Abstract
Women’s greater vulnerability to intrusive memories following trauma may be partially explained by the influence of ovarian hormones on memory consolidation processes. Contributing to accumulating research examining the influence of ovarian hormones on the development of intrusive memories, we hypothesized that cyclical fluctuations in estradiol and progesterone, not merely absolute levels, contribute to this risk. We further hypothesized that hormonal contraceptives, which effectively eliminate fluctuations and keep ovarian hormones at chronic low levels, can convey protective effects against memory intrusions following analogue trauma exposure. We examined the development of memory intrusions following trauma film stressor exposure among men (n = 27), hormonal contraceptive (HC) users (n = 41), and naturally cycling (NC) women in the early follicular (EF; n = 24), late follicular (n = 20), ovulatory window (n = 14), and luteal phases (n = 21) for 5 days to assess whether low ovarian hormone levels convey a protective effect for women. Contrary to hypotheses, this study found no support for this prospect; rather, exposure to stressors during the window around ovulation increased the risk for more frequent intrusive memories. Enhanced stress responsivity may have particular effects on ovulation, promoting evolutionary fitness.
1. Introduction
Women are disproportionately affected by posttraumatic stress disorder (PTSD) [1,2,3]. Although men are more likely to experience a significant traumatic event in their lifetime (61% vs. 51%), women are more than twice as likely to develop PTSD [4], almost four times as likely to experience chronic PTSD [5], and report more severe re-experiencing symptoms, such as sudden and uncontrollable trauma-related recollections [1,6]. Proposed explanations for this disparity include gender differences in post-trauma cognitions [7], women’s greater tendency toward self-blame [8], and variations in the types of traumas typically experienced by men and women [9]. However, none of these factors is sufficient to account for the magnitude of the gender gap [1,8].
Biological mechanisms may offer more explanatory power. Research points to the role of stress-response hormones—particularly cortisol (CORT) and noradrenaline (NA)—in the trauma memory consolidation process [10,11,12,13] and PTSD symptom development [14,15]. These hormones are involved in emotional memory over-consolidation, the process by which memories with vivid, sensory, and affective qualities are encoded, later contributing to intrusive symptoms [14,15,16].
Importantly, this process appears to differ for men and women. Trauma memories vary by sex [3,9,17], and unique contributions of biology to peri-traumatic memory processes may explain the substantial variance in PTSD gender distribution [18,19,20,21,22]. The secretion of stress-responsive CORT and NA is influenced by the ovarian hormones of estradiol and progesterone, which fluctuate across the menstrual cycle (See Figure 1) [22,23,24,25,26,27,28]. Men who have low levels of estradiol and progesterone do not display cyclic variability in their release of CORT or NA in response to stress [29]. Women, however, appear to experience higher levels of circulating stress-response hormones and are at greater risk for memory over-consolidation following exposure to stressors at specific stages of the menstrual cycle [25,26,30,31]. Researchers have reported that elevated sex steroids predict cortisol elevation in response to stress [28,32], which, in turn, predicts more frequent memory intrusions [26].
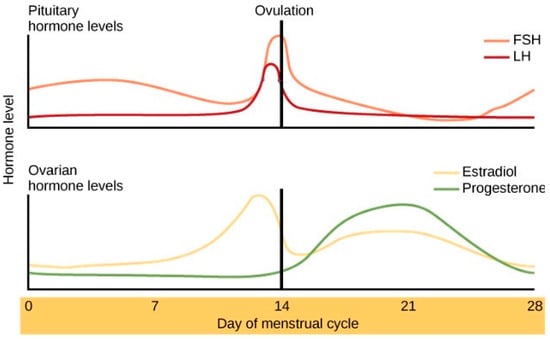
Figure 1.
Ovarian hormones across the menstrual cycle. Reprinted from Human Biology by Christine Miller, 2020 (Original: modification of work by Mikael Häggström) accessed on 10 March 2025 and retrieved from https://humanbiology.pressbooks.tru.ca/chapter/20-8-menstrual-cycle/.
However, the literature has been equivocal regarding which ovarian hormone—and which phase of the cycle—contributes most to this risk. Some studies suggest high levels of progesterone increase risk [17,27,28,33], while others implicate estradiol [26,34,35]. Similarly, because steady low-level ovarian hormones may protect against stress-responsive hypothalamic–pituitary–adrenal (HPA) and autonomic arousal [36,37,38,39], some researchers have speculated that low-level hormones may protect against the development of memory intrusions [40,41,42]. Stress exposure during the early follicular phase (EF, when both progesterone and estradiol are maintained at their lowest levels for approximately five days) may pose less risk for the over-consolidation of vivid details of stressful events, relative to the rest of the cycle [41,43], but evidence of any protective effect in this period has not been consistently found [44]; this may be due, in part, to methodological differences in procedure (i.e., most studies combine the distinct low and high hormone periods of the follicular phase).
Generally, there is a lack of consensus on a critical timeframe (and corresponding hormone levels) that contributes to vulnerability to intrusion development in women. Indeed, attempts to define a critical window have classified much of the menstrual cycle as a vulnerability period, with different research groups identifying varying risk phases [25,26,27,30,31]. Rather than absolute levels of estradiol and progesterone, it may be the cyclical fluctuations themselves that increase women’s risk for heightened stress responsivity and excessive memory consolidation [45]. For example, the cyclical binding and unbinding of estrogen to neuronal receptors may disrupt the glucocorticoid system, increasing women’s reactivity to stress [46]. Through a similar mechanism, hormonal fluctuations have also been linked to women’s greater risk for anxiety disorders tied to the body’s stress response system [47,48].
Hormonal contraceptives (HCs), which suppress natural fluctuations in estradiol and progesterone and maintain chronically low levels of both hormones [49], can provide a useful comparison group [37,38,39,43,50]. HC use has been shown to dampen corticosteroid and noradrenergic activation during stress and, in some cases, reduce memory over-consolidation. HC users have been found to recall fewer negative details after stress exposure [49,51,52], and taking HCs or emergency contraceptives, which have a similar mechanism [53] after sexual assault, is linked to fewer PTSD re-experiencing symptoms six months later [53]. Moreover, HC use has been found to interact with early post-trauma oxytocin administration to promote faster recovery in those with severe posttraumatic symptoms [54]. However, findings are again not uniform; other studies suggest that HCs augment the stress response [55], increase memory intrusions, and slow extinction learning [56,57].
The reviewed literature alludes to the complexity of this work, which has produced contradictory findings [44,58,59] across research questions related to menstrual cycle status and the risk of re-experiencing. A complete understanding of the function of ovarian hormone levels on emotional memory over-consolidation is likely multi-determined and implicates other genetic and biologic factors [60]. More likely than either ovarian hormone levels or fluctuations contributing to an increased risk alone is the prospect that both high levels and periodic fluctuations convey some cumulative effects together, playing a role in memory intrusions. Further investigations as to whether low levels of both estradiol and progesterone (in the context of naturally cycling women in the EF and among women on HCs) may be protective against the development of intrusions following stressor exposure are warranted.
Additionally, some contradictions in this literature may stem from methodological differences. Many studies rely on retrospective recall (rather than the real-time recording) of intrusions over a set period. Additionally, some trauma analogues lack external validity. For instance, exposure to a sad story or negative image may not induce enough stress to trigger memory intrusions in most participants, making the results more reflective of anxiety proneness than susceptibility to traumatization. The findings of memory fragments characterized by hypermnesia (unusually enhanced, detailed memory) coupled with gaps in recall for the larger context or timeline of trauma in PTSD [61] have also led some researchers to use qualifying memory details as opposed to the gist (i.e., overall theme or event summary [62]) as a proxy for intrusive re-experiencing [63,64]. While intrusive memories are vividly sensory, intrusion frequency (rather than characterizing intrusions as gist or detail) is considered the most generalizable measure of traumatic re-experiencing [64,65]. The presumed link between intrusion frequency and memory detail in stressful event recall has received little empirical attention.
1.1. The Present Study
The present study aimed to further elucidate the role of the menstrual cycle and HC use in the development of post-trauma intrusive memories. This study was designed to examine the frequency of emotional memory intrusions following analogue trauma exposure and its putative relationship to hormonal status in HC users and naturally cycling women. Cycle phase divisions among naturally cycling women were operationalized to capture specific windows around peak ovarian hormones and to compare the cycle’s lowest and most elevated hormone levels without the noise incurred by fluctuations within the larger phases. The theoretical prospect that low levels of ovarian hormones may be protective (for which prior empirical support is mixed) was tested.
