Abstract
There is evidence that the quality of life and mental health of pregnant women change during pregnancy. To evaluate the impact of physical activity on the quality of life and mental health of pregnant women with obesity or overweight, a systematic review was performed using six electronic databases (PubMed, Cochrane (CENTRAL), ScienceDirect, Scielo, BVS and PEDro). In total, 205 articles were collected, and after screening in accordance with the PRISMA declaration, six randomized clinical trials were selected. Methodological quality was assessed using the Cochrane RoB2 tool and a narrative synthesis of the results was performed. Physical activity interventions did not demonstrate statistically significant results on the quality of life and mental health of pregnant women with obesity or overweight. The effects of physical activity during pregnancy for women with obesity or overweight are varied due to the diversity of interventions implemented. Nonetheless, a discernible positive association emerges between stringent adherence to the prescribed physical activity regimen and enhanced physical well-being, weight management, and heightened aerobic capacity. In order to ascertain more definitive conclusions, rigorous clinical trials are needed that take into account the heterogeneity of interventions and ensure adequate adherence to the protocol.
1. Introduction
Pregnancy has the potential to trigger behavioral changes in women [1,2,3]. This is because during this period anatomical, biochemical, physiological and hormonal changes are triggered, affecting mood and emotions, which can impact quality of life during pregnancy [1,2,3].
Quality of life, defined as “individuals’ perceptions of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns”, is intrinsically linked to each individual’s personal context and value system [4]. It is a multidimensional concept that encompasses physical, psychological and social aspects. Quality of life perceived by pregnant women is essential to the concept of perinatal health, and can be influenced by clinical and non-clinical events that are significant for women during this period [1]. In what concerns psychological aspects, mental health complications during pregnancy are frequent and can be intrinsically related to overweight and obesity [2], which are common conditions in women of reproductive age [5]. It is estimated that in 2016, around 55% of adult women had body mass indexes (BMIs) categorized as overweight and obesity [6], and this number is projected to increase, therefore being classified as a medical condition of pandemic dimensions [7].
Overweight pregnant women are those whose pre-pregnancy BMI is equal to or greater than 25 kg/m2, and pregnant women with obesity are those whose pre-pregnancy BMI is equal to or greater than 30 kg/m2 [8]. Excessive weight and obesity when associated with pregnancy can increase the risk of cardiovascular comorbidities, such as gestational diabetes, pregnancy-induced hypertension, pre-eclampsia and heart disease [9,10]. In addition, it can result in premature birth [11], increase the risk of caesarean section and lead to a longer hospital stay after delivery [9,10]. Maternal comorbidities directly affect the fetus, significantly increasing its vulnerability to cardiovascular complications [12], leading to a higher incidence of fetal distress, stillbirth and neonatal death, and predisposing it to develop overweight or obesity throughout life [13]. Studies indicate that children born to women with overweight or obesity face a higher chance of developing obesity themselves [13,14].
Physical activity (PA) during pregnancy can play a major role in reducing the impact of these comorbidities on women’s health [15,16,17]. International organizations recommend that all women without contraindications perform regular PA during pregnancy and postpartum, corresponding to at least 150 min of moderate-intensity aerobic PA per week [16,18,19]. The benefits associated with PA during pregnancy include improvements in cardiovascular function, a sense of well-being and sleep quality [20], a reduction in the risk of excessive weight gain, gestational diabetes [16], pre-eclampsia, varicose veins, thrombosis and low back pain [21]. It also reduces levels of fatigue, stress, anxiety and depression and has a positive impact on the mother’s quality of life after giving birth [3,20,21].
Some authors have evaluated the impact of PA on the lives of pregnant women and the positive effects on the mother’s well-being and quality of life, indicating that regular practice reduces fatigue and negative feelings, as well as providing greater satisfaction with health [3,20,21]. However, to date, the effect that this intervention could have on the quality of life of overweight or obese pregnant women is unknown.
Therefore, the aim of this study is to conduct a systematic review on the effects of PA on the quality of life of a specific population of overweight and obese pregnant women.
