Daytime urinary incontinence in children and adolescents. Lancet Child Adolesc. Health 2019, 3, 492–501. [Google Scholar] [CrossRef]
- Oldenhod, A.P.; Linde, J.M.; Hofmeester, I.; Steffens, M.G.; Kloosterman-Eijgenraam, F.J.; Blanker, M.H. Managing children with daytime urinary incontinence: A survey of Dutch general practitioners. Neurourol. Urodyn. 2022, 41, 234–240. [Google Scholar]
- Robson, L.M. Diurnal enuresis. Pediatr. Rev. 1997, 18, 407–412. [Google Scholar] [CrossRef]
- Linderholm, B.E. The Cystometric Findings in Enuresis. J. Urol. 1966, 96, 718–722. [Google Scholar] [CrossRef]
- Schulman, S.L.; Quinn, C.K.; Plachter, N.; Kodman-Jones, C. Comprehensive Management of Dysfunctional Voiding. Pediatrics 1999, 103, e31. [Google Scholar] [CrossRef]
- Hellerstein, S.; Linebarger, J.S. Voiding dysfunction in pediatric patients. Clin. Pediatr. 2003, 42, 43–49. [Google Scholar] [CrossRef]
- Gomez Rincon, M.; Leslie, S.W.; Daley, S. Enuresis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Heymen, S. Psychological and cognitive variables affecting treatment outcomes for urinary and fecal incontinence. Gastroenterology 2004, 126, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Sebesta, E.M.; Connors, E.L.; Rourke, E.; Reynolds, W.S.; McKernan, L.C. Psychosocial factors in neurogenic lower urinary tract dysfunction. Pediatr. Nephrol. 2021, 35, 1567–1575. [Google Scholar]
- Van Herzeele, C.; Walle, J.V. Incontinence and psychological problems in children: A common central nervous pathway? Pediatr. Nephrol. 2016, 31, 689–692. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, M.; Mendez-Gallart, R.; García Mérida, M.; Somoza-Argibay, I. Influence of constipation on enuresis. An. Pediatría (Engl. Ed.) 2021, 95, 108–115. [Google Scholar] [CrossRef] [PubMed]
- McGrath, K.H.; Caldwell, P.H.; Jones, M.P. The frequency of constipation in children with nocturnal enuresis: A comparison with parental reporting. J. Paediatr. Child Health 2008, 44, 19–27. [Google Scholar] [CrossRef]
- Whale, K.; Cramer, H.; Joinson, C. Left behind and left out: The impact of the school environment on young people with continence problems. Br. J. Health Psychol. 2018, 23, 253–277. [Google Scholar] [CrossRef]
- Yitik Tonkaz, G.; Deliağa, H.; Çakir, A.; Tonkaz, G.; Özyurt, G. An evaluation of parental attitudes and attachment in children with primary monosymptomatic nocturnal enuresis: A case-control study. J. Pediatr. Urol. 2023, 19, 174.e1–174.e5. [Google Scholar] [CrossRef]
- Ramakrishnan, K. Evaluation and treatment of enuresis. Am. Fam. Physician 2008, 78, 489–496. [Google Scholar]
- Palma, P.L.; Marzuillo, P.; Di Sessa, A.; Guarino, S.; Capalbo, D.; Marrapodi, M.M.; Buccella, G.; Cameli, S.; Miraglia del Giudice, E.; Torella, M.; et al. From Clinical Scenarios to the Management of Lower Urinary Tract Symptoms in Children: A Focus for the General Pediatrician. Healthcare 2023, 11, 1285. [Google Scholar] [CrossRef]
- Wiener, J.S.; Scales, M.T.; Hampton, J.; King, L.R.; Surwit, R.; Edwards, C.L. Long-term efficacy of simple behavioral therapy for daytime wetting in children. J. Urol. 2000, 164, 786–790. [Google Scholar] [CrossRef]
- Allen, H.A.; Austin, J.C.; Boyt, M.A.; Hawtrey, C.E.; Cooper, C.S. Initial trial of timed voiding is warranted for all children with daytime incontinence. Urology 2007, 69, 962–965. [Google Scholar] [CrossRef]
- Reddy, S.M.; Gray, H.; Barry, T.; Bessell, B.; Shalaby, M.; Woodward, M.; Awad, K. Efficacy of Biofeedback in Paediatric Urology Patients: A Single Centre Experience. J. Pediatr. Surg. 2024, 59, 295–298. [Google Scholar] [CrossRef]
- Rhodes, C. Effective management of daytime wetting. Paediatr. Nurs. 2000, 12, 14–17. [Google Scholar] [CrossRef]
- Ibrahim Elgohary, H.M.; Elfahl, A.M.; Elkholy, M.N. The combined effect of biofeedback training and osteopathic procedures for the treatment of functional daytime urinary incontinence: A prospective, randomized clinical trial. J. Bodyw. Mov. Ther. 2025, 42, 289–296. [Google Scholar] [CrossRef]
