Abstract
The Italian National Immunization Plan (NIP) states that public health today aims at immunizing adolescents of both sexes for maximum protection against all HPV-related vaccine-preventable diseases. Nowadays, the vaccination offer to primary cohorts is reaffirmed with continued free vaccination up to at least 26 years of age for females and up to at least 18 years of age for males. The Italian NIP 2023–2025 recommends HPV vaccination for at-risk categories, including individuals with HIV, men who have sex with men (MSM), women treated for intermediate- or high-grade lesions, and travelers. Catch-up vaccination is recommended for women at least up to 26 years of age, also using the appropriate occasion of the call for the first screening for the prevention of cervical cancer as an opportunity for vaccination, and for men at least up to and including 18 years of age if they have not been previously vaccinated or have not completed the vaccination cycle. In summary, the Italian vaccination offer has been extended to additional cohorts in order to reduce the burden of HPV-related diseases and to improve vaccination coverage of the Italian population.
1. Introduction
HPV is classified within the Papillomaviridae, a family of nonenveloped double-stranded DNA viruses. Papillomavirus virions consist of non-enveloped icosahedral capsids with an approximate diameter of 55 nm that contain a double-stranded circular DNA genome of about 8 kb [1]. The genetic information of HPV encodes both structural and nonstructural proteins. The structural proteins (L) comprise a major (L1) and a minor (L2) capsid protein. The first one is shared among most HPV types while the L2 protein displays significant variability. Conversely, the nonstructural proteins are labeled as E proteins, characterized by their presence in infected cells but absence in the mature virion [2].
Papillomavirus can be transmitted through different means. Both interindividual transmission and autoinoculation are methods of transmission. Interindividual transmission between two individuals can happen through sexual contact, perinatally, or via skin-to-skin contact (e.g., through hands) [3,4]. In addition, individuals can transmit the infection even when they show no signs or symptoms. Sexual transmission is the most common route, establishing HPV as one of the prevalent sexually transmitted infections.
There are over 200 HPV genotypes; among these, HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 have carcinogenic potential. Globally, HPV 16, in particular, is the most widespread genotype. When HPV affects the anogenital tract, both asymptomatic forms and benign lesions can occur. The most common lesions, often attributed to infection by HPV 6 and 11, are genital warts. Other benign lesions that may progress or go into remission include low-grade dysplasias, which can affect the uterine cervix as well as the anus, vagina, vulva, or penis [5].
HPV infections can be self-limiting (indeed, the majority of them resolve within 6 months to 1 year [6,7]) or progress if the virus persists. Particularly if it is a high-risk genotype, persistent infection that does not resolve can lead to the development of dysplasia and, if not adequately treated, even evolve into invasive carcinoma. It has been known that HPV is related to cervical cancer since the 1980s when Zur Hausen and his collaborators found papillomavirus DNA in most cases of cervical cancer [8].
Follow-up epidemiological investigations validated that a substantial number of cases of cervical cancer may be associated with the sexual transmission of approximately 12 oncogenic, high-risk (HR) HPV types, particularly HPV 16 and HPV 18 [9,10,11]. The types of cancers associated with HPV infection have a significant epidemiological impact. HPV is indeed the most common factor linked to the development of cervical cancer, but it also plays a substantial role in the occurrence of vulvar tumors, accounting for a range of 25 to 43% [2]. Additionally, HPV is responsible for 33–48% of penile tumors [5], and in the case of anal cancer, HPV is present in approximately 67% of cases [6]. Vaginal cancer has a direct association with HPV infection, with 90% of vaginal cancers being of squamous nature [12]. In the United States alone, there were 12,600 reported cases from 2012 to 2016, and over 70% of oropharyngeal cancers have been linked to HPV [7,13].
In contrast to women, in males, HPV infection tends to manifest clinically as anogenital warts, causing significant morbidity and increasing HPV transmission rates [14]. The rising identification of neoplastic lesions in various areas, including the genitals (anus, penis, vulva, and vagina) and non-genital tract (oropharynx), underscores the importance of focusing on both male and female individuals. Adopting a gender-inclusive preventive strategy offers advantages in addressing the diverse manifestations of HPV-related health concerns [12]. The preventive HPV vaccines available commercially consist of L1 VLPs from several HPV types.