A decision was made not to collapse across the remainder of the menstrual cycle for analysis when comparing women in the EF phase to other naturally cycling women for several reasons. While the low hormone EF phase is hypothesized to be protective against developing emotional memory intrusions, this prospect relies heavily on research that high levels of ovarian hormones enhance intrusion frequency, such that the EF phase may not be protective relative to the entirety of the cycle. There is also disagreement in the literature that implicates higher levels of ovarian hormones as to whether vulnerability is due to estradiol or progesterone (implicating distinct windows of risk). Furthermore, while both high estradiol and high progesterone levels have been identified as factors informing intrusion development within specific studies, it is unclear whether the risk is additive (with rising levels being associated with increasingly greater stress responsivity and intrusions) or dependent on some threshold (e.g., 75% of the peak preovulatory estradiol level). Thus, collapsing across the cycle equates to dismissing the variance among groups in the mechanisms (estradiol and progesterone) presumed to underlie this process, which may prevent the detection of any protective effect.
The same logic applies to the comparison of HC use with potential discrete windows of high risk among naturally cycling women. This is particularly relevant if high levels are found to be greater determinants of risk than fluctuations in predicting more frequent intrusions. In addition, the low-level ovarian hormone conditions (HC and EF) were not combined because (a) this is one of the few studies to assess whether stressor exposure during the EF buffers against intrusions; (b) it is unknown whether the additional presence of synthetic ovarian hormones in HC users may differentially impact the memory consolidation process; and (c) combining the low ovarian hormone groups would prevent identifying fluctuations from levels, preventing the comparison of the two groups in terms of their putative protective utility.
The frequency and detail of intrusive memories were compared among the following groups over a 5-day period: the EF, late follicular, ovulatory, and luteal phrases, HC users, and men (a comparison group).
1.2. Hypotheses
We expected that both low-hormonal groups would experience fewer and less detailed intrusions relative to all other groups of women. Specifically, we hypothesized that women in the late follicular, luteal, and ovulatory phases would all display more frequent and detailed intrusions than women in both the EF and HC groups, supporting the protective effect of low-hormone groups. Additionally, we hypothesized that HCs would convey a greater protective advantage (i.e., associated with fewer intrusions) than the EF phase. A statistically significant finding for the protective nature of HCs (which keeps both estradiol and progesterone at chronically low levels and largely inhibit fluctuations) compared to all other groups, including EF, would support a model of risk in which both absolute levels and fluctuations in ovarian hormones influence stress memory over-consolidation. Finally, memory detail, relative to gist, is often used as a proxy for re-experiencing within this literature; yet, the relationship between intrusion frequency and the degree of detail has not been assessed empirically. Thus, the exploratory aim of this study was to examine the relationship between intrusion frequency and the prevalence of detailed memories relative to gist.
2. Material and Methods
This study was approved by the Institutional Review Board (Pennsylvania State University IRB: PRAMS 41329).
2.1. Research Design
The mixed-methods study utilized a between-subjects experimental design. Specifically, it employed a trauma film paradigm methodology. This is a common trauma analogue procedure in which a highly distressing film is shown to a nonclinical sample [66]. This method is used to simulate a low-grade version of the stress reactivity that occurs in response to a traumatic event, allowing researchers to examine putative etiological factors implicated in heightened stress responses and the development of intrusive re-experiencing [64]. The trauma film paradigm [67] is capable of inducing analogue PTSD symptoms in a laboratory setting for up to a week [68]. The procedure consists of watching a film clip that simulates traumatic life events illustrating “death, threatened death, actual or threatened serious injury, or actual or threatened sexual violence” [69] and is effective in evoking autonomic stress responses [64,66,68] and subsequent re-experiencing symptoms [64,66,70], similar to those associated with post-trauma sequelae [68]. The trauma film paradigm has been (a) widely used in research examining peri-traumatic cognitive processes and memory intrusions [68,71,72,73] and (b) validated as a trauma simulation process [64]. The mixed methods approach (which included the collection of hormonal and physiological data, validated self-report instruments, and qualitative memory records) enabled the researchers to ensure the accurate classification of menstrual cycle grouping and reactivity to the stimulus (confirming its experience as a stressor as well as examining the frequency and content of intrusive memories across naturally cycling women, HC users, and men.
Cycle phase determinations for group assignment. Naturally, cycling women were categorized into phases based on self-reported cycle day and length. Phase determinations were made as per previous research [74] but amended to capture the low hormone early follicular phase, categorize the entirety of the cycle, and correct for potential self-reported errors. The first day of menses was designated +1 and the prior day −1, categorizing the following groups: (a) days −2 to +5, early follicular phrase, capturing the lowest levels of estradiol and progesterone across the cycle; (b) days +6 to −17, late follicular phrase, defined by elevated estradiol and low progesterone; (c) days −16 to −12, ovulatory phrase, capturing peak estradiol and an early rise in progesterone, representing the fertile window for the majority of women [75]; and (d) days −11 to −3, luteal phrase, capturing the progesterone peak. Phase classifications deviated slightly from prior research. Previous divisions have included either the premenstrual (defined by −3 to +1; e.g., [72]) or early follicular (days 1–5 [31]) periods, as indicated by specific research aims. As the objective of the current study was to capture the lowest levels of estradiol and progesterone, and research shows that hormone levels are comparable from two days premenstrual through the early follicular phase [76], all 7 days were included and labelled “early follicular” for conceptual clarity. As such, the luteal phase was truncated by 2 days. Additionally, the ovulatory phase was extended by one day from traditional measurements [72] to include one day prior (−16), thus enhancing the chances of appropriately capturing the cycle’s estradiol peak, given gynecological research indicating that the majority of women ovulate anywhere between 16 and 12 days before their next menstrual period [77,78]. These changes allowed for the clustering of the lowest levels, moderate levels, and highest levels of hormones, respectively.
2.2. Participants and Recruitment
Participants
Participants were undergraduate students recruited from the psychology department’s “subject pool” at Pennsylvania State University (N = 151). Following mass screening, naturally cycling women across the menstrual cycle (n = 81), women using HCs (n = 41), and a comparison group of men (n = 29) consented to participate. Exclusion criteria assessed during mass screening included irregular menstrual cycles among naturally cycling women, regular smoking, endocrine disorders, current psychotropic medication use, or a BMI above 30 based on self-reported height and weight, all due to the potential hormonal confounds these conditions may have posed to relevant outcome data. Individuals who screened positive for PTSD based on established liberal criteria using the Posttraumatic Diagnostic Scale (PDS) [79] were also excluded, as participation may have been symptom-exacerbating and confounded the typical process of intrusion development following trauma-film paradigm exposure. Similarly, women who experienced sexual assault were excluded in response to a single-item screener question, as the laboratory stressor contained relevant interpersonal violent content.
Eligible undergraduates were called or emailed about the study. Interested participants received additional information, were further screened during a telephone interview with a research assistant, and women using HCs and men were scheduled as available. Naturally, cycling women tracked, recorded, and self-reported the dates of two episodes of menses before being scheduled for their study visit via the projected cycle phase per established recommendations [80,81]. Efforts were made to schedule naturally cycling women across the entirety of their cycle.
2.3. Experimental Session Procedure
Participants were asked not to eat or drink for an hour before presenting for a one-hour laboratory session. Because they may have denied previous trauma during screening for confidentiality reasons, during informed consent procedures, every participant was told that the study stimulus contained graphic physical and sexual content, and staff urged them not to participate should this be relevant. Following consent, participants rinsed their mouths with water and were instructed in the saliva collection technique by a research assistant. Specifically, participants let saliva pool in their mouths and then gently drooled through a straw into a Salivette tube until at least 2 mL of saliva was obtained (i.e., passive drool technique [82]). The salivary sample was collected before the initiation of any other procedures. Participants were then hooked up to an automated vital signs monitor, which took their heart rate and blood pressure recordings at two-minute intervals throughout the duration of the lab session. Participants filled out baseline self-report questionnaires reflecting their mood and current anxiety before film exposure. A minimum of five heart rate and blood pressure readings were taken to establish a baseline and habituate participants to the recording device prior to starting the film. Participants viewed a ten-minute film stressor depicting graphic physical and sexual violence. Participants were asked to watch the screen for the duration and to try not to avert their gaze or obstruct their vision with their hands. Heart rate and blood pressure readings continued to be taken for a minimum of 4 min after the film concluded while participants filled out brief post-film questionnaires. Finally, participants were given instructions and a pocket-sized paper diary to record any spontaneous intrusive recollections related to the clip content experienced over a 5-day period.
Analogue Trauma Film Stimulus. A ten-minute film clip featuring a compilation of scenes from the Gaspar Noe movie Irreversible, containing graphic physical and sexual violence, was used in this study. This film has been previously validated as a stressor [83,84], effectively producing an average of 7.2 intrusive memories among men and women during the week following viewing [82]. This trauma film stimulus is both relatable and realistic, including graphic physical assault between two men and scenes of a young woman leaving a party, walking alone at night, and then being physically and sexually assaulted by a stranger. As such, this stressor includes the context of the trauma (as opposed to scenes from an emergency room) and depicts activities relevant to undergraduate students that may be more resonant.