2. Results
2.1. Literature Search and Selection
The literature search initially identified 205 records in six databases: 16 in PubMed, 159 in the Cochrane Central Register of Controlled Trials (CENTRAL), 9 in ScienceDirect, 15 in Scielo, 4 in the Virtual Health Library (BVS) and 2 in the Physiotherapy Evidence Database (PEDro). Of these, 24 were duplicates. After screening the titles, abstracts and full text of the remaining 181 studies, 93 articles were excluded, and the full text of 88 publications was screened (reason 1: not RCT; reason 2: not written in Portuguese or English; reason 3: sample without pregnant women; reason 4: sample without pregnant women with overweight or obesity; reason 5: the intervention without PA or exercise; reason 6: outcomes without quality of life evaluation), from which six randomized controlled clinical trials were selected for inclusion in the review, and none of the selected articles were written in Portuguese. Figure 1 shows the process of searching for and selecting the studies in accordance with the PRISMA statement [22].
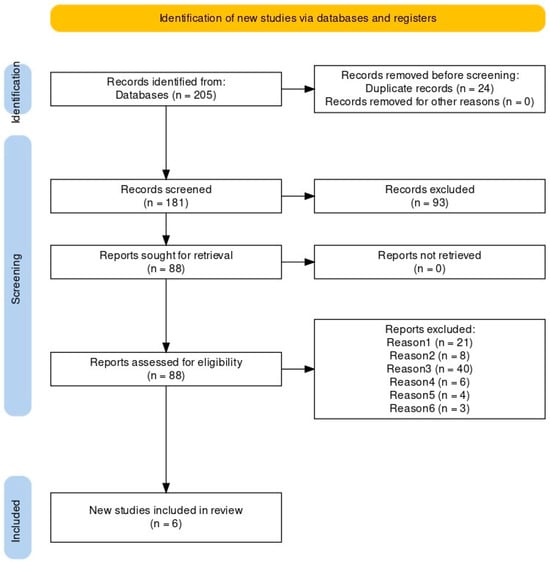
Figure 1.
PRISMA flowchart reporting the process of searching for and selecting the studies.
2.2. Characteristics of the Included Studies
The six studies [23,24,25,26,27,28] were published between 2013 [27] and 2022 [26]. Three studies were carried out in Europe [25,26,27], and the other three took place in the United States of America [23], Australia [24] and New Zealand [28]. All studies used hospital infrastructures, with five of them taking place in a public setting [24,25,26,27,28] and one in a private setting [23].
A detailed summary of the characteristics of each study can be found in Table 1.

Table 1.
Summary of findings: characteristics of the studies.
2.3. Assessment of Methodological Quality
The results of version 2 of the Cochrane tool for assessing the risk of bias in randomized clinical trials (RoB2) [29] are shown in Figure 2.
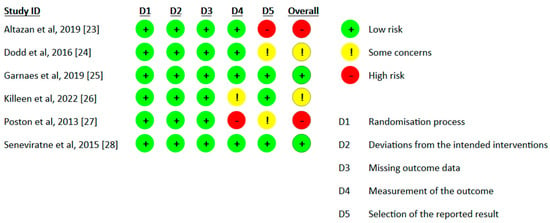
Figure 2.
Risk of bias among included studies [23,24,25,26,27,28].
Of the six studies included, two were considered to be at “high risk of bias” [23,27] two were considered to be of “some concern” [24,26] and two were considered to be at “low risk of bias” [25,28]. The domains for which two of the publications were assessed with a “high risk of bias” are the “selection of reported results” domain, due to the studies representing secondary measures and not being part of the a priori power analysis [23] , and the “measurement of results” domain, due to the use of dummy variables for physical activity data when baseline values were absent [27]. The same domains also reveal “some concerns” in three clinical trials due to missing information related to the selection of the data presented [24,27] and the evaluators’ knowledge of the intervention due to its direct nature [26]. On the other hand, the domains relating to the “randomization process”, “deviations from the intended interventions” and “missing outcome data” present a “low risk of bias” in all six articles selected for review.
2.4. Characteristics of the Sample
The total sample comprised 2684 individuals, with an average age of approximately 30.6 years, and the number of participants in each study ranged from 54 [23] to 1933 [24].
The average gestational age at the start of the interventions was 12.5 weeks, ranging from 10 [23] to 20 weeks [28], and only four of the analyzed articles mentioned these data [23,24,27,28].
