Abstract
Background: Phosphodiesterase type 5 inhibitors (PDE5i), particularly tadalafil and sildenafil, are the first-line therapies for erectile dysfunction (ED). After the patent expiration of branded tadalafil in 2017, generic formulations became available. Despite equivalent efficacy, skepticism persists regarding the effectiveness and safety of generics. The SHIFT study aimed to evaluate the non-inferiority of a generic tadalafil (Dalerpen) compared with branded and other generic tadalafil in terms of clinical efficacy and patient satisfaction. Methods: A prospective, multicenter study was conducted involving 247 patients treated with tadalafil (either 5 mg or 20 mg) for ED. Patients switched from branded or other generic tadalafil to Dalerpen. Baseline and follow-up assessments included the International Index of Erectile Function—Erectile Function Domain (IIEF-EF) (primary endpoint), Sexual Encounter Profile (SEP-2 and SEP-3), and International Prostatic Symptom Score (IPSS). A one-month follow-up was performed. Results: A total of 247 patients were included in the final analysis. After switching to Dalerpen, significant improvements were observed in both IIEF-EF (18.8 ± 5.6 vs. 16.7 ± 5.4, p < 0.001) and IPSS scores (10.4 ± 6.7 vs. 11.2 ± 6.3, p < 0.001), though the minimal clinically important difference (MCID) was not reached. SEP-3 scores also significantly increased (3 ± 1.2 vs. 2 ± 1.1, p < 0.001). Multivariate analysis identified baseline IIEF, IPSS scores, and post-treatment IPSS as predictors of IIEF-EF improvement (p < 0.001). Switching to Dalerpen was an independent predictor of both IIEF-EF and IPSS improvement. No new adverse events were reported. Conclusions: The SHIFT study demonstrates that Dalerpen is non-inferior to branded tadalafil in terms of clinical efficacy, offering a reliable and cost-effective therapeutic option. Educating patients on bioequivalence and addressing concerns regarding generic drugs are essential to facilitate therapeutic switches.
1. Introduction
Erectile dysfunction (ED) is defined as the consistent inability to attain and maintain a penile erection sufficient for satisfactory sexual performance [1].
In Italy, approximately 3 million individuals are affected by ED [2]. These data derive from an epidemiological study conducted on 2000 subjects, which reported an overall prevalence of ED of 12.8%. The study also confirmed a significant increase in ED prevalence with advancing age (2% between 18 and 30 years and 48% over 70 years).
The introduction of phosphodiesterase type 5 inhibitors (PDE5is) has revolutionized the treatment of erectile dysfunction (ED). To date, these drugs represent the first-line therapy, demonstrating efficacy in over 80% of unselected patients [3,4]. Sildenafil and tadalafil are the most commonly used PDE5is [5], both of which have shown comparable efficacy in treating ED. A recent study [6] from a large online prescription platform (OPP) confirmed a preference for tadalafil over sildenafil among patients who had tested both PDE5is.
In 2002, tadalafil was introduced to the Italian market as Cialis (Ely Lilly, Indianapolis, US; 2003). After the patent expired in 2017, several equivalent generic drugs became available. Generic medications have the potential to significantly reduce healthcare costs compared with their branded counterparts. The increased utilization of generic drugs at the prescribing and dispensing levels represents a viable strategy to contain rising pharmaceutical expenditures. Cost savings associated with generic substitution have been linked to improved medication adherence, with potential downstream benefits on clinical outcomes. However, their adoption is often hampered by scepticism among both physicians and patients regarding their effectiveness and safety. Clinicians may harbour reservations about generics. In a large-scale survey, approximately 23% questioned the efficacy of generic drugs, and nearly half expressed concerns about their quality [7]. These beliefs can influence prescribing behaviour and reporting practices, thereby affecting both the actual and perceived safety and effectiveness of generics. Concerns persist among patients regarding the perceived association between lower cost and reduced quality of generics. For example, patients with negative views toward generics may be more inclined to report adverse events, introducing a perception-related bias into post-market data. This feeling often arises from a lack of understanding or misinformation about bioequivalence, as well as marketing strategies employed by branded drug manufacturers. A bioequivalent drug is a medication that contains the same qualitative and quantitative amount of the active substance (thus containing the same quantity and quality of the active ingredient), the same pharmaceutical form as the reference medicine, and an identical release method (pharmaceutical equivalent). Bioequivalence is demonstrated through appropriate bioavailability studies. To determine bioequivalence after a single dose, the parameters to be analysed are AUC (0-t), or, when relevant, AUC (0–72 h) and Cmax. For these parameters, the 90% confidence interval for the ratio of the test is considered. To be inside the acceptance interval, the lower bound should be ≥80.00% when rounded to two decimal places and the upper bound should be ≤125.00% when rounded to two decimal places [8]. Critics often argue that, while pharmaceutical companies are required to undertake extensive clinical trials for branded drugs, generic drug manufacturers only need to prove bioequivalence, rather than clinical outcome equivalence. Therapeutic equivalence is not directly tested, but is presumed based on tests of bioequivalence. Regulatory efforts should therefore aim to minimize the risk of therapeutic nonequivalence without undermining the economic advantages of generic formulations. While bioequivalent generics approved by the regulator and manufactured according to regulatory standards have not demonstrated evidence of therapeutic divergence, a residual risk of nonequivalence remains when switching from originator products to generics or between generics. Such discrepancies are more readily detectable in institutional settings than in routine care, where therapeutic failure may be misattributed to disease progression [9].