2.4. Measures
The Posttraumatic Diagnostic Scale (PDS; [77]) is a widely used, well-validated self-report measure of PTSD symptom severity. It includes items to assess relevant trauma history, current symptoms across domains, and associated distress and functional impairment [77]. The PDS was administered during the mass screening of the undergraduate subject pool; individuals screening positive for PTSD were excluded from study participation.
Salivary Assays. Salivary samples were frozen at −40 °C [79], and a proportion of samples were assayed for estradiol and progesterone levels, including 56% of naturally cycling women and 12% of hormonal contraceptive users. These assays were used as a manipulation check to confirm the phase projections derived from self-reported menstrual cycle tracking. The vast majority of assays were allocated to naturally cycling women (n = 45), given the propensity of HCs to keep both ovarian hormones at continuous low levels. Samples were prioritized for assay as follows: (1) To facilitate the comparison of low ovarian hormones with elevations, the ovulatory window (n = 11; 79% assayed) was overrepresented, ensuring the appropriate capture of the estradiol peak and early luteal rise in progesterone levels; (2) the late follicular (n = 12; 60% assayed) and luteal phases (n = 14; 66% assayed) were prioritized next to confirm that their hormonal profiles were distinguishable (on average) from the ovulatory group and each other; and (3) EF samples were chosen last (n = 8; 33% assayed), as women were expected to most accurately report this phase, given that all but 3 women (2 at day −2, 1 at day −1) were menstruating during participation. Furthermore, both hormones were expected to be low and exhibit less overlap in estradiol and progesterone levels than across the other phases. Samples were assayed using DRG International Salivary Kits. The salivary concentrations of circulating estradiol and progesterone have been validated as methods to assess the cycle phase, particularly when used in conjunction with self-reports [85] and were, thus, used to confirm women’s self-reported phase.
Physiological Reactivity. An automated heart rate and blood pressure monitor measured physiological status before, during, and after the trauma-film exposure; readings were automatically taken at 2 min intervals for a minimum of 24 min during the laboratory visit. Frequently used in research, these monitors are highly reliable [86] and ensure the standardization of cardiovascular measures, minimizing errors common in manual devices [84].
State-Trait Anxiety Inventory (STAI [87]). The STAI-state anxiety subscale is a 20-item measure designed to assess individuals’ degree of anxiety, including feelings of autonomic arousal “right now, at this moment” in a given circumstance. Each item (e.g., “I feel tense” and “I am worried”) is rated on a 4-point Likert scale ranging from “not at all” to “very much so”. Total scores range from 20 to 80, with higher scores indicative of greater current feelings of situational anxiety. The STAI is the most widely used measure of anxiety and has evidenced excellent psychometrics [88]. Within this sample, the STAI demonstrated good internal consistency (α = 0.82). The STAI was administered at baseline and following exposure to the trauma film to assess for changes in anxiety.
Positive and Negative Affect Schedule. PANAS-X [89]. The PANAS-X is a 60-item measure assessing the current endorsement of 11 positive and negative affective states: fear, sadness, guilt, hostility, shyness, fatigue, surprise, joviality, self-assurance, attentiveness, and serenity. It is well-validated and widely used in the assessment of moods or evaluative feelings regarding specific subjects or situations [90]. Each effect consists of 3 to 8 requisite items (e.g., “serenity” includes “calm,” “relaxed,” and “at ease”), rated on a Likert scale ranging from 1 “very slightly or not at all” to 5 “extremely.” The PANAS-X demonstrates good convergent validity (r = 0.85–0.91) [88]. For this study, participants were asked to rate their current effect following exposure to the film clip only. The 10-item negative affectivity subscale showed good internal consistency (α = 0.86), and the 10-item positive affectivity subscale showed acceptable (α = 0.70) internal consistency.
Memory Diary. Considered the most appropriate method for accurate recordings in the trauma film literature [68], the memory diary included printed instructions defining an intrusion (i.e., a spontaneous, not deliberately recalled, thoughts, images, or dreams), and each page was devoted to recording a single intrusion. Some participants alternately requested to record intrusions in the note apps of their phones, into which they copied the basic instructions from the diary and proceeded to use mobile versions instead of paper. The paper diary was provided for personal use, regardless, and all participants transferred recorded information for each intrusion onto a secure website containing identical questions. Researchers retrieved all data from the website; paper diaries were not collected.
2.5. Data Preparation
Ovarian Hormone Outliers Check. The mean cycle length for naturally cycling women was 29.06 (SD = 3.5) days. Given the substantial normative variation in estradiol and progesterone levels across women at various cycle phases and research indicating that hormones tend to be at the higher end of cycle phase norms in younger women [78,79], no participants were reassigned to a different phase based on the assay results. A single participant, with among the lowest levels of progesterone among all individuals sampled, despite reporting being in the mid-luteal phase (the progesterone peak), was excluded based on deviation from the established norms (guidelines established by Salimetrics LLC) as their cycle phase could not be reasonably validated.
Stimulus Manipulation Check. The effectiveness of the stimulus was verified by physiological and psychological symptom measures. See Table 1. To be considered a stimulus responder, participants had to meet at least one criterion in any response category (biological, physiological, or affective). Biological responses were defined as (1) a peak heart rate of at least 85 bpm, with an increase of at least 5 bpm from baseline, and (2) a change of 20 mm Hg in systolic or diastolic blood pressure. Participants who reported sweating, light-headedness, rapid breathing, or chest tightness were also considered responders. Psychological responses were defined by significant changes in anxiety from baseline to post-stimulus or moderate endorsements of guilt or fear on the PANAS-X subscales. Anxiety changes were assessed for clinical significance using the reliable change index (RCI), where a score exceeding 1.96 indicated significant change. A score of 18 or higher on the PANAS-X hostility or fear subscale was also used to define responsivity. Most participants showed some reactivity in at least one area, and there were only two non-responders whose data did not affect the analysis or effect sizes and were retained.

Table 1.
Manipulation check: number and percentage of responders to analogue trauma film.
Intrusion Coding. A trained graduate or undergraduate research assistant reviewed each recorded memory intrusion (1–3 sentences long) to confirm validity, as defined by overt association with the trauma film. Intrusions unassociated with the film, broadly defined, were excluded (n = 5). Individual intrusions were categorized as primarily gist or detailed memories based on an amalgam of previously operationalized procedures [41,62,91]. Specifically, gist referred to narrative elements central to the events of the film without which the story would be incomplete (e.g., the woman was attacked by a man in a tunnel; the man punched through the car window to attack the driver). Details were descriptive elements unessential to the plot (e.g., the woman was wearing a yellow dress; the tunnel was lit by red lights). Intrusions were classified as either gist or detailed memories by four trained coders. At least three of the four independent coders agreed on the classification of each intrusion. When agreement could not be reached, the intrusions were discussed by the study personnel to determine consensus.
2.6. Analyses
A one-way analysis of variance (ANOVA) was conducted to examine whether intrusion frequency differed as a function of hormonal status grouping. Five potential outliers were detected (1 man, 2 naturally cycling women, and 2 HC users) [92]. Analyses revealed the same pattern of results with and without outliers; both were statistically significant and produced identical effect sizes. To present more conservative results and minimize standard errors, outliers were assigned the next highest value of their individual groups.
The percentages of memories that were coded as detailed, relative to gist, were calculated for each participant. Given the violations of homogeneity of variance in group distributions regarding the percentage of gist and detailed memories, a Welch Robust Test for Equality of Means was run to assess the differences in the percentage of detail memories experienced across groups. To analyze group differences in the prevalence of detailed memories, original scores were used to calculate the percentages for the 5 participants who were outliers based on the number of intrusions.
3. Results
3.1. Final Sample
Two participants withdrew before study completion, and two participants were excluded due to technical errors during the laboratory session. As such, the final sample included 147 participants (27 men, 41 HC users, and 79 naturally cycling women). The average age at participation was 18.83 (SD = 1.057) years, and participants ranged from 18 to 24 years of age. Most participants, 119 (77.27%), identified as White; 7 identified as Black (4.55%); 18 (11.69%) identified as Asian; 2 identified as Hispanic (1.30%); 5 identified as Mixed Race (3.23%); and 3 declined to answer (1.94%).
3.2. Descriptive Statistics: Relevant Ovarian Hormone Levels by Group
The final group sizes of naturally cycling women were as follows: early follicular (EF; n = 24), late follicular (n = 20), ovulatory window (n = 14), and luteal phases (n = 21). Ovarian hormone assays reflected the expected trend of capturing peak estradiol during the ovulatory phase, the highest levels of progesterone during the luteal phase, lower levels of both hormones during the early follicular phase, and the lowest levels of both hormones among HC users (n = 41). The mean group levels of estradiol and progesterone across conditions are plotted in Figure 2 and listed in Table 2. There were no significant differences in estradiol levels across the groups; ovulatory estradiol levels among women were compared to levels among women in the EF phase (p = 0.239; d = 3.02), women in the late follicular phase (p = 0.965; d = 0.78), women in the luteal phase (p = 0.687; d = 1.36), and among HC users (p = 0.215; d = 4.11). Luteal progesterone differed significantly from progesterone levels among HC users (p = 0.002; d = 5.07), women in the EF phase (p = 0.001; d = 4.82), women in the late follicular phase (p = 0.004; d = 3.43), and women in the ovulatory window (p = 0.032; d = 2.53).