- Burns, L. Viscero-somatic and somato-visceral spinal reflexes. J. Am. Osteopath. Assoc. 1907, 100, 249–258. [Google Scholar]
- Volcheck, M.M.; Graham, S.M.; Fleming, K.C.; Mohabbat, A.B.; Luedtke, C.A. Central sensitization, chronic pain, and other symptoms: Better understanding, better management. Clevel. Clin. J. Med. 2023, 90, 245–254. [Google Scholar] [CrossRef]
- Jessen, A.S.; Hagstroem, S.; Borch, L. Comparison and characteristics of children successfully treated for daytime urinary incontinence. J. Pediatr. Urol. 2022, 18, 24.e1–24.e9. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Oh, M.M. Updates of Overactive Bladder in Pediatrics. Int. Neurourol. J. 2023, 27, 3–14. [Google Scholar] [CrossRef]
- Van Arendonk, K.J.; Knudson, M.J.; Austin, J.C.; Cooper, C.S. Improved Efficacy of Extended Release Oxybutynin in Children with Persistent Daytime Urinary Incontinence Converted from Regular Oxybutynin. Urology 2006, 68, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Wefer, J.; Truss, M.C.; Jonas, U. Tolterodine: An Overview. World J. Urol. 2001, 19, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Reinberg, Y.; Crocker, J.; Wolpert, J.; Vandersteen, D. Therapeutic Efficacy of Extended Release Oxybutynin Chloride, and Immediate Release and Long Acting Tolterodine Tartrate in Children with Diurnal Urinary Incontinence. J. Urol. 2003, 69, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Langtry, H.D.; McTavish, D. Terodiline. Drugs 1990, 40, 748–761. [Google Scholar] [CrossRef]
- Elmér, M.; Nørgaard, J.P.; Djurhuus, J.C.; Adolfsson, T. Terodiline in the Treatment of Diurnal Enuresis in Children. Scand. J. Prim. Health Care 1988, 6, 119–124. [Google Scholar] [CrossRef]
- Ramsay, S.; Naud, É.; Simonyan, D.; Moore, K.; Bolduc, S. A randomized, crossover trial comparing the efficacy and safety of fesoterotidine and oxybutynin extended-release in children with overactive bladder: The FOXY study. Can. Urol. Assoc. J. 2020, 14, E59–E64. [Google Scholar] [CrossRef]
- Kelleher, C. A Review of Solifenacin in the Treatment of Urinary Incontinence. Ther. Clin. Risk Manag. 2008, 4, 117–128. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, H. Efficacy and Safety of Solifenacin Combined with Biofeedback in Children with Overactive Bladder. BMC Urol. 2024, 24, 97. [Google Scholar] [CrossRef]
- Wuest, M.; Witte, L.P.; Michel-Reher, M.B.; Propping, S.; Braeter, M.; Strugala, G.J.; Wirth, M.P.; Michel, M.C.; Ravens, U. The muscarinic receptor antagonist Propiverine exhibits α(1)-adrenoceptor antagonism in human prostate and porcine trigonum. World J. Urol. 2011, 29, 149–155. [Google Scholar] [CrossRef]
- Marschall-Kehrel, D.; Feustel, C.; de Geeter, C.P.; Stehr, M.; Radmayr, C.; Sillén, U.; Strugala, G. Treatment with Propiverine in Children Suffering from Nonneurogenic Overactive Bladder and Urinary Incontinence: Results of a Randomized Placebo-Controlled Phase 3 Clinical Trial. Eur. Urol. 2009, 55, 729–738. [Google Scholar] [CrossRef]
- Fayez, R.; Gupta, V. Imipramine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Franco, I.; Arlen, A.M.; Collett-Gardere, T.; Zelkovic, P.F. Imipramine for Refractory Daytime Incontinence in the Pediatric Population. J. Pediatr. Urol. 2018, 14, 58.e1–58.e5. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.R. Randomized Double-Blind, Active-Controlled Phase 3 Study to Assess 12-Month Safety and Efficacy of Mirabegron, a β3-Adrenoceptor Agonist, in Overactive Bladder. Eur. Urol. 2013, 63, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Fryer, S.; Nicoara, C.; Dobson, E.; Griffiths, M.; McAndrew, H.F.; Kenny, S.E.; Corbett, H.J. Effectiveness and Tolerability of Mirabegron in Children with Overactive Bladder: A Retrospective Pilot Study. J. Pediatr. Surg. 2020, 55, 316–318. [Google Scholar] [CrossRef]
- Chapple, C.R. Mirabegron in Overactive Bladder: A Review of Efficacy, Safety, and Tolerability. Neurourol. Urodyn. 2013, 33, 17–30. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).