Randomized controlled trials comparing generic and branded drugs are rarely conducted for economical, marketing and clinical reasons. In the absence of these trials, retrospective data remain the main tool to obtain clinical information on the outcomes of generic medications.
To our knowledge, no prospective trial comparing generic and branded tadalafil has been performed. These premises guided the SHIFT study, aimed at evaluating the non-inferiority of treatment with a generic tadalafil (Dalerpen, EcuPharma srl, Milan, Italy, 2019) compared with other tadalafil, including the branded one (Cialis), in terms of clinical efficacy.
2. Materials and Methods
We designed a non-randomized, self-controlled, observational, prospective study. Patients were recruited by 35 expert andrologists affiliated with the Italian Society of Andrology (SIA), evenly distributed across Italy, between March 2023 and October 2023.
Informed consent was obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Regione Calabria (Sezione Area Centro, protocol code n.23 on 20 January 2022).
Consecutive patients over 18 years of age who had been treated with another tadalafil formulation (not Dalerpen) at 20 mg or 5 mg for ED for at least three months were included. Patients taking tadalafil either daily or as needed were eligible. Subjects were required to declare that they were sexually active, engaging in at least one intercourse per week, while taking the prescribed therapy. Exclusion criteria included the use of other medications or devices for the treatment of ED, the use of tadalafil 5 mg for benign prostatic hyperplasia without ED, any severe adverse events previously reported during tadalafil use, absolute contraindications to PDE5 inhibitors, hypogonadism, Peyronie’s disease, phimosis, balanoposthitis, or contraindications to sexual activity.
At the baseline visit, all patients were offered the option to switch from their current tadalafil formulation to Dalerpen at the same dosage and regimen previously taken, regardless of perceived effectiveness or side effects. Patients were not influenced in their decision to switch, and declining the change did not affect the therapeutic relationship. The follow-up visit was conducted 30 days after the therapy switch.
During the baseline visit, patients underwent a thorough medical history, a complete andrological physical examination, and blood tests to assess lipid profile, glycemic profile, total testosterone, and total PSA. They also completed the International Index of Erectile Function—Erectile Function Domain (IIEF-EF), Sexual Encounter Profile (SEP-2 and SEP-3), and International Prostatic Symptom Score (IPSS) questionnaires. At follow-up, patients repeated the blood tests and questionnaires, and responded to a closed-ended question regarding their satisfaction (“What is your perception of the impact of the therapy switch on your erectile function?”: Improvement, Stability, or Worsening). Andrologists were also asked to identify, during patient interviews, any factors that might hinder or facilitate the therapy change. All adverse events related to tadalafil use before and after the switch were recorded. The IIEF-EF was chosen as the primary outcome.
Statistics
The Shapiro–Wilk test was used to assess the normality of data distribution. Quantitative variables were reported as means and standard deviations (SDs), while qualitative variables were presented as absolute and relative frequencies. Based on the previous literature, a minimal clinically important difference (MCID) of 4 points in IIEF-EF was assumed. A conservative non-inferiority margin (Δ) of 3 points was selected, representing the maximum acceptable loss of efficacy after switching to Dalerpen. Assuming a standard deviation of 6 points for the within-subject difference and an intra-subject correlation of 0.5, the adjusted standard deviation for the paired analysis was estimated at 6.0.