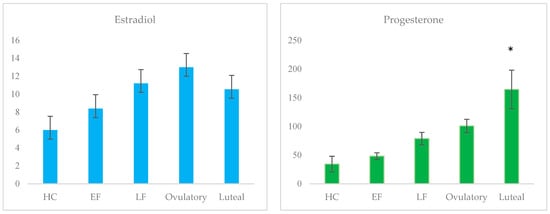
Figure 2.
Mean salivary progesterone and estradiol levels across groups (pg/mL); * denotes statistically significant difference.

Table 2.
Mean estradiol and progesterone levels among groups.
3.3. Intrusive Memories
The mean number of intrusions across participants was 4.06 (SD = 3.13), with a range from 0 to 12 (95% CI: 3.55–4.57). Participants (including untransformed outliers) reported a total of 649 intrusions over the 5-day period; 91.16% reported at least one intrusion. A one-way ANOVA illustrated that a significant group effect was observed: F (5,146) = 2.28; p = 0.050; η2 = 0.07. Post hoc tests using Fisher’s LSD revealed that the ovulatory group (M = 6.5, SD = 3.48) differed significantly from all other groups: early follicular (M = 4.17, SD = 3.25; d = 0.69), late follicular (M = 3.45, SD = 2.67; d = 0.98), and luteal phrases (M = 4.05, SD = 3.03; d = 0.75), HC users (M = 3.98, SD = 3.31; d = 0.74), and men (M = 3.30, SD = 2.55; d = 1.05). The mean number of intrusions across groups is plotted in Figure 3.
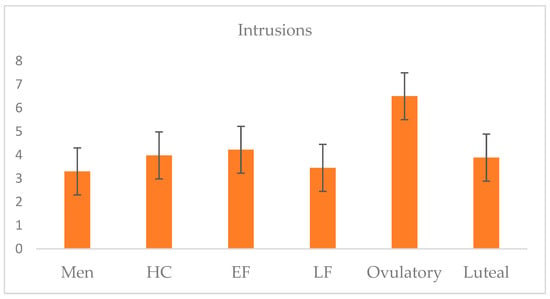
Figure 3.
Five-day intrusion frequency means across groups of naturally cycling women, HC users, and men.
3.4. Gist and Detail Memories
Of all the intrusions reported, 386 (59.48%) were gist memories, and 263 (40.52%) were detailed memories. The number of intrusions was positively correlated with the percentage of detailed intrusions (r = 0.221; p < 0.001); the number of intrusions was not significantly correlated with the percentage of gist intrusions (r = 0.061; p = 0.441). On average, 33.08% (SD = 35.00) of a participant’s intrusions were classified as detailed rather than gist memories. Among the participants in the EF phase, an average of 24.61% (SD = 32.87) of intrusions were detailed; in the late follicular phase, 49.25% of intrusions were detailed (SD = 44.22); in the ovulatory window, 39.08% of intrusions were detailed (SD = 27.01); in the luteal phase, 25.21% of intrusions were detailed (SD = 23.94); in HC users, 30.00% of intrusions were detailed (SD = 33.63); and in men, 36.34% (SD = 39.86) of intrusions were detailed. A one-way ANOVA revealed no significant difference across groups with regard to the percentage of memories that were detailed rather than gist memories: Welch’s F (5, 56.98) = 1.394; p = 0.240; η2 = 0.04. The percentages of detailed intrusions across groups are plotted in Figure 4.
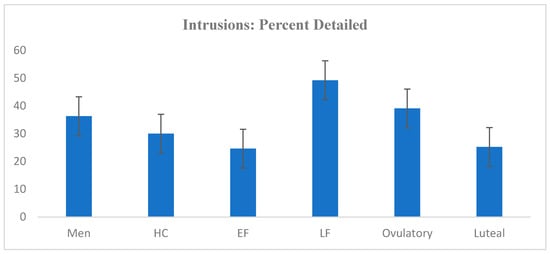
Figure 4.
Average percentage of intrusions classified as detailed across groups.
4. Discussion
We hypothesized that ovarian hormone elevations and fluctuations contribute to the risk of developing memory intrusions. As HCs keep hormones at low levels and suppress fluctuations, we hypothesized that women using HCs would have the fewest, least detailed intrusions. Contrary to study predictions (and some previous research [30,42,51,53]), neither the low hormone EF phase nor HC use provided protection following exposure to stressors. Relative to all other naturally cycling women, those in the EF and HC groups did not exhibit fewer memory intrusions or a smaller percentage of detailed intrusions. As such, this study provides no evidence that ovarian hormone fluctuations, or the suppression thereof, facilitate or protect against intrusive memories. These findings diverge from research findings that HC use is associated with less detailed memories of emotional events [42,51,93] and findings that HCs may be protective against the development of recurring symptoms [53,54]. Notably, this study’s findings also do not corroborate alternate research suggesting that HC use conveys heightened risk [56,57]. As the EF phase was not found to be protective, this research also does not indicate that low-level ovarian hormones mitigate against memory intrusions.
The unexpected finding that women in the ovulatory phase displayed significantly more intrusions than all other groups implies a unique window of vulnerability, corresponding to the time estradiol peaks and progesterone begins rising. This finding corroborates, in part, with research implicating elevated estradiol and the ratio of estradiol to progesterone in driving risk for memory over-consolidation [35]. A prior study assessing emotional memory intrusions following stressor exposure during distinct periods of the menstrual cycle found support for elevated risk during a “post-ovulatory” early luteal window [35]. In contrast to others [72], this group defined a somewhat earlier ovulatory and luteal phase (operationalizing days −9 to −13 as the early luteal phase). As such, there are 2 days of overlap between this study’s ovulatory phase and the aforementioned study’s early luteal phase. Further, 27% (n = 4) of the present study’s ovulatory group participated on cycle days that overlapped with the early luteal phase, providing partial corroboration that a legitimate window of risk exists [35]. As neither our research group nor theirs assayed estradiol and progesterone throughout the entirety of the cycle, both studies relied on estimates, using likelihood windows from self-reported menstrual tracking and study participation day hormone levels. As such, both study windows could capture various participants during ovulation, right after, or right before. Regardless, both sets of findings support the general prospect that the time around ovulation seems to be a significant period, driving the risk for emotional memory over-consolidation.
An ovulatory risk period has potential theoretical implications. The day of ovulation represents a woman’s fertility peak. From an evolutionary standpoint, the period around ovulation may serve as a critical window during which threats are salient for women and stress responsivity, as indicated by emotional memory over-consolidation, is enhanced. Providing additional support that women may be hypersensitive to environmental threats during the ovulatory period, some research suggests that women can most accurately identify anger and fear, accompanied by enhanced amygdala activity, right before ovulation [94,95]. This ability to perceive threat dissipates across the luteal phase, as both amygdala activity and accurate threat identification are negatively correlated with progesterone levels [96].
As neuroendocrine and sympathetic nervous system activity is presumed to underlie memory over-consolidation, this may be a functional byproduct of enhanced ovulatory stress sensitivity. Stress hyper-sensitivity may be a means to avoid conception under perilous environmental conditions. Indeed, ovulation is largely under neuroendocrine control [97,98], and the intricate process of co-regulation between the HPA and HPG axes suggests that this crosstalk was developed specifically to enhance evolutionary fitness [99]. The prevention of ovulation by neuroendocrine responses to chronic external stressors, hypothalamic anovulation, is not uncommon [100], and high levels of salivary alpha-amylase (a norepinephrine metabolite) have been found in women unable to conceive [101]. Even single episodes of stress can disrupt ovulatory processes; a higher NE release during the ovulatory window has been found to temporarily reduce fertility across those days exclusively [102]. This study and Soni et al.’s (2013) support enhanced risk for emotional memory intrusions around ovulation [35]; whether this risk occurs in response to stress right before, during, and/or right after ovulation has yet to be identified. Should this vulnerability window capture peri-ovulation, the presence of emotional memory intrusions from the hours to days after acute stress exposure may serve as reminders of an unsafe environment long after the hyper-aroused body has returned to homeostasis.