In terms of inclusion criteria, all the studies included pregnant women who were overweight or obese. One of the studies [27] had a sample made up only of pregnant women with a BMI ≥ 30 kg/m2, another study [25] focused only on pregnant women with a BMI ≥ 28 kg/m2 and in the remaining four studies the BMI considered was ≥25 kg/m2 [23,24,26,28].
Four studies included pregnant women over the age of 18 [16,23,25,28], two of which set an age limit of 40 [23,28] and one of 45 [26].
All the studies defined single pregnancy as an inclusion criterion, and the gestational age of inclusion differed between the studies, varying overall between 10 and 20 weeks. Two of the studies only specified the maximum gestational age as an eligibility criterion, at 12 [23] and 20 weeks [28], while the other studies considered gestational ages within the following time ranges: between 10 and 15 weeks [26], between 10 and 20 weeks [24], between 12 and 18 weeks [25] and between 15 and 17 weeks of gestation [27].
There were other requirements considered for inclusion in the studies, such as knowledge of the English language [23,26], authorization from the obstetrician and no clinical history of psychological disorders [23], or the requirement for women to own a smartphone [26].
2.5. Characteristics of the Interventions
Four studies were based on the premise of behavioral change through nutritional and lifestyle counseling, aimed at acquiring healthy habits relating to diet and PA practice [23,24,26,27]. The different interventions were based on advised diets, combined with recommendations for practicing various types of PA, such as walking [24,27] or recreational activities [24]. Two studies used smartphone apps to interact with the participants and monitor the progress of the interventions, serving as a source of information and motivation [23,26]. None of the studies reported pre-pregnancy physical activity levels.
The other two studies based their intervention on the prescription of structured PA supervised by a physiotherapist [25] or an exercise physiologist [28]. Both prescriptions included aerobic exercise, on a treadmill [25] or stationary bike [28]. One consisted of an entirely home-based exercise program [28]. The other was carried out in face-to-face sessions and also included muscular resistance exercises, including pelvic floor muscle training, and an autonomous home exercise program [25].
2.6. Measures of Study Results
Outcome assessments were carried out at various times, from the start of the clinical trials and during pregnancy to the postpartum period.
Several measuring instruments were used to assess quality of life, including the EuroQol questionnaire (EQ-5D) (Poston et al., 2013) [27], the MOS 36-Item Short-Form Health Survey (SF-36) [26], the Rand 12-Item Short Form (SF-12) [23] and the WHO QOL-BREF [28].
With regard to mental health, the risk of depression was assessed using the Edinburgh Postnatal Depression Scale (EPDS) [24,25] and the Beck Depression Inventory II (BDI-II) [23], anxiety was assessed with the Spielberger State-Trait Inventory Self-Evaluation Questionnaire (STAI) (Dodd et al., 2016) [24] and psychological well-being was assessed using the Psychological General Well-Being Index (PGWBI) questionnaire [25] and the World Health Organization Well-Being Index (WHO-5) [26].
Other measuring instruments were used to assess various outcomes, such as calibrated scales to monitor gestational weight gain [23,28], laboratory analysis to draw up the participants’ cardiometabolic profiles [26] , heart rate monitors to monitor aerobic fitness and exercise adherence [28], accelerometers and the Recent Physical Activity Questionnaire (RPAQ) to record physical activity [27] , a 24 h food recall and a food frequency questionnaire to monitor diet [27], logbooks and pedometers to check behaviors related to meeting predefined SMART goals [27] , a 10-point Likert scale to analyze the participants’ satisfaction with their pregnancy and childbirth experiences [24], and a question taken from the MOS 36-Item Short-Form Health Survey (SF-36) to report their self-perception of general health status [25].
2.7. Narrative Synthesis of the Results of the Selected Studies
Dietary counseling and physical activity interventions showed no significant effect on quality of life when compared with that under standard prenatal care [23,24,27] , with two studies recording a deterioration in quality of life throughout pregnancy [24,27]. The same was true for exercise prescription [25,28]. However, in this case, greater adherence to the protocol was associated with better post-intervention physical quality of life [28]. Adverse effects of physical activity in pregnant women have not been reported by any authors.