With α = 0.05 and 80% power, a minimum of 26 patients per subgroup was required to demonstrate non-inferiority. Paired t-tests were used to compare continuous variables within the same group at different time points, while unpaired t-tests or one-way ANOVA were used to compare continuous variables between groups. Categorical variables were compared using the chi-squared test. Multivariate linear regression models were applied to identify independent predictors of changes in IIEF-EF and IPSS scores. A p-value < 0.05 was considered statistically significant. All analyses were performed using RStudio version 2024.12.1+563.
3. Results
A total of 247 patients completed the study. Of these, 126 (51%) were treated with tadalafil at 5 mg, whereas 121 (49%) received tadalafil at 20 mg. The switch was made from both branded and other generic tadalafil, as shown in Table 1.

Table 1.
Type of tadalafil treatment at baseline.
The mean age of patients was 61.2 ± 10.5 years. The baseline characteristics of enrolled subjects are listed in Table 2.

Table 2.
Baseline characteristics of enrolled patients.
At the one-month follow-up after the therapy switch, IIEF-EF’s mean and standard deviation improved compared with the baseline (18.8 ± 5.6 vs. 16.7 ± 5.4; p < 0.001). The IPSS scores were significantly reduced compared with the baseline (vs 10.4 ± 6.7 vs 11.2 ± 6.3; p < 0.001), but this reduction was not clinically meaningful (Table 3 and Figure 1). The MCID was not reached for both questionnaires. SEP-3 also showed a significant increase after treatment (3 ± 1.2 vs. 2 ± 1.1 p < 0.001). No new adverse events have been reported in the study group.

Table 3.
Main results: baseline vs. 1 month after therapy switch.
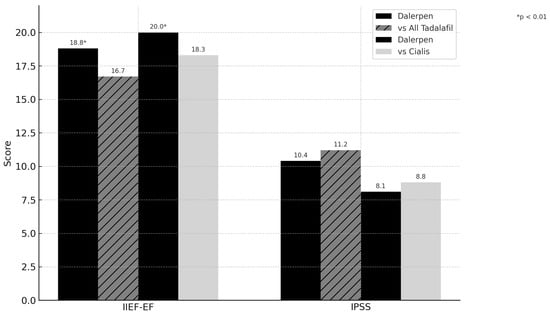
Figure 1.
IIEF-EF and IPSS changes after 1 month follow-up.
A sub-analysis of patients who switched from the branded drug revealed a significant increase in IIEF-EF (20.0 ± 5.6 vs. 18.3 ± 5.5 p < 0.001), whereas no changes in IPSS score were observed (8.1 ± 6.1 vs. 8.8 ± 5.9 p = 0.018) (Figure 1). Multivariate analysis showed that baseline IIEF and IPSS scores, as well as post-treatment IPSS, were independent predictors of IIEF-EF improvement (p < 0.001). Baseline and post-treatment PSA and IIEF-EF after therapy were independent predictors of IPSS improvement (p < 0.001). Switching treatment to Dalerpen was found to be an independent predictor for both IIEF-EF and IPSS improvement, but no correlation was found between the type of tadalafil used at baseline and changes in the questionnaires (p > 0.05).
Overall, 89% of subjects reported improvement or stability in erectile function one month after the therapy switch. Patient satisfaction regarding the therapy switch is summarized in Table 4.

Table 4.
Patient satisfaction after therapy switch.
Barriers to therapy switch were scepticism about changing an effective therapy (60%), the habit of using a specific drug (22%), fear of adverse events with the new drug (18%), and reluctance to continue therapy with tadalafil due to previous inefficacy or side-effects with same drug (10%). Patients dissatisfied with the current treatment, experiencing adverse reactions, or motivated by cost reduction (particularly when choosing a generic formulation over a branded drug) are identified as optimal candidates for therapeutic switch. Explaining the characteristics of the prescribed generic drug in terms of efficacy, administration modalities, and side-effects, illustrating the concept of bioequivalence, and addressing the economic issues related to chronic tadalafil therapy were considered fundamental aspects of patient counselling for favouring the switch.
4. Discussion
The SHIFT study aimed to evaluate the non-inferiority of the generic tadalafil (Dalerpen) compared with branded and other generic formulations in terms of clinical efficacy and patient satisfaction in treating erectile dysfunction (ED). Dalerpen was approved by the Italian Medicines Agency (AIFA) in 2019 for the treatment of erectile dysfunction and signs and symptoms of BPH [10]. Bioequivalence studies conducted have demonstrated minimal variability in the main pharmacokinetic parameters (Cmax and AUC) compared with the originator. Furthermore, the excipients used in this formulation are almost entirely comparable to those of the branded product [10]. These reasons led us to select this drug for the current study. Our findings demonstrate that switching from branded or other generic tadalafil to Dalerpen does not negatively impact clinical outcomes of treatment. The primary outcomes of this study indicated that both IIEF-EF and IPSS significantly improved after switching to Dalerpen, suggesting that the generic formulation of tadalafil, Dalerpen, not only maintains the efficacy observed with branded counterparts, but may also enhance patient-reported outcomes.