As average progesterone levels were fairly high among the ovulatory sample, it is likely that for some women, the days immediately following ovulation, rather than ovulation itself, were captured. However, this would not preclude the possibility of an evolutionary mechanism at play. Following fertilization, the egg has several days to implant within the uterus, and research supports the fact that this process can be disrupted by glucocorticoids [103,104] and, to a lesser extent, norepinephrine [105] under stressful circumstances. Furthermore, very high levels of cortisol in the days after successful implantation have been associated with spontaneous abortion [106]. The salience of emotional memory intrusions, should they occur immediately after ovulation, may serve as a reminder of threat, facilitating vigilance in a woman who has just conceived. Indeed, luteal phase women are faster to respond to and engage with emotional and social cues [107,108]—potentially geared toward fetal protection [109,110]. Overall, this study provides limited support for estradiol and progesterone themselves as predictors of emotional memory over-consolidation, implicating heightened stress response processes related to ovulation instead. The increased frequency of intrusive memories following acute stressor exposure may be one of several means by which elevations in cortisol and noradrenaline promote evolutionary fitness, in this case, potentially via cognitive threat salience and resultant behavioural vigilance.
This reasoning is consistent with recent reviews, suggesting a more nuanced interpretation of prior contradictory findings across research. Attempting to reconcile discrepant findings, authors have proposed that (a) high progesterone levels seem to enhance the risk of re-experiencing trauma [44]; (b) high estradiol levels may be a factor that enhances learning potential and thus promote both fears of learning and extinction in different contexts [58]; (c) individual variability in ovarian hormone levels likely contributes to mixed findings; and (d) research should also focus on small specific windows of the menstrual cycle, in addition to other factors, to understand relative risk and protection [44,56,60].
As an exploratory aim, we also examined the often-presumed relationship between intrusion frequency and the prevalence of detailed (relative to gist) intrusions. Upon forced recall tests, researchers frequently presented gist memories as evidence of low risk for re-experiencing and representative of a lack of memory over-consolidation. Based on these findings in the literature, it would be highly unlikely for such a large percentage of intrusions, as was apparent in these data, to be gist-based. As such, the fact that more than half of the intrusive memories in this study were gist-based challenges the assumptions that re-experiencing is always best captured by remembered detail. Furthermore, while a significant relationship between the number of intrusions and detailed content was supported, this association was small. Using detail associated with recall for emotional experiences may be a methodologically insubstantial way of extrapolating the risk of re-experiencing trauma. To our knowledge, this is the first study to assess the relationship between the frequency and detail of intrusive memories and does so within a nonclinical population. This finding demands replication; empirical validation of the assumptions with which trauma research operates is crucial for the advancement of the field.
This study has several strengths. The stimulus was realistic and produced visceral reactions in many participants; thus, extreme emotional stress reactivity (as evident in trauma) may have been better approximated. This is supported by the manipulation check, as participants exhibited a high degree of physiological and emotional responsivity, both objectively measured and self-reported. Furthermore, to improve the accuracy and generalizability of the data, real-time intrusion recording procedures were employed rather than retrospective estimates, and the recording period lasted for 5 days following exposure to the stressor.
This study also suffers from several limitations. Among those more common to the literature, group sizes were relatively small, and the study’s primary finding is based on the smallest group size (n = 14), potentially diminishing the generalizability of the results to the larger population. Moreover, estradiol levels did not differ significantly across groups; however, effect size estimates suggest that this was due to insufficient statistical power. Next, the study population was an undergraduate convenience sample. The use of a nonclinical sample precludes investigation into whether the process of emotional memory over-consolidation may operate differently in those at higher risk for psychopathology. As perhaps the most significant limitation, group designation relied on self-reported menstrual cycle data. Attempts were made to combat potential errors by having women track their cycles for two consecutive months and assaying estradiol and progesterone to confirm the likelihood of the projected phase. However, only a portion of salivary samples were assayed for estradiol and progesterone levels. Despite this, only one participant exhibited levels inconsistent with their projected phase; thus, there is nothing to suggest any inherent flaw in this methodology, engendering systematic bias in the results. Given the normative inter- and intra-individual variability associated with fluctuating estradiol and progesterone levels, the collection of circulating ovarian hormone levels over an entire menstrual cycle is undoubtedly the most accurate way to make phase determinations. Moreover, the ability to consistently assay for both ovarian and stress-response (e.g., CORT, NA) hormones across a cycle, considering hormone interrelationships and examining them as predictors of reactivity to and sequelae developing after exposure to a stressor, would provide the most comprehensive examination of these factors. Within this sample, both ovarian hormones tended to represent the higher norms for each phase, and substantial overlap in levels was evident across phases, as is consistent with research on younger women. Indeed, a tenet of ovarian hormone research is that variability in levels, in particular what constitutes high and low levels, is a function of the individual [111].
Given that research regarding how HCs impact stress and memory processes is still nascent [56], this study may also suffer from unknown limitations. HC users were homogenous in being on combined-type oral contraceptives (containing synthetic estradiol and progesterone). However, the relationship between levels of circulating ovarian hormones and neural levels is largely un-explicated. It is possible, for example, that taking HCs depresses plasma levels of estradiol while elevating levels in the brain, altering the cascade of HPA activity and memory processes. It is also possible that factors such as pill type, the presence of specific levels of exogenous ovarian hormones, and duration of HC use influence memory via CORT and NA regulation. Failure to control for these factors, in addition to cursory knowledge of the influence of HCs on neurobiological processes beyond those implicated in reproductive health, prevents drawing the firm conclusion that HCs never exhibit a protective effect. Indeed, it is possible that certain contraceptive types used for a certain duration of time may be protective. Additionally, study findings indicate an enhanced risk for emotional memory over-consolidation during ovulation, the very process that HCs are designed to suppress. Thus, in that regard, HCs do provide a protective effect. Finally, relevant to this study in particular, there was no way to monitor HC use (including the time of day that women took their pills), and assays among this group were particularly limited (12% of HC users had their saliva assayed). Thus, the influence of medication (non)adherence cannot be ruled out.
5. Conclusions
This research contributes to the accumulating literature examining the menstrual cycle on vulnerability for stress reactivity and emotional memory processes, suggesting that the window around ovulation is a period of particular vulnerability for enhanced stress reactivity. The replication of this research utilizing full cycle assay data and a larger sample of NC women (particularly in the ovulatory phase) is necessary to draw any conclusions about vulnerability toward stress and intrusion development across the menstrual cycle. To extend this literature, future research may assess changes from rest in circulating levels of CORT and other neuroendocrine stress indicators (e.g., salivary alpha-amylase release) following stimulus exposure, to confirm whether the theory of emotional memory over-consolidation is the appropriate paradigm from which to understand intrusive memory development in naturally cycling women. Given research supporting the direct effects of estradiol on neural areas implicated in memory generally, it is also possible that a distinctive mechanism is at play. Consistent with the tenet of equifinality, stress-responsivity during the window around ovulation may account for one distinctive pathway toward risk for intrusive memory development and PTSD, more generally, following trauma exposure.