After the call issued in 2018 by the Director General of the World Health Organization (WHO), Dr. Tedros Adhanom Ghebreyesus, to scale up prevention, detection, and treatment for HPV, in 2020, WHO adopted the Global Strategy to accelerate the elimination of cervical cancer as a public health problem. This is the first global health strategy aimed at eliminating cancer as a public health problem, with the goal of vaccinating 90% of girls against HPV by the age of 15 years [15]. Despite being a vaccine-preventable and treatable disease, especially if diagnosed early, cervical cancer’s estimated age-standardized incidence rate for 2020 is still high (13.3/100,000 women). This cancer is ranked 5th among the most common cancers worldwide and it is responsible for hundreds of thousands of deaths each year and has a strong impact in terms of health, social, and economic variables [16]. For cervical cancer, the term “elimination as a public health problem” globally indicates an incidence of less than 4 per 100,000 women-years in each country. The following 90-70-90 targets must be met by 2030 for countries to be on the path towards cervical cancer elimination: (a) 90% of girls fully vaccinated with the HPV vaccine by age 15 years, (b) 70% of women screened with a high-performance test by 35 years of age and again by 45 years of age, and (c) 90% of women identified with cervical disease received treatment (90% of women with precancer treated, and 90% of women with invasive cancer managed) [15].
The objective of the article is to comprehensively address various issues inherent to the HPV virus. These include the prevalence of HPV, its connections with cervical cancer and other diseases, the efficacy of HPV vaccines, immunization strategies, and global efforts to reduce the burden of disease associated with HPV, with a focus on the state of the art in Italy. The overall goal is to summarize key information on HPV, emphasizing its importance at the global and national levels, and to support the importance of preventive strategies, particularly vaccination, in order to mitigate the negative impact of HPV on public health.
2. Global and Italian HPV Epidemiology
Worldwide, cervical cancer is the fourth most frequent cancer in women, with an estimated 604,127 new cases reported in 2020. It is the second most common female cancer in women aged 15 to 44 years [13]. Among deaths from malignancies globally, about 5% are closely associated with HPV infection [17]. Also, according to World Health Organization (WHO) estimates, the annual number of new cases of cervical cancer is expected to increase from 570,000 to 700,000 in the coming years (estimated from 2018 to 2030). Over the same period, the annual number of deaths will increase from 311,000 to 400,000. Most of these deaths will be registered in low- and middle-income countries due to the fact that no HPV vaccines or adequate screening campaigns are yet available in these countries. Of the estimated 342,000 deaths from cervical cancer in 2020, about 90% of these occur in low- and middle-income countries [15].
About 70% of global cervical cancer cases and about 50% of high-grade dysplasias are associated with HPV genotypes 16 and 18 [18]. The prevalence of HPV infection in the genital region has been thoroughly examined through extensive epidemiological studies focusing on cervical infection [19,20,21,22]. In a study conducted in the United States to assess the likelihood of acquiring human papillomavirus (HPV), it was found that over 80% of individuals, including both men and women, acquire HPV before the age of 45, and half of these infections will be of high-risk types [23]. Furthermore, according to some studies, 50% of infections that contribute to the development of cervical cancer are contracted before the age of 21 [24].
In any case, although different collection and laboratory testing methodologies among countries complicate a comprehensive assessment of the prevalence of cervical HPV infection, the highest point prevalence is found before age 25. After this age, in the range of 35 to 50 years, the prevalence tends to decline, standing between 15 and 20 percent, and then stabilizing or declining further in the older age groups. However, some countries, such as those in Central and South America, show a second peak in the older age groups (over 40 years), or at any rate, as in sub-Saharan African countries, where there is no decrease in HPV prevalence after age 30 [15,25,26]. It is now widely acknowledged that serotypes 16 and 18, primarily, and less frequently other high-risk types, contribute to the majority of malignancies in the oropharyngeal and anogenital regions [27]. Regarding the prevalence trend of HPV infection in males, although it has been studied less extensively compared to females [28,29,30], it appears to remain more stable over the years. This differs from the female prevalence, where a decrease is observed with aging. The rate of HPV infection in men may be stable across their lifetimes in contrast to the decline observed in women as they age [31,32].