In the field of mental health, lifestyle interventions have not been shown to have a significant impact on changes in the risk of depression [23,24,27] and anxiety [24] or on levels of psychological well-being reported by the participants [26]. The same was true of supervised exercise, the effects of which were not statistically significant, either on psychological well-being or on symptoms of postpartum depression, and low adherence to exercise protocols and small sample sizes may have contributed to this result [25].
In what concerns gestational weight gain, an intervention based on the prescription of physical exercise [28] showed no differences between the groups. On the other hand, a behavioral and lifestyle counseling intervention showed positive results in weight control throughout pregnancy, with the experimental group achieving a gestational weight gain of percentage of <25.5% compared with the control group [23]. Greater gestational weight gain was also associated with worsening mood, depressive symptoms and lower physical health-related quality of life during pregnancy, returning to baseline values or slightly better values in the postpartum period [23].
Lifestyle change interventions contributed to other outcomes, such as participants’ improved knowledge about healthy eating choices and exercise during pregnancy, and greater confidence about their own health and that of their baby [24]. An increase in self-reported physical activity during pregnancy was also observed, although these data was not corroborated via objective evaluation [27]. In turn, prescribed physical exercise showed positive effects in relation to the aerobic fitness and maximum workload of the participants [28] (Senevirate et al., 2016).
3. Methods
3.1. Registration
This article was developed in accordance with the PRISMA statement [22], and the respective protocol was registered in the PROSPERO database (ID385430).
3.2. Review Question
Despite the existence of research on the impact of exercise on the quality of life of pregnant women [1,3], we were unable to find any studies that explicitly analyzed pregnant women with overweight or obesity. Therefore, our research question was as follows: “Does physical activity influence quality of life of pregnant women with pre-gestational overweight or obesity?”. Relative to this, the primary outcome we intend to study is quality of life, with secondary outcomes relating to mental health in pregnancy.
3.3. Research Strategy
A systematic search was carried out between November and December 2022, using six electronic databases: PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), ScienceDirect, Scielo, Virtual Health Library (VHL) and Physiotherapy Evidence Database (PEDro).
The PICO strategy of the research is in line with the review question and covers the population of pregnant women with obesity and pre-gestational overweight (BMI ≥ 25 kg/m2), and interventions that include physical activity and/or physical exercise, making a comparison with standard interventions (medical consultations, ultrasounds, clinical analysis, etc.), with outcomes on quality of life.
The keywords derived from the PICO elements were used in different combinations with Boolean operators and combined the following terms (medical subject headings (MeSH)): “obesity” OR “obese” OR “overweight” OR “excessive weight” AND “pregnancy” OR “pregnant” OR “maternal” OR “antenatal” OR “gestational” OR “prenatal” OR “gravid” AND “physical activity” OR “exercise” OR “movement” OR “workout” OR “training” OR “exercising” OR “fitness” AND “quality of life” OR “QoL” OR “wellbeing” OR “happiness” OR “standard of living”.
The searches were limited to articles published between December 2012 and December 2022 and written in English or Portuguese, which are listed in the Supplementary Material (Table S1).
3.4. Eligibility Criteria
The eligibility criteria for the articles were as follows: (a) randomized clinical trials (to evaluate the effectiveness of PA and exercise); (b) written in English or Portuguese; (c) on adult pregnant women (≥18 years); (d) with pre-gestational overweight or obesity (BMI ≥ 25 kg/m2); (e) without any medical contraindication to the practice of PA [15,19]; (f) who performed any type of PA.
Exclusion criteria were as follows: (a) articles not including pregnant women with pregestational overweight or obesity; (b) not assessing quality of life; (c) with interventions that do not include physical activity and/or exercise. Data on the exclusion criteria for the studies can be found in Supplementary Material (Table S1).
3.5. Data Collection
Two reviewers (DS and IF) collected and analyzed the information from each article, collecting data on the respective studies and their characteristics, looking at quality of life as the primary outcome and gestational weight gain and mental health in pregnancy as secondary outcomes.
3.6. Screening and Selection of Studies
The studies were selected in a three-stage process. First, two reviewers (DS and IF) independently searched different databases and exported the results to EndNote20. After removing duplicates, the titles and abstracts were analyzed according to the eligibility criteria, followed by a screening of the full text for the consideration of inclusion. Disagreements between the two reviewers were resolved through a discussion at each stage, and, in the absence of consensus, a third reviewer was consulted (DB).