Author Contributions
Conceptualization, K.A.D. and A.D.M.; methodology, K.A.D. and A.D.M.; validation, K.A.D., A.D.M., A.T. and D.M.M.; formal analysis, K.A.D. and A.D.M.; investigation, K.A.D.; resources, A.D.M.; data curation, K.A.D. and A.T.; writing—original draft preparation, K.A.D., A.D.M. and A.T.; writing—review and editing, D.M.M. and K.A.D.; visualization, D.M.M.; supervision, A.D.M. project administration, K.A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Pennsylvania State University (PRAMS 41329) in 2014.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author due to privacy concerns.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Olff, M. Sex and Gender Differences in Post-Traumatic Stress Disorder: An Update. Eur. J. Psychotraumatol. 2017, 8, 1351204. [Google Scholar] [CrossRef]
- Haering, S.; Seligowski, A.V.; Linnstaedt, S.D.; Michopoulos, V.; House, S.L.; Beaudoin, F.L.; An, X.; Neylan, T.C.; Clifford, G.D.; Germine, L.T.; et al. Disentangling Sex Differences in PTSD Risk Factors. Nat. Ment. Health 2024, 2, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, D.M.; Berke, E.T. Gender- and Sex-Based Contributors to Sex Differences in PTSD. Curr. Psychiatry Rep. 2020, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Chiu, W.T.; Demler, O.; Merikangas, K.R.; Walters, E.E. Prevalence, Severity, and Comorbidity of 12-Month DSM-IV Disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Tolin, D.F.; Foa, E.B. Sex Differences in Trauma and Posttraumatic Stress Disorder: A Quantitative Review of 25 Years of Research. Psychol. Bull. 2006, 132, 959–992. [Google Scholar] [CrossRef] [PubMed]
- Hourani, L.; Williams, J.; Bray, R.M.; Wilk, J.E.; Hoge, C.W. Gender Differences in Posttraumatic Stress Disorder and Help Seeking in the U.S. Army. J. Women’s Health 2016, 25, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Tolin, D.F.; Foa, E.B. Gender and PTSD: A Cognitive Model. In Gender and PTSD; Kimerling, R., Ouimette, P., Wolfe, J., Eds.; The Guilford Press: New York, NY, USA, 2002; pp. 76–97. [Google Scholar]
- Kucharska, J. Sex Differences in the Appraisal of Traumatic Events and Psychopathology. Psychol. Trauma Theory Res. Pract. Policy 2017, 9, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Rønning, L.; Zelkowitz, R.L.; Piccirillo, M.L.; Liu, J.; Thomas, J.L.; Guler, J.; van Zuiden, M. Gender Differences in Early Posttraumatic Stress Disorder Symptoms: A Network Analysis. Eur. J. Psychotraumatol. 2025, 16, 2448385. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Stevens, J.S.; Michopoulos, V. Neuroendocrine Pathways Underlying Risk and Resilience to PTSD in Women. Front. Neuroendocrinol. 2019, 55, 100790. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A. Post-Traumatic Stress Disorder: A State-of-the-Art Review of Evidence and Challenges. World Psychiatry 2019, 18, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.M.; Gaffey, A.E. Hormones and emotion: Stress and beyond. In Handbook of Cognition and Emotion; Robinson, M.D., Watkins, E., Harmon-Jones, E., Eds.; The Guilford Press: New York, NY, USA, 2013; pp. 69–94. [Google Scholar]
- Nursey, J.; Phelps, A.J. Stress, Trauma, and Memory in PTSD. In Stress: Concepts, Cognition, Emotion, and Behavior: Handbook of Stress; Fink, G., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 169–176. [Google Scholar] [CrossRef]
- Nicholson, E.L.; Bryant, R.A.; Felmingham, K.L. Interaction of Noradrenaline and Cortisol Predicts Negative Intrusive Memories in Posttraumatic Stress Disorder. Neurobiol. Learn. Mem. 2014, 112, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y.; Stalla, G.; Stalla, J.; Wotjak, C.T.; Anderzhanova, E. Norepinephrine and Corticosterone in the Medial Prefrontal Cortex and Hippocampus Predict PTSD-like Symptoms in Mice. Eur. J. Neurosci. 2015, 41, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, A.; Clark, D.M. A Cognitive Model of Posttraumatic Stress Disorder. Behav. Res. Ther. 2000, 38, 319–345. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.K.; Kleim, B.; Nicholson, E.L.; Zuj, D.V.; Cushing, P.J.; Gray, K.E.; Clark, L.; Felmingham, K.L. Sex differences in intrusive memories following trauma. PLoS ONE 2018, 13, e0208575. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, M.; Hinks, M.; Hallett, D.; Blundell, J.; Sweeney, E.; Thorpe, C.M.; Walling, S.G.; Swift-Gallant, A. Evidence that Ovarian Hormones, but Not Diet and Exercise, Contribute to the Sex Disparity in Post-Traumatic Stress Disorder. J. Psychiatr. Res. 2023, 168, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.J.; Matthews, A.; Bruno, R.; Felmingham, K.L. Modulation of the endocannabinoid system by sex hormones: Implications for posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2018, 94, 302–320. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, L.V.; Sharp, T.H.; Olff, M.; Seedat, S.; Halligan, S.L. Sex-Based Contributors to and Consequences of Post-traumatic Stress Disorder. Curr. Psychiatry Rep. 2023, 25, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Kornfield, S.L.; Hantsoo, L.; Epperson, C.N. What Does Sex Have to Do with It? The Role of Sex as a Biological Variable in the Development of Posttraumatic Stress Disorder. Curr. Psychiatry Rep. 2018, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Pooley, A.E.; Benjamin, R.C.; Sreedhar, S.; Eagle, A.L.; Robison, A.J.; Mazei-Robison, M.S.; Breedlove, S.M.; Jordan, C.L. Sex Differences in the Traumatic Stress Response: The Role of Adult Gonadal Hormones. Biol. Sex Differ. 2018, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Bayer, J.; Schultz, H.; Gamer, M.; Sommer, T. Menstrual-Cycle Dependent Fluctuations in Ovarian Hormones Affect Emotional Memory. Neurobiol. Learn. Mem. 2014, 110, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Walder, D.J.; Statucka, M.; Daly, M.P.; Axen, K.; Haber, M. Biological Sex and Menstrual Cycle Phase Modulation of Cortisol Levels and Psychiatric Symptoms in a Non-Clinical Sample of Young Adults. Psychiatry Res. 2012, 197, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Maki, P.M.; Mordecai, K.L.; Rubin, L.H.; Sundermann, E.; Savarese, A.; Eatough, E.; Drogos, L. Menstrual Cycle Effects on Cortisol Responsivity and Emotional Retrieval Following a Psychosocial Stressor. Horm. Behav. 2015, 74, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Chervonsky, L.; Felmingham, K.L.; Bryant, R.A. The Role of Estrogen in Intrusive Memories. Neurobiol. Learn. Mem. 2013, 106, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ertman, N.; Andreano, J.M.; Cahill, L. Progesterone at Encoding Predicts Subsequent Emotional Memory. Learn. Mem. 2011, 18, 759–763. [Google Scholar] [CrossRef]
- Felmingham, K.L.; Fong, W.C.; Bryant, R.A. The Impact of Progesterone on Memory Consolidation of Threatening Images in Women. Psychoneuroendocrinology 2012, 37, 1896–1900. [Google Scholar] [CrossRef]
- Kudielka, B.M.; Kirschbaum, C. Sex Differences in HPA Axis Responses to Stress: A Review. Biol. Psychol. 2025, 69, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Andreano, J.M.; Arjomandi, H.; Cahill, L. Menstrual Cycle Modulation of the Relationship between Cortisol and Long-Term Memory. Psychoneuroendocrinology 2008, 33, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Ferree, N.K.; Kamat, R.; Cahill, L. Influences of Menstrual Cycle Position and Sex Hormone Levels on Spontaneous Intrusive Recollections Following Emotional Stimuli. Conscious. Cogn. 2011, 20, 1154–1162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Montero-López, E.; Santos-Ruiz, A.; García-Ríos, M.C.; Rodríguez-Blázquez, M.; Rogers, H.L.; Peralta-Ramírez, M.I. The Relationship between the Menstrual Cycle and Cortisol Secretion: Daily and Stress-Invoked Cortisol Patterns. Int. J. Psychophysiol. 2018, 131, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Krinke, E.; Held, U.; Steigmiller, K.; Felmingham, K.; Kleim, B. Sex Hormones and Cortisol During Experimental Trauma Memory Consolidation: Prospective Association with Intrusive Memories. Eur. J. Psychotraumatol. 2022, 13, 2040818. [Google Scholar] [CrossRef] [PubMed]