In Italy, women aged 15 years and older, at risk of developing cervical cancer, account for 26.7 million women. It is estimated that each year, 3152 women receive a cervical cancer diagnosis and 1011 deaths occur from the disease. Cervical cancer is ranked as the 15th most common female cancer; the same is ranked as the fourth most prevalent cancer in 15–44-year-old women. In the general population, cervical HPV 16/18 infection is present in about 4.1% of women, and HPV 16/18 are responsible for 72.2% of invasive cervical cancers [33]. Mortality rates for all HPV-related cancers remain high despite the implementation of secondary preventive interventions, such as free Pap tests and free HPV tests for screening. The primary screening methods for cervical cancer include the Pap test and the Human Papillomavirus DNA (HPV-DNA) test. Historically, the Pap test has been administered every three years to women aged 25 to 64. However, recent scientific evidence has shown that the HPV-DNA test, conducted every five years, is more cost-effective for women over 30. All regions are actively working to adopt the HPV-DNA test model. Between the ages of 25 and 30, the recommended screening is still the Pap test, to be performed every three years. The new screening test focuses on detecting high-risk HPV infections and should not be conducted before the age of 30 [34]. The death ratio is high for cervical cancer (24% within 5 years), as well as for oropharynx cancer (75% in both genders) and anal cancer (86% and 89% in women and men, respectively) [33].
The most known HPV cancer is undoubtedly cervical cancer, for which HPV infection is a necessary condition, but the tropism of the virus is not limited to the cervical mucosa. In fact, the virus can also cause infections of the vulva and vagina in women, penile cancer in males, and anal, oropharyngeal, and esophageal infections and cancers in both sexes (Table 1).

Table 1.
New cases/year of HPV-related cancers in Italy (estimates for 2020; modified from ref. [35]).
The virus is not only responsible for lesions that lead to cancer over time but also for lesions such as genital warts or laryngeal papillomatosis, which represent, due to their frequency and the high risk of chronicity and recurrence, an important burden for the patient and society [33,35]. Most of the diagnosed infections are related to the oncogenic HR-HPV genotypes. In detail, HPV 16/18 cause approximately 70% of all cervical cancers worldwide, while a further 20% are related to HPV 31, 33, 45, 52, and 58 [36]. The ‘low-risk’ genotypes, on the other hand, are mainly HPV 6 and HPV 11 and are most commonly responsible for benign or low-grade proliferative lesions such as epidermodysplasia verruciformis in the skin, condylomata in the genital mucosa, or papillomas in the respiratory, oral, and conjunctival mucosa [18,37].
3. European and Italian Target Schedules
In Europe, the European Medicines Agency (EMA) authorized the first HPV vaccines in 2006. After the first two approved vaccines (one bivalent that contained the high-risk HPV genotypes 16 and 18 and the other tetravalent that also included types 6 and 11, which are more commonly responsible for benign lesions such as condylomas), the EMA gave the green light to a nonavalent vaccine that includes additional genotypes (31, 33, 45, 52) in addition to the previous ones. With regard to protection from precancerous lesions, all of these vaccines have shown efficacy in preventing precancerous lesions caused by genotypes 16 and 18 by more than 90 percent, with the advantage of the nonavalent vaccine of also protecting against dysplasias caused by the additional genotypes with more than 90 percent efficacy [38].
In addition to their efficacy, the approved vaccines have shown safety in all pre-authorization clinical trials and are presently under post-marketing surveillance, continuously affirming their safety [39].
The Italian National Immunization Plan (NIP) stated, on the basis of new and important scientific evidence, that public health today aims at immunizing adolescents of both sexes for optimal protection against all vaccine-preventable HPV-related diseases. The nine-valent HPV vaccine therefore represents the most effective prevention tool available today for the widest coverage against HPV-related diseases in both sexes, as it contains the nine HPV types: 6, 11, 16, 18, 31, 33, 45, 52, and 58 [40].
For individuals 9 to 14 years of age, the nine-valent vaccine should be administered according to a two-dose (0, 6–12 months) or three-dose (0, 2, 6 months) schedule. For individuals 15 years of age and older, the nine-valent vaccine should be administered according to a three-dose (0, 2, 6 months) schedule [41].
Data regarding the European routine cohort recommendations show that adolescents are the primary target of vaccination campaigns. Several countries also offer the HPV vaccination for other groups of the population [38].
4. Italian Immunization Plans and Multicohort Strategy
In Italy, the 2017–2019 NIP proposed vaccination at the age of 12 years. Starting in 2015, Italian regions began extending vaccination to include males. Vaccination was also recommended for women at the age of 25 during the first call for cervical cytology screening (Pap test). Additionally, in the 2017–2019 NIP, a recommendation for anti-HPV vaccination in at-risk categories was introduced, including men who have sex with men [42]. In the new 2023–2025 NIP, one of the scopes is to reinforce the prevention of cervical cancer and other HPV-related diseases. The NIP confirms the low level of HPV vaccine coverage (32,22% in female and 26,75% in male cohort in 2009 compared to the 95% expected) (Figure 1) [43]. The vaccination coverage trend shows a significant reduction compared to the pre-pandemic period. The full cycle coverage in the cohort of 15-year-olds (2005 cohort in 2021, who turn 16 in the year of the survey) used by the WHO as a reference in its statistics is 70.55%, up from the value for the same age group in the previous year (63.84%) [42,44].