3.7. Certainty of the Evidence and Risk of Bias
The risk of bias of the articles included in the review was assessed by two reviewers independently (DS and IF), using version 2 of the Cochrane tool for assessing the risk of bias in randomized clinical trials (RoB2) [29]. This tool assesses different types of bias that may be present in this type of study and is based on five domains: “randomization process”, “deviations from intended interventions”, “missing outcome data”, “measurement of outcomes” and “selection of reported outcomes” [29].
The domains include signaling questions that aim to elicit information relevant to assessing the risk of bias. The assessments of each domain are classified as follows: “low risk of bias”, “some concern” or “high risk of bias” [29].
The sum of the assessment of the five domains results in the “overall risk of bias” for a given study, and it is considered to be at a “low risk of bias” if it is assessed with this result in all domains; to be of “some concern” if the study presents this assessment in at least one domain, but is not at a high risk of bias for any of them; and to be of a “high risk of bias” if the study is assessed in this way in at least one domain, or if it presents some concerns in several domains [29].
4. Discussion
As far as we know, this study is the first systematic review to investigate the effects of PA on the quality of life of pregnant women with overweight or obesity. There is a systematic review that evaluates PA on quality of life of pregnant women [3], others that address the influence of various factors on quality of life of pregnant women [1,2] and another that analyzes the effectiveness of PA on various pregnancy-related outcomes [30], but none of them specifically focused on the population subgroup of our research.
As in the systematic review by Chan, Yeung & Law [30], the results of this study are inconsistent with regard to the beneficial effects of PA on the quality of life of pregnant women. Some authors mentioned in this review found an increase in quality of life in women who practiced PA, while others found no positive effects, and this was mainly due to the fact that the exercise/physical activity intervention was not defined and varied between studies.
In contrast, the systematic reviews by Liu et al. (2019) and Boutib et al. (2022) on the influencing factors of PA reflect an increase in the quality of life of pregnant women, with normative weight, who practice PA [1,3]. In their research, Boutib et al. (2022) considered several factors that could affect the quality of life of pregnant women, such as low back pain, sleep quality, gestational weight gain, diabetes mellitus, nausea and vomiting, exercise adherence and social support [1].
In 2018, Lagadec and co-authors [2] concluded that non-obese pregnant women have a lower quality of life compared with the general population, and compared with women in the same age group. Quality of life decreased with increasing limitations and decreasing PA practice throughout pregnancy, with the third trimester showing the most significant decrease [2]. Maternal obesity and psychological factors are associated with a decrease in the quality of life of pregnant women [1], and this may be one of the main factors for the inconclusive results in this review.
Depression and anxiety during and after pregnancy have an impact on the short- and long-term health problems of mother and child [31]. Our review found no statistically significant effects of structured PA on either psychological well-being or symptoms of postpartum depression, and poor adherence to intervention protocols and small sample sizes may have had some influence on these results [32].
The systematic review and meta-analysis by Davenport et al. (2018) [33] showed that although prenatal structured PA has benefits on depression or depressive symptoms during pregnancy, these results did not extend to the postpartum period. This seems to corroborate our findings to some extent. However, during the gestational period, these interventions were associated with a 67% reduction in the odds of developing prenatal depression and a reduction in the severity of prenatal depressive symptoms. This effect is similar to that found for psychological treatments in pregnant women with depression, is directly related to the amount of exercise and is seen more in pregnant women who are supervised during exercise [31].
Lifestyle change interventions, such as PA and nutritional counseling, have also not been shown in this review to have a significant impact on the levels of psychological well-being reported by the participants [26] or on changes in the risk of depression [23,24,27] and anxiety [24].
These results are contradictory to those of a meta-analysis by Nakamura et al. (2019) [34] that indicate that a minimum of 30 min of moderate-intensity PA one to four times a week can contribute to reducing the risk of postpartum depression. They concluded that practicing PA during pregnancy has been shown to reduce the risk of prenatal depression, which is predictive of postpartum depression [34].
Structured PA prevents and treats anxiety and depression in non-pregnant populations, but it is still poorly understood whether or not this impact is replicated in mental health problems during pregnancy [31], especially in the overweight or obese subgroup.