The improvement in erectile and urinary function considering only patients that switched from the branded tadalafil is statistically, but not clinically, significant, but it is enough to consider Dalerpen non-inferior compared with the branded drug. The MCID was not reached, so we have to carefully evaluate the results, and no conclusions can be drawn on a possible better efficacy of Dalerpen over other tadalafils. The positive change reported by patients could be likely attributable to the perception of taking a “new”, “different”, or “better” drug compared with the previous one. Additionally, it may be influenced by the time dedicated by the prescribing physician, involving more attentive counseling and a more detailed explanation of the characteristics and strengths of the proposed medication.
The FDA and other regulatory authorities considers that their approval standards for generic drugs are sufficiently robust to ensure that these products deliver therapeutic outcomes equivalent to those of their brand-name counterparts. On this basis, the agency considers it appropriate to switch between brand-name and generic formulations, without expecting a loss in efficacy or an increase in adverse effects. Consequently, routine clinical monitoring beyond standard care is not deemed necessary following such substitutions. This guidance applies broadly, including to medications used in high-risk settings, such as those for cardiac arrhythmias, transplant immunosuppression, or epilepsy, as well as to drugs characterized by a narrow therapeutic index, where small differences in dose or blood concentration can carry clinical significance [11]. Therefore, depending on the regulatory framework of each country, pharmacists may be authorized to substitute reference drugs with generic alternatives, including the possibility to freely switch among different generic versions of the same medication. But it is important to consider that pharmacokinetic variability among different generic formulations of the same reference drug may pose clinical concerns when switching from one generic to another, a risk often associated with the so-called “biocreep” phenomenon. In fact, while generics are required to demonstrate bioequivalence to the branded drug, they are not necessarily bioequivalent to one another. This lack of transitivity means that, although both Generic A and Generic B are bioequivalent to the branded formulation, they are not inherently bioequivalent to each other [12].
The SHIFT study, however, highlights that Dalerpen demonstrated non-inferiority not only to the branded tadalafil, but also to other generics, suggesting that the specific formulation and excipients of Dalerpen may play a role in achieving comparable efficacy.
The present study aligns with prior research that demonstrated no significant clinical differences between generic and branded drugs across various therapeutic areas [13]. Similar findings have been reported in the context of cardiovascular, hypoglycemic, and antihyperlipidemic drugs, where generics showed equivalent clinical outcomes compared with their branded counterparts [14,15]. Nonetheless, lingering skepticism about the efficacy of generics persists among some patients and healthcare providers, which could influence adherence and satisfaction, and some trials indicate that patient perceptions about generics could often affect therapeutic outcomes [16,17,18]. In addition, a “nocebo” effect in taking a generic drug rather than the branded ones could lead to an unsatisfactory therapeutic effect [19]. Adequate counselling is the key to improving patients’ satisfaction regarding treatment expectations and outcomes. This is clearly evidenced by the responses of andrologists, who highlighted that the difficulties in proposing a change in therapy are related to patients’ beliefs, which can be easily overcome through proactive discussion. Underlining the advantages of taking a generic drug while dispelling doubts, concerns, and prejudices about this type of therapy can facilitate acceptance. Effective physician–patient communication plays a critical role in the successful transition to generic therapies, particularly in areas such as sexual health, where patient perceptions significantly influence treatment adherence and satisfaction. Integrating counselling into routine clinical care should involve clearly explaining the regulatory standards of bioequivalence, addressing individual patient concerns, and emphasizing the therapeutic equivalence of the prescribed generic. Moreover, presenting the switch as part of a shared decision-making process, rather than a cost-driven substitution, may foster greater patient trust and acceptance. Practical strategies may include dedicating a few focused minutes during the consultation to compare the generic with the branded formulation, using simple language to explain pharmacological similarity, and explicitly addressing misconceptions about quality and efficacy. By embedding these communication practices into standard care, clinicians can enhance therapeutic engagement, reduce the potential for nocebo effects, and ultimately improve clinical outcomes in patients initiating or switching to generic medications.