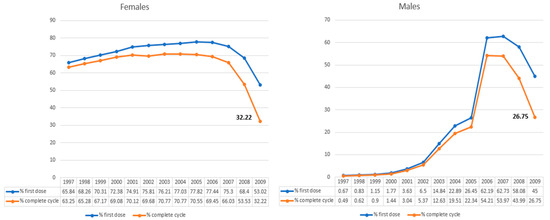
Figure 1.
HPV vaccination coverage as of 31 December 2021, in Italy (modified from ref. [43]).
As per Italian NIP, the vaccination offer to primary cohorts is reaffirmed with continued free vaccination up to at least 26 years of age for females and up to at least 18 years of age for males. The Italian NIP 2023–2025 recommends HPV vaccination for at-risk groups, including HIV-positive individuals, men who have sex with men (MSM), women with high-grade cervical lesions, and people who travel for tourism, study, or work [42]. Catch-up vaccination is recommended for women at least up to 26 years of age, also using the appropriate occasion of the call for the first screening for the prevention of cervical cancer as an opportunity for vaccination, and for men at least up to and including 18 years of age if they have not been previously vaccinated or have not completed the vaccination cycle [42]. Those with human papillomavirus (HPV)-related disease continue to face the risk of subsequent HPV infection and associated disease after treatment of specific lesions. Preventive HPV vaccines have shown benefits in preventing subsequent HPV-related disease when given prior to or shortly following treatment [45].
5. Update on Nine-Valent and Four-Valent HPV Vaccines
The clinical development of the nine-valent HPV vaccine included several phase III clinical trials conducted in females and males, in adolescent and adult populations, with two- and three-dose schedules and with coadministration with other vaccines [46,47,48,49,50,51]. For some of these trials, long-term follow-up (LTFU) data are available [52,53]. One of the most recent trials describes immunogenicity, effectiveness, and safety after a ten-year follow-up. Geometric mean antibody titers peaked at month 7, decreased sharply at months 7–12, and then gradually declined up to month 126. Seropositivity rates for every nine-valent vaccine type remained high after 10 years (126 months), notwithstanding the used test (≥81% and ≥95% by competitive and immunoglobin G-Luminex immunoassay, respectively). No cases of high-grade intraepithelial neoplasia or condyloma in males or females related to HPV types included in the nine-valent vaccine were observed after 11 years of follow-up. Incidence rates of HPV-related 6-month persistent infection due to nine-valent vaccine types were low in both genders (54.6 and 52.4 per 10,000 person-years in males and females, respectively). Immunogenicity and effectiveness of the nine-valent vaccine remained high through ∼10 years post three doses in 9–15-year-old boys and girls [54]. Real-world effectiveness and impact data for the nine-valent HPV vaccine are limited to two studies at this time. The first of these, the trial of Giuliano et al., assessed the efficacy of the nine-valent HPV vaccine against cervical, vulvar, and vaginal disease caused by all of the nine vaccine HPV types and the prevention of related cervical surgeries compared with a historic placebo population. The analysis included three international, randomized, double-blind studies that were conducted using the same methodology. Among women who tested negative for 14 HPV types before vaccination, the incidence of high-grade CIN and cervical definitive therapy related to the nine HPV types was reduced by 98.2% and 97.8%, respectively [55].
The second study, the trial of Kjaer et al., reported the results of an interim analysis that evaluated the effectiveness of the nine-valent HPV vaccine at 8 years out of a planned long-term follow-up (LTFU) of 14 years from the start of vaccination. The results report no new cases of HPV type 6/11/16/18/31/33/45/52/58-related CIN (any grade), AIS, cervical cancer, vulvar cancer, or vaginal cancer from the start of the LTFU study [52].