Studies show that the majority of pregnant women do not comply with recommended guidelines for practicing PA, and factors such as tiredness, pain and a growing belly are pregnancy-related barriers [35]; although these are not contraindications during a healthy pregnancy, they tend to contribute to a decrease in PA levels during the gestational period [34]. However, pregnancy represents a window of opportunity to prevent or limit any negative outcomes, as it is a period when women are more receptive to learning and may be more able to incorporate changes into their routine, namely changes in PA habits. The use of digital media to prescribe exercise for obese pregnant women appears to have a favorable impact on physical activity levels and may reduce the barriers to physical inactivity during pregnancy [36]. Therefore, these behavioral changes, which are sometimes so difficult to implement in the general population, can be more easily adopted by women during the pregnancy period, and they have even become a key predictor for enabling women to adopt a healthy lifestyle [34,37]. Furthermore, it has been shown that receiving PA recommendations led to 40.5% (95% CI 38.4–42.4) of women choosing to remain active during pregnancy [38]. On the other hand, the use of smartphone app technology has also been shown to play a key role in facilitating a beneficial change in behavior that can lead to better health outcomes for the mother and fetus [37].
From a public health perspective, behavior change should be enhanced by providing information on the benefits of physical activity for mental health in pregnancy, in particular group physical activity, which could provide additional social support in reducing depressive symptoms and preventing postpartum depression [34].
This research also has some limitations, such as the restricted inclusion of randomized clinical trials, which excludes any other type of study. Research was limited to the English and Portuguese languages only, and the generalizability of the results can be debated. In four of the six studies included, physical activity was not the only factor to be assessed, and other factors also had an influence on the participants’ quality of life [23,24,26,27]. We can also consider variability of measurement instruments for assessing symptoms of depression and quality of life a limitation, as well as the lack of a defined structure in the physical activity intervention. Confounding variables such as pregnant women’s income and comorbidities associated with pregnancy were not analyzed and may influence quality of life during pregnancy. In addition, the gestational age taken into account in the various clinical trials did not include the beginning of pregnancy, so it can be said that none of the studies included data on the quality of life of pregnant women during pregnancy in its entirety. Furthermore, the adherence rate was low, which could contribute to a possible bias in the results.
5. Conclusions
PA does not seem to have an effect on the quality of life and mental health of overweight or obese pregnant women. However, it was not possible to obtain quantitative results from this systematic review due to the heterogeneity of the interventions. In this area, there is a need to develop rigorous clinical trials to establish the direct impact of physical activity and exercise interventions on the quality of life and mental health of this population. It would be important to define a structured exercise regime with intensity, frequency, type and time and to use the same assessment instruments to evaluate the different outcomes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/women4020010/s1, Table S1: Database search results for 13 December 2022.
Author Contributions
All authors made substantive intellectual contributions to the development of this manuscript. D.B., D.R.S. and I.H.F. researched the literature, analyzed the articles and drafted the manuscript. F.S.-R., C.B.A. and P.C.S. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Boutib, A.; Chergaoui, S.; Marfak, A.; Hilali, A.; Youlyouz-Marfak, I. Quality of Life during Pregnancy from 2011 to 2021: Systematic Review. Int. J. Women’s Health 2022, 14, 975–1005. [Google Scholar] [CrossRef] [PubMed]
- Lagadec, N.; Steinecker, M.; Kapassi, A.; Magnier, A.M.; Chastang, J.; Robert, S.; Gaouaou, N.; Ibanez, G. Factors influencing the quality of life of pregnant women: A systematic review. BMC Pregnancy Childbirth 2018, 18, 455. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Gou, W.-H.; Wang, J.; Chen, D.-D.; Sun, W.-J.; Guo, P.-P.; Zhang, X.-H.; Zhang, W. Effects of exercise on pregnant women’s quality of life: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 242, 170–177. [Google Scholar] [CrossRef] [PubMed]
- WHO. Programme on Mental Health: WHOQOL User Manual; Division of Mental Health and Prevention of Substance Abuse World Health Organization: Geneva, Switzerland, 2012. [Google Scholar] [CrossRef]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 8 March 2023).