To our knowledge, the SHIFT study is the first to demonstrate the non-inferiority of a generic Tadalafil formulation compared with the branded version, not only in terms of pharmacokinetic bioequivalence, but also from a clinical efficacy perspective. This is a significant advancement, as most studies on generics focus solely on bioequivalence, rather than clinical outcomes. The complete overlap in excipients between Dalerpen and the branded product likely contributes to the consistent efficacy observed, further supporting the practical interchangeability of these formulations. This finding reinforces the notion that, when generic formulations maintain excipient consistency with the branded product, clinical outcomes are more predictable and reliable.
One of the strengths of the SHIFT study is its real-world design, involving diverse clinical settings and multiple centers, which enhances the generalizability of the findings. The use of validated questionnaires (IIEF-EF, SEP-2, SEP-3, and IPSS) ensures robust, standardized outcome measures. However, the non-randomized nature of this study may introduce selection bias, and the absence of a control group limits the ability to make direct efficacy comparisons. In addition, an observer bias could be hypothesized in this trial. It is also plausible that this study may have been subject to observer bias, which we aimed to mitigate through thorough counselling provided prior to treatment modification.
Psychological factors related to patient attitudes towards generics also pose a potential bias, as evidenced by studies on drug adherence and satisfaction after switching from branded to generic formulations [15]. Finally, treatment with Dalerpen occurred after at least three months of tadalafil treatment, and the enhanced efficacy could be derived from the prolonged therapy; the observation period was probably too short to draw conclusions. This study represents an initial step towards encouraging the andrology community to promote research comparing generic and branded drugs, as well as comparisons between different generic formulations. To build on these findings, future randomized controlled trials should focus on the long-term outcomes of switching from branded to generic tadalafil, particularly examining patient adherence and satisfaction. Beyond clinical evaluation, pharmacokinetic assessment through AUC estimation at baseline and after an appropriate follow-up period may provide further valuable insights. Additionally, exploring the role of excipient consistency in achieving therapeutic equivalence between generics and branded formulations could provide further insights into optimizing generic drug use.
5. Conclusions
The SHIFT study demonstrates that the generic formulation Dalerpen is a valid therapeutic option for patients previously treated with branded or other generic tadalafil, showing no inferior clinical efficacy. Addressing patient concerns about generic medications through education on bioequivalence is essential to facilitate the therapeutic switch.
Author Contributions
Conceptualization, A.P., T.C. and D.A.; methodology, T.C. and F.P.; software, C.P.; validation, A.P., C.C., G.P. and L.B.; formal analysis, D.A. and C.M. (Celeste Manfredi); data curation, D.A. and N.S.; writing—original draft preparation, D.A., C.M. (Carlos Miacola) and C.M. (Celeste Manfredi); writing—review and editing, D.A., C.M. (Celeste Manfredi) and C.M. (Carlos Miacola); visualization, C.C., I.O. and M.B.; supervision, T.C. and A.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out by Italian Society of Andrology (SIA) and received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Regione Calabria (Sezione Area Centro, protocol code n.23 on 20 January 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
“The SHIFT study Group”: Laura Abba, Francesco Barillaro, Carlos Cambara Zuniga, Marco Capece, Francesco Cattaneo, Danilo Centrella, Sara Cesaraccio, Michele Del Zingaro, Dante Di Domenico, Marina Di Mauro, Paolo Di Palma, Danilo Di Trapani, Francesco Gaeta, Giuseppe Garofano, Piergiorgio Greco, Sanie Hoxa, Massimo Iafrate, Stefano Lauretti, Piero Letizia, Giovanni Liguori, Andrea Morelli, Elena Morini, Matteo Pippia, Salvatore Privitera, Dario Pugliese, Cesare Regina, Oreste Risi, Michele Rizzo, Lorenzo Ruggera, Gabriele Tulone, Valerio Vagnoni, Manuel Valentini, Niand cola Zanovello.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- NIH Consensus Development Panel on Impotence. NIH Consensus Conference. Impot. JAMA 1993, 270, 83–90. [Google Scholar]