- Franke, L.K.; Miedl, S.F.; Danböck, S.K.; Lohse, J.; Liedlgruber, M.; Bürkner, P.-C.; Pletzer, B.; Wilhelm, F.H. Estradiol during (analogue-) trauma: Risk- or protective factor for intrusive re-experiencing? Psychoneuroendocrinology 2022, 143, 105819. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.; Curran, V.H.; Kamboj, S.K. Identification of a Narrow Post-Ovulatory Window of Vulnerability to Distressing Involuntary Memories in Healthy Women. Neurobiol. Learn. Mem. 2013, 104, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Hantsoo, L.; Jagodnik, K.M.; Novick, A.M.; Baweja, R.; di Scalea, T.L.; Ozerdem, A.; McGlade, E.C.; Simeonova, D.I.; Dekel, S.; Kornfield, S.L.; et al. The role of the hypothalamic-pituitary-adrenal axis in depression across the female reproductive lifecycle: Current knowledge and future directions. Front. Endocrinol. 2023, 14, 1295261. [Google Scholar] [CrossRef] [PubMed]
- Høgsted, E.S.; Borgsted, C.; Dam, V.H.; Lundgaard, K.; Jensen, J.S.; Nielsen, N.E.A.; Andersen, M.; Hansen, A.S. Stress-Hormone Dynamics and Working Memory in Healthy Women Who Use Oral Contraceptives versus Non-Users. Front. Endocrinol. 2021, 12, 731994. [Google Scholar] [CrossRef] [PubMed]
- Boisseau, N.; Enea, C.; Diaz, V.; Dugué, B.; Corcuff, J.B.; Duclos, M. Oral Contraception but Not Menstrual Cycle Phase Is Associated with Increased Free Cortisol Levels and Low Hypothalamo-Pituitary-Adrenal Axis Reactivity. J. Endocr. Investig. 2013, 36, 955–964. [Google Scholar] [CrossRef]
- Roche, D.J.; King, A.C.; Cohoon, A.J.; Lovallo, W.R. Hormonal Contraceptive Use Diminishes Salivary Cortisol Response to Psychosocial Stress and Naltrexone in Healthy Women. Pharmacol. Biochem. Behav. 2013, 109, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Ferree, N.K.; Cahill, L. Post-Event Spontaneous Intrusive Recollections and Strength of Memory for Emotional Events in Men and Women. Conscious. Cogn. 2009, 18, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.E.; Ahmed, I.; Cahill, L. Sex and Menstrual Cycle Phase at Encoding Influence Emotional Memory for Gist and Detail. Neurobiol. Learn. Mem. 2013, 106, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.E.; Segal, S.K.; Worden, I.V.; Yim, I.S.; Cahill, L. Hormonal Contraception Use Alters Stress Responses and Emotional Memory. Biol. Psychol. 2013, 92, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Andreano, J.M.; Cahill, L. Sex Influences on the Neurobiology of Learning and Memory. Learn. Mem. 2009, 16, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.K.; Ney, L.J.; Honan, C.; Felmingham, K.L. Gonadal Steroid Hormones and Emotional Memory Consolidation: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2021, 130, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Beltz, A.M.; Moser, J.S. Ovarian Hormones: A Long Overlooked but Critical Contributor to Cognitive Brain Structures and Function. Ann. N. Y. Acad. Sci. 2020, 1464, 156–180. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.J.; Handa, R.J. Estrogen Impairs Glucocorticoid Dependent Negative Feedback on the Hypothalamic-Pituitary-Adrenal Axis via Estrogen Receptor Alpha within the Hypothalamus. Neuroscience 2009, 159, 883–895. [Google Scholar] [CrossRef]
- Li, S.H.; Graham, B.M. Why Are Women So Vulnerable to Anxiety, Trauma-Related and Stress-Related Disorders? The Potential Role of Sex Hormones. Lancet Psychiatry 2017, 4, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.M.; Newhouse, P.A. Estrogen, Stress, and Depression: Cognitive and Biological Interactions. Annu. Rev. Clin. Psychol. 2019, 15, 399–423. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, J.M.; Heller, C.; Kheloui, S.; Ismail, N.; Raval, A.P.; Schuh, K.M.; Tronson, N.C.; Leune, B. Beyond Birth Control: The Neuroscience of Hormonal Contraceptives. J. Neurosci. 2024, 44, e1235242024. [Google Scholar] [CrossRef] [PubMed]
- Gervasio, J.; Zheng, S.; Skrotzki, C.; Pachete, A. The effect of oral contraceptive use on cortisol reactivity to the Trier Social Stress Test: A meta-analysis. Psychoneuroendocrinology 2022, 136, 105626. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.E.; Ertman, N.; Lakhani, Y.S.; Cahill, L. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiol. Learn. Mem. 2011, 96, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Person, B.; Oinonen, K.A. Emotional Memory in Oral Contraceptive Users: Negative Stimuli Are More Forgettable. Psychol. Rep. 2020, 123, 2282–2304. [Google Scholar] [CrossRef] [PubMed]
- Ferree, N.K.; Wheeler, M.; Cahill, L. The Influence of Emergency Contraception on Post-Traumatic Stress Symptoms Following Sexual Assault. J. Forensic Nurs. 2012, 8, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Engel, S.; van Zuiden, M.; Frijling, J.L.; Koch, S.B.J.; Nawijn, L.; Schumacher, S.; Knaevelsrud, C.; Veltman, D.J.; Olff, M. Patterns of Recovery From Early Posttraumatic Stress Symptoms After a Preventive Intervention With Oxytocin: Hormonal Contraception Use Is a Prognostic Factor. Biol. Psychiatry 2019, 85, e71–e73. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, V.L.; Pötzl, L.; Wolf, O.T.; Merz, C.J. Hormonal contraceptive usage influences stress hormone effects on cognition and emotion. Front. Neuroendocrinol. 2022, 67, 101012. [Google Scholar] [CrossRef] [PubMed]
- Maslahati, T.; Schultebraucks, K.; Galve Gómez, M.; Hellmann-Regen, J.; Otte, C.; Wingenfeld, K.; Roepke, S. Effects of oral contraceptives on intrusive memories: A secondary analysis of two studies using the trauma film paradigm in healthy women. Eur. J. Psychotraumatol. 2023, 14, 2282003. [Google Scholar] [CrossRef] [PubMed]
- Spalek, K.; Loos, E.; Schicktanz, N.; Domes, G.; Schwabe, L.; Wolf, O.T. Women using hormonal contraceptives show increased valence ratings and memory performance for emotional information. Neuropsychopharmacology 2019, 44, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.M.; Walker, R.S.; Zoellner, L.A. Estrogen, Progesterone, and the Menstrual Cycle: A Systematic Review of Fear Learning, Intrusive Memories, and PTSD. Clin. Psychol. Rev. 2018, 66, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Mu, E.; Thomas, E.H.X.; Kulkarni, J. Menstrual Cycle in Trauma-Related Disorders: A Mini-Review. Front. Glob. Women’s Health 2022, 3, 910220. [Google Scholar] [CrossRef] [PubMed]
- Nillni, Y.I.; Rasmusson, A.M.; Paul, E.L.; Pineles, S.L. The Impact of the Menstrual Cycle and Underlying Hormones in Anxiety and PTSD: What Do We Know and Where Do We Go From Here? Curr. Psychiatry Rep. 2021, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Oyarzún, J.P.; Packard, P.A. Stress-induced gist-based memory processing: A possible explanation for overgeneralization of fear in posttraumatic stress disorder. J. Neurosci. 2012, 32, 9771–9772. [Google Scholar] [CrossRef]
- Adolphs, R.; Denburg, N.L.; Tranel, D. The Amygdala’s Role in Long-Term Declarative Memory for Gist and Detail. Behav. Neurosci. 2001, 115, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.Y.; La Marca, R.; Steptoe, A.; Brewin, C.R. Heart rate, startle response, and intrusive trauma memories. Psychophysiology 2014, 51, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Brewin, C.R.; Gregory, J.D.; Lipton, M.; Burgess, N. Intrusive images in psychological disorders: Characteristics, neural mechanisms, and treatment implications. Psychol. Rev. 2010, 117, 210–232. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.A.; Brewin, C.R.; Hennessy, R.G. Trauma Films, Information Processing, and Intrusive Memory Development. J. Exp. Psychol. Gen. 2004, 133, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Chouinard-Gaouette, L.; Blanchette, I. Validation of a trauma film: Emotional responses, intrusive memories and concept activations. Cognit. Ther. Res. 2024, 49, 403–414. [Google Scholar] [CrossRef]
- Lazarus, R.S.; Opton, E.M., Jr.; Nomikos, M.S.; Rankin, N.O. The Principle of Short-Circuiting of Threat: Further Evidence. J. Pers. 1965, 33, 622–635. [Google Scholar] [CrossRef] [PubMed]
- James, E.L.; Lau-Zhu, A.; Clark, I.A.; Visser, R.M.; Hagenaars, M.A.; Holmes, E.A. The Trauma Film Paradigm as an Experimental Psychopathology Model of Psychological Trauma: Intrusive Memories and Beyond. Clin. Psychol. Rev. 2016, 47, 106–142. [Google Scholar] [CrossRef] [PubMed]
- Substance Abuse and Mental Health Services Administration. Table 3.14, DSM-IV to DSM-5 Posttraumatic Stress Disorder Comparison. In Impact of the DSM-IV to DSM-5 Changes on the National Survey on Drug Use and Health; Substance Abuse and Mental Health Services Administration (US): Rockville, MD, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519704/table/ch3.t14/ (accessed on 10 June 2025).