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- IOM (Institute of Medicine). Weight Gain During Pregnancy: Reexamining the Guidelines Committee to Reexamine IOM Pregnancy Weight Guidelines; The National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- ACOG. Practice Bulletin Obesity in Pregnancy. Obstet. Gynecol. 2015, 126, 1118–1119. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.M.; Grivell, R.M.; Nguyen, A.-M.; Chan, A.; Robinson, J.S. Maternal and perinatal health outcomes by body mass index category. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Durst, J.K.; Sutton, A.L.M.; Cliver, S.P.; Tita, A.T.; Biggio, J.R. Impact of Gestational Weight Gain on Perinatal Outcomes in Obese Women. Am. J. Perinatol. 2016, 33, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, C.; Gaudet, L.; Cassir, G.; Nowik, C.; McLeod, N.L.; Jacob, C.; Walker, M. Guideline No. 391-Pregnancy and Maternal Obesity Part 1: Pre-conception and Prenatal Care. J. Obstet. Gynaecol. Can. 2019, 41, 1623–1640. [Google Scholar] [CrossRef]
- O’Reilly, J.R.; Reynolds, R.M. The risk of maternal obesity to the long-term health of the offspring. Clin. Endocrinol. 2013, 78, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shen, Z.; Zhan, Y.; Wang, Y.; Ma, S.; Zhang, S.; Liu, J.; Wu, S.; Feng, Y.; Chen, Y.; et al. Effects of pre-pregnancy body mass index and gestational weight gain on maternal and infant complications. BMC Pregnancy Childbirth 2020, 20, 390. [Google Scholar] [CrossRef] [PubMed]
- ACOG. Committee Opinion number 804: Physical activity and exercise during pregnancy and Postpartum Period. Obstet. Gynecol. 2020, 135, 178–188. [Google Scholar] [CrossRef]
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.-M.; Davies, G.A.; Poitras, V.; Gray, C.; Garcia, A.J.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. No. 367-2019 Canadian Guideline for Physical Activity throughout Pregnancy. J. Obstet. Gynaecol. Can. 2018, 40, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, D.S.; Carvalho, C.B.; Conde, M.; Mota, J.A.; Santos, P.C. Effectiveness of a structured exercise intervention in gestational weight gain in pregnant women with overweight and obesity: A systematic review with meta-analysis. Int. J. Gynecol. Obstet. 2023, 162, 811–822. [Google Scholar] [CrossRef] [PubMed]
- ACOG. Committee Opinion number 650: Physical Activity an Exercise During Pregnancy and the Postpartum Period. Obstet. Gynecol. 2015, 126, e135–e142. [Google Scholar] [CrossRef]
- ACSM. ACSM Guideline for Exercise Testing and Prescription; Wolters Kluwer Health: Philadelphia, PA, USA, 2018. [Google Scholar]
- Rhodes, R.E.; Janssen, I.; Bredin, S.S.D.; Warburton, D.E.R.; Bauman, A. Physical activity: Health impact, prevalence, correlates and interventions. Psychol. Health 2017, 32, 942–975. [Google Scholar] [CrossRef] [PubMed]
- Prather, H.; Spitznagle, T.; Hunt, D. Benefits of Exercise During Pregnancy. Am. Acad. Phys. Med. Rehabil. 2012, 4, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Altazan, A.D.; Redman, L.M.; Burton, J.H.; Beyl, R.A.; Cain, L.E.; Sutton, E.F.; Martin, C.K. Mood and quality of life changes in pregnancy and postpartum and the effect of a behavioral intervention targeting excess gestational weight gain in women with overweight and obesity: A parallel-arm randomized controlled pilot trial. BMC Pregnancy Childbirth 2019, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.M.; Newman, A.; Moran, L.J.; Deussen, A.R.; Grivell, R.M.; Yelland, L.N.; Crowther, C.A.; McPhee, A.J.; Wittert, G.; Owens, J.A.; et al. The effect of antenatal dietary and lifestyle advice for women who are overweight or obese on emotional well-being: The LIMIT randomized trial. Acta Obstet. Gynecol. Scand. 2016, 95, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Garnæs, K.K.; Helvik, A.S.; Stafne, S.N.; Mørkved, S.; Salvesen, K.; Salvesen; Moholdt, T. Effects of supervised exercise training during pregnancy on psychological well-being among overweight and obese women: Secondary analyses of the ETIP-trial, a randomised controlled trial. BMJ Open 2019, 9, e028252. [Google Scholar] [CrossRef] [PubMed]