- Parazzini, F.; Menchini Fabris, F.; Bortolotti, A.; Calabrò, A.; Chatenoud, L.; Colli, E.; Landoni, M.; Lavezzari, M.; Turchi, P.; Sessa, A.; et al. Frequency and determinants of erectile dysfunction in Italy. Eur. Urol. 2000, 37, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Hatzimouratidis, K.; Salonia, A.; Adaikan, G.; Buvat, J.; Carrier, S.; El-Meliegy, A.; McCullough, A.; Torres, L.O.; Khera, M. Pharmacotherapy for erectile dysfunction: Recommendations from the Fourth International Consultation for Sexual Medicine (ICSM 2015). J. Sex. Med. 2016, 13, 465–488. [Google Scholar] [CrossRef] [PubMed]
- Hatzimouratidis, K.; Amar, E.; Eardley, I.; Giuliano, F.; Hatzichristou, D.; Montorsi, F.; Vardi, Y.; Wespes, E.; European Association of Urology. Guidelines on male sexual dysfunction: Erectile dysfunction and premature ejaculation. Eur. Urol. 2010, 57, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Ma, M.; Xie, W.; Yang, X.; Huang, Y.; Sun, T.; Luo, Y.; Huang, J. Direct comparison of tadalafil with sildenafil for the treatment of erectile dysfunction: A systematic review and meta-analysis. Int. Urol. Nephrol. 2017, 49, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, C.; Franco, A.; Ditonno, F.; Mathur, R.; Franco, G.; Lombardo, R.; Russo, G.I.; DECillis, S.; Fiori, C.; Arcaniolo, D.; et al. Treatment preferences of patients with erectile dysfunction: A systematic review of randomized controlled trials. Minerva Urol. Nephrol. 2024, 76, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Shrank, W.H.; Liberman, J.N.; Fischer, M.A.; Girdish, C.; Brennan, T.A.; Choudhry, N.K. Physician perceptions about generic drugs. Ann. Pharmacother. 2011, 45, 31–38. [Google Scholar] [CrossRef] [PubMed]
- EMA Guidelines on the Investigation of Bioequivalence—Ref. CPMP/EWP/QWP/1401/98 Rev. 1. 2010. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (accessed on 19 October 2024).
- Rheinstein, P.H. Therapeutic inequivalence. Drug Saf. 1990, 5 (Suppl. S1), 114–119. [Google Scholar] [CrossRef] [PubMed]
- RCP Dalerpen. Available online: https://api.aifa.gov.it/aifa-bdf-eif-be/1.0.0/organizzazione/7214/farmaci/45641/stampati?ts=RCP (accessed on 19 October 2024).
- Henney, J.E. From the food and drug administration. JAMA 1999, 282, 1995. [Google Scholar] [CrossRef] [PubMed]
- Gozzo, L.; Caraci, F.; Drago, F. Bioequivalence, Drugs with Narrow Therapeutic Index and The Phenomenon of Biocreep: A Critical Analysis of the System for Generic Substitution. Healthcare 2022, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Hartung, D.M.; Middleton, L.; Svoboda, L.; McGregor, J.C. Generic substitution of lamotrigine among medicaid patients with diverse indications: A cohort-crossover study. CNS Drugs 2012, 26, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Reichardt, B.; Dunkler, D.; Hronsky, M.; Winkelmayer, W.C.; Bucsics, A.; Strohmaier, S.; Heinze, G. Comparative effectiveness of branded vs. generic versions of antihypertensive, lipid-lowering and hypoglycemic substances: A population-wide cohort study. Sci. Rep. 2020, 10, 5964. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.H.; Benner, J.S.; Girase, P.; Benigno, M.; Axelsen, K.; Liu, L.Z.; Nichol, M.B. Generic and therapeutic statin switches and disruptions in therapy. Curr. Med. Res. Opin. 2009, 25, 1247–1607. [Google Scholar] [CrossRef] [PubMed]
- Shrank, W.H.; Cox, E.R.; Fischer, M.A.; Mehta, J.; Choudhry, N.K. Patients’ perceptions of generic medications. Health Aff. 2009, 28, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Iosifescu, A.; Halm, E.A.; McGinn, T.; Siu, A.L.; Federman, A.D. Beliefs about generic drugs among elderly adults in hospital-based primary care practices. Patient Educ. Couns. 2008, 73, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Shrank, W.H.; Stedman, M.; Ettner, S.L.; DeLapp, D.; Dirstine, J.; Brookhart, M.A.; Fischer, M.A.; Avorn, J.; Asch, S.M. Patient, physician, pharmacy, and pharmacy benefit design factors related to generic medication use. J. Gen. Intern. Med. 2007, 22, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Faasse, K.; Martin, L.R. The Power of Labeling in Nocebo Effects. Int. Rev. Neurobiol. 2018, 139, 379–406. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).