However, the effectiveness of the four-valent HPV vaccine is relevant to the nine-valent HPV vaccine since the vaccines are manufactured similarly and contain four of the same HPV L1 VLPs [56]. Several dozen studies have been published on four-valent HPV vaccine effectiveness around the world. A recent systematic review of the literature summarized the results of many of these studies. The authors systematically reviewed observational studies on HPV vaccination within MEDLINE, EMBASE, and Google Scholar from 2016 to 2020, involving 14 years of follow-up data. A total of 138 peer-reviewed publications reporting on the impact of the HPV vaccine or its effectiveness were identified. Targets considered included infection rates at various anatomical sites and the incidence of various HPV-related disease outcomes. The results suggest that the large-scale implementation of HPV vaccination programs globally has helped decrease genital infections and caused significant reductions in the incidence of HPV-related diseases. HPV vaccination programs continue to support a marked reduction in new HPV infections and corresponding disease outcomes. As infections with high-risk HPV types can now be protected against by immunization, near-complete disease elimination may become an achievable target, coupled with cervical screening and treatment for those with the disease [57].
The nine-valent HPV vaccine safety profile has been established by several studies [58]. The World Health Organization (WHO) Global Advisory Committee on Vaccine Safety (GACVS) has issued several reports, the most recent in June 2017. The Committee concluded that, since their last review, there is still no evidence to suggest a causal association between the HPV vaccine and complex regional pain syndrome (CRPS), postural orthostatic tachycardia syndrome (POTS), or the diverse symptoms that include pain and motor dysfunction [59].
6. Italian Strategies to Increase HPV Vaccination Coverage in the Post-Pandemic Period
Despite the strong evidence supporting this vaccination, in Italy, the HPV vaccination coverage in eleven-year-old girls and boys, which was already far from the 95% target in previous years, was further reduced in 2020 (birth cohort 2008) due to the strong impact of the pandemic on activities and vaccinations, which has been particularly marked for adolescents. A similar impact has also been seen on cervical cancer screening activities, highlighting the need to implement all possible actions in the coming years to ensure the effective relaunch of HPV vaccination, aiming to achieve the coverage target HPV vaccination of ≥95% in adolescents and the progressive reduction in the incidence of cervical and uterine cancer [43].
According to NIP 2023–2025, the most important actions are (a) relaunching and strengthening the national HPV vaccination campaign, including the active involvement of the local scientific community, specialists, and scientific and civil societies; (b) promoting vaccination through widening access to vaccination services, scheduling open days and catch-up activities, extending the active and free provision of the vaccine to cohorts at least to the age of initiation of cervical cancer screening, offering free-of-charge vaccines for males at least up to and including 18 years of age, maintaining free vaccination over time for beneficiary cohorts, and adopting tools and technologies to support active outreach and appointment management; (c) developing an effective communicative campaign to oppose vaccination hesitancy; and (d) integrating primary and secondary prevention [42].
Among the actions implemented to recover the vaccination delays caused by the SARS-CoV-2 pandemic, some regions have strengthened the recommendations in favor of co-administration of the HPV vaccine with other vaccines recommended by age [60].
7. Discussion and Future Perspectives
HPV was ranked in the latest American Association for Cancer Research (AACR) report as the second most cancer-causing pathogen in the world [61]. For this reason, in 2020, the WHO adopted the first global health strategy to eliminate this cancer as a public health problem (target: 90% of girls fully vaccinated against HPV by the age of 15) [15]. Regarding future perspectives, numerous studies in the literature have evaluated the immunological response following a single dose of the HPV vaccine. The results indicate a sustained high immune response, even ten years after the administration of the four-valent vaccine dose [62]. In a recent randomized trial, the Dose Reduction Immunobridging and Safety Study (DoRIS) demonstrated that a robust immune response is attainable with a single dose of either bivalent, quadrivalent, or nonavalent HPV vaccine. These findings underscore the potential of a single-dose vaccination strategy to achieve the elimination of cervical cancer, particularly in economically disadvantaged countries. Furthermore, it suggests that adopting a single-dose approach can contribute to cost reduction and enhance vaccine accessibility globally [63]. The Global Strategy and its related targets represent a unique opportunity to drive long-term, sustainable advocacy and save lives [64].
In the Italian scenario, although the HPV vaccination is not among the mandatory ones, according to Law 119/2017, it is an Essential Level of Care (LEA) [65]. As of December 2021, HPV vaccination coverage in Italy does not reach the 95% target set by the 2023–2025 NIP in any cohort of both genders. As per the 2023–2025 NIP, the vaccination offer has been extended to additional cohorts in order to decrease the HPV-related diseases’ burden and improve vaccination coverage of the Italian population. In Italy, the NIP 2023–2025 provides vaccination against HPV with a two-dose vaccination schedule at the age of 12 for girls and boys up to 14 years of age and a three-dose vaccination course starting from 15 years of age [42].
For instance, France launched a vaccination campaign against genital human papillomavirus (HPV) for all girls and boys in secondary schools, as the country has one of the lowest vaccine rates