- Killeen, S.L.; Yelverton, C.A.; Geraghty, A.A.; Kennelly, M.A.; Eakins, S.; Farrell, L.; Fagan, J.F.; Mehegan, J.; McAuliffe, F.M. The Edmonton Obesity Staging System and pregnancy outcomes in women with overweight or obesity: A secondary analysis of a randomized controlled trial. Clin. Obes. 2022, 12, e12510. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Briley, A.L.; Barr, S.; Bell, R.; Croker, H.; Coxon, K.; Essex, H.N.; Hunt, C.; Hayes, L.; Howard, L.M.; et al. Developing a complex intervention for diet and activity behaviour change in obese pregnant women (the UPBEAT trial); assessment of behavioural change and process evaluation in a pilot randomised controlled trial. BMC Pregnancy Childbirth 2013, 13, 148. [Google Scholar] [CrossRef]
- Seneviratne, S.; Jiang, Y.; Derraik, J.; McCowan, L.; Parry, G.; Biggs, J.; Craigie, S.; Gusso, S.; Peres, G.; Rodrigues, R.; et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: A randomised controlled trial. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.W.H.; Au Yeung, E.; Law, B.M.H. Effectiveness of Physical Activity Interventions on Pregnancy-Related Outcomes among Pregnant Women: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 1840. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.H.; McCurdy, A.P.; Mottola, M.F.; Skow, R.J.; Meah, V.L.; Poitras, V.J.; Garcia, A.J.; Gray, C.E.; Barrowman, N.; Riske, L.; et al. Impact of prenatal exercise on both prenatal and postnatal anxiety and depressive symptoms: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Garnæs, K.K.; Mørkved, S.; Salvesen, Ø.; Moholdt, T. Exercise Training and Weight Gain in Obese Pregnant Women: A Randomized Controlled Trial (ETIP Trial). PLoS Med. 2016, 13, e1002079. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.H.; Ruchat, S.M.; Poitras, V.J.; Jaramillo Garcia, A.; Gray, C.E.; Barrowman, N.; Skow, R.J.; Meah, V.L.; Riske, L.; Sobierajski, F.; et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; van der Waerden, J.; Melchior, M.; Bolze, C.; El-Khoury, F.; Pryor, L. Physical activity during pregnancy and postpartum depression: Systematic review and meta-analysis. J. Affect. Disord. 2019, 246, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.C.; Abreu, S.; Moreira, C.; Santos, R.; Ferreira, M.; Alves, O.M.A.; Moreira, P.; Mota, J. Physical Activity Patterns During Pregnancy in a Sample of Portuguese Women: A Longitudinal Prospective Study. Iran. Red Crescent Med. J. 2016, 18, e22455. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, D.; Bobadilla-Agouborde, C.; Festas, C.; Carvalho, C.; Abdalla, P.P.; Amezcua-Prieto, C.; Naia-Entonado, Z.; Mesquita, C.C.; Mota, J.; Santos, P.C. Feasibility, Clinical Efficacy, and Maternal Outcomes of a Remote Exercise Program in Pregnant Women with Obesity: The GROB Randomized Control Pilot Study. Clin. Exp. Obstet. Gynecol. 2024, 51, 70. [Google Scholar] [CrossRef]
- Roche, D.; Rafferty, A.; Holden, S.; Killeen, S.L.; Kennelly, M.; McAuliffe, F.M. Maternal Well-Being and Stage of Behaviour Change during Pregnancy: A Secondary Analysis of the PEARS Randomised Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 34. [Google Scholar] [CrossRef]
- Rial-Vázquez, J.; Vila-Farinas, A.; Varela-Lema, L.; Santiago-Pérez, M.I.; Rey-Brandariz, J.; Candal-Pedreira, C.; Pérez-Ríos, M. Actividad física en el embarazo y puerperio: Prevalencia y recomendaciones de los profesionales sanitarios. Aten. Primaria 2023, 55, 102607. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).