- Holmes, E.A.; Bourne, C. Inducing and Modulating Intrusive Emotional Memories: A Review of the Trauma Film Paradigm. Acta Psychol. 2008, 127, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-Y.; La Marca, R.; Steptoe, A.; Brewin, C.R. Biological responses to trauma and the development of intrusive memories: An analog study with the trauma film paradigm. Biol. Psychol. 2014, 103, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.A.; Niehaus, K.E.; Duff, E.P.; Di Simplicio, M.C.; Clifford, G.D.; Smith, S.M.; Holmes, E.A. First Steps in Using Machine Learning on fMRI Data to Predict Intrusive Memories of Traumatic Film Footage. Behav. Res. Ther. 2014, 62, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Iyadurai, L.; Visser, R.M.; Lau-Zhu, A.; Porcheret, K.; Horsch, A.; Holmes, E.A.; James, E.L. Intrusive memories of trauma: A target for research bridging cognitive science and its clinical application. Clin. Psychol. Rev. 2019, 69, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Klump, K.L.; Keel, P.K.; Racine, S.E.; Burt, S.A.; Neale, M.; Sisk, C.L.; Boker, S.; Hu, J.Y. The Interactive Effects of Estrogen and Progesterone on Changes in Emotional Eating across the Menstrual Cycle. J. Abnorm. Psychol. 2013, 122, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.L. The Endocrinology of the Menstrual Cycle. Methods Mol. Biol. 2014, 1154, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.G.; Carr, B.R. The Normal Menstrual Cycle and the Control of Ovulation. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2018. [Google Scholar]
- Arélin, K.; Mueller, K.; Barth, C.; Rekkas, P.V.; Kratzsch, J.; Burmann, I.; Villringer, A.; Sacher, J. Progesterone Mediates Brain Functional Connectivity Changes during the Menstrual Cycle—A Pilot Resting State MRI Study. Front. Neurosci. 2015, 9, 44. [Google Scholar] [CrossRef]
- Pletzer, B. Editorial: From Sex Differences in Neuroscience to a Neuroscience of Sex Differences: New Directions and Perspectives. Front. Neurosci. 2015, 9, 330. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Foa, E.B.; McLean, C.P.; Zang, Y.; Zhong, J.; Powers, M.B.; Kauffman, B.Y.; Knowles, K. Psychometric Properties of the Posttraumatic Diagnostic Scale for DSM-5 (PDS-5). Psychol. Assess. 2016, 28, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Hampson, E.; Young, E.A. Methodological Issues in the Study of Hormone-Behavior Relations in Humans: Understanding and Monitoring the Menstrual Cycle. In Sex Differences in the Brain: From Genes to Behavior; Becker, J.B., Berkley, K.J., Geary, N., Hampson, E., Herman, J.P., Young, E.A., Eds.; Oxford University Press: New York, NY, USA, 2008; pp. 63–78. [Google Scholar]
- Hampson, E. A Brief Guide to the Menstrual Cycle and Oral Contraceptive Use for Researchers in Behavioral Endocrinology. Horm. Behav. 2020, 119, 104655. [Google Scholar] [CrossRef] [PubMed]
- Gildner, T.E. Reproductive Hormone Measurement from Minimally Invasive Sample Types: Methodological Considerations and Anthropological Importance. Am. J. Hum. Biol. 2021, 33, e23535. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.D.; Nehmy, T.; Seymour, M. The Effect of Cognitive Load and Hyperarousal on Negative Intrusive Memories. Behav. Res. Ther. 2023, 45, 2652–2663. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.D.; Cain, N.; Nehmy, T.; Seymour, M. The Influence of Thought Suppression and Cognitive Load on Intrusions and Memory Processes Following an Analogue Stressor. Behav. Ther. 2009, 40, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; McRae-Clark, A.L.; Carlson, S.; Lynch, W.J.; Saladin, M.E.; Gray, K.M.; Spratt, S. Determining menstrual phase in human biobehavioral research: A review with recommendations. Exp. Clin. Psychopharmacol. 2016, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Shimbo, D.; Carey, R.M.; Charleston, J.B.; Gaillard, T.; Jamerson, K.A.; McEvoy, J.W.; Smith, S.M.; Stanley, S.; Weber, M.A.; et al. Measurement of Blood Pressure in Humans: A Scientific Statement From the American Heart Association. Hypertension 2019, 73, e35–e66. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Knowles, K.A.; Olatunji, B.O. Specificity of Trait Anxiety in Anxiety and Depression: Meta-Analysis of the State-Trait Anxiety Inventory. Clin. Psychol. Rev. 2020, 82, 101928. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, A.; González-Robles, A.; Mor, S.; Mira, A.; Quero, S.; García-Palacios, A.; Baños, R.M.; Botella, C. Positive and Negative Affect Schedule (PANAS): Psychometric Properties of the Online Spanish Version in a Clinical Sample with Emotional Disorders. BMC Psychiatry 2020, 20, 56. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Y.; Zhao, P.; Yang, J. Effects of Level of Processing on Emotional Memory: Gist and Details. Cogn. Emot. 2011, 25, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Shevlyakov, G.; Andrea, K.; Choudur, L.; Smirnov, P.; Ulanov, A.; Vassilieva, N. Robust versions of the Tukey boxplot with their application to detection of outliers. In Proceedings of the 2013 IEEE International Conference on Acoustics, Speech and Signal Processing, Vancouver, BC, Canada, 26–31 May 2013; pp. 6506–6510. [Google Scholar] [CrossRef]
- Mordecai, K.L.; Rubin, L.H.; Eatough, E.; Glynn, L.M.; Johnson, L.; Sandman, C.A.; Cahill, L. Cortisol reactivity and emotional memory after psychosocial stress in oral contraceptive users. J. Neurosci. Res. 2017, 95, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Derntl, B.; Kryspin-Exner, I.; Fernbach, E.; Moser, E.; Habel, U. Emotion Recognition Accuracy in Healthy Young Females Is Associated with Cycle Phase. Horm. Behav. 2008, 53, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Osório, F.L.; de Paula Cassis, J.M.; Machado de Sousa, J.P.; Poli-Neto, O.; Martín-Santos, R. Sex Hormones and Processing of Facial Expressions of Emotion: A Systematic Literature Review. Front. Psychol. 2018, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Derntl, B.; Windischberger, C.; Robinson, S.; Lamplmayr, E.; Kryspin-Exner, I.; Gur, R.C.; Moser, E.; Habel, U. Facial Emotion Recognition and Amygdala Activation Are Associated with Menstrual Cycle Phase. Psychoneuroendocrinology 2008, 33, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Berga, S.; Naftolin, F. Neuroendocrine Control of Ovulation. Gynecol. Endocrinol. 2012, 28 (Suppl. 1), 9–13. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E. Chapter 7—Neuroendocrine Control of the Menstrual Cycle. In Yen and Jaffe’s Reproductive Endocrinology, 8th ed.; Strauss, J.F., Barbieri, R.L., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 149–166.e5. [Google Scholar] [CrossRef]
- Acevedo-Rodriguez, A.; Kauffman, A.S.; Cherrington, B.D.; Borges, C.S.; Roepke, T.A.; Laconi, M. Emerging insights into hypothalamic-pituitary-gonadal axis regulation and interaction with stress signalling. J. Neuroendocrinol. 2018, 30, e12590. [Google Scholar] [CrossRef] [PubMed]
- Berga, S.L. Stress-induced anovulation. In Stress: Physiology, Biochemistry, and Pathology; Academic Press: Cambridge, MA, USA, 2019; pp. 213–226. [Google Scholar]
- Lynch, C.D.; Sundaram, R.; Maisog, J.M.; Sweeney, A.M.; Buck Louis, G.M. Preconception stress increases the risk of infertility: Results from a couple-based prospective cohort study—The LIFE study. Hum. Reprod. 2014, 29, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Louis, G.M.B.; Lum, K.J.; Sundaram, R.; Chen, Z.; Kim, S.; Lynch, C.D.; Schisterman, E.F.; Pyper, C. Stress reduces conception probabilities across the fertile window: Evidence in support of relaxation. Fertil. Steril. 2011, 95, 2184–2189. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-N.; Li, L.; Hou, G.; Wang, Z.-B.; Hou, Y.; Liu, Z.-H.; Schatten, H.; Sun, Q.-Y. Glucocorticoid exposure affects female fertility by exerting its effect on the uterus but not on the oocyte: Lessons from a hypercortisolism mouse model. Hum. Reprod. 2018, 33, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.T.; Zhang, H.Y.; Chen, S.T.; Li, M.Y.; Fu, T.; Yang, Z.M. The detrimental effects of stress-induced glucocorticoid exposure on mouse uterine receptivity and decidualization. FASEB J. 2020, 34, 14200–14216. [Google Scholar] [CrossRef] [PubMed]
- Smeenk, J.M.J.; Verhaak, C.M.; Vingerhoets, A.J.J.M.; Sweep, C.G.J.; Merkus, J.M.W.M.; Willemsen, S.J.; van Minnen, A.; Straatman, H.; Braat, D.D.M. Stress and outcome success in IVF: The role of self-reports and endocrine variables. Hum. Reprod. 2005, 20, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Nepomnaschy, P.A.; Welch, K.B.; McConnell, D.S.; Low, B.S.; Strassmann, B.I.; England, B.G. Cortisol levels and very early pregnancy loss in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 3938–3942. [Google Scholar] [CrossRef] [PubMed]
- Derntl, B.; Hack, R.L.; Kryspin-Exner, I.; Habel, U. Association of Menstrual Cycle Phase with the Core Components of Empathy. Horm. Behav. 2013, 63, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Pilarczyk, J.; Schwertner, E.; Wołoszyn, K.; Kuniecki, M. Phase of the Menstrual Cycle Affects Engagement of Attention with Emotional Images. Psychoneuroendocrinology 2019, 104, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Wolohan, F.D.A.; Bennett, S.J.V.; Crawford, T.J. Females and attention to eye gaze: Effects of the menstrual cycle. Exp. Brain Res. 2013, 227, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, L.; Wang, X. The Effect of Menstrual Cycle Phases on Approach-Avoidance Behaviors in Women: Evidence from Conscious and Unconscious Processes. Brain Sci. 2022, 12, 1417. [Google Scholar] [CrossRef] [PubMed]
- Gloe, L.M.; Russman Block, S.; Klump, K.L.; Beltz, A.M.; Moser, J.S. Determining menstrual cycle phase: An empirical examination of methodologies and recommendations for improvement in behavioral and brain sciences. Horm. Behav. 2023, 155, 105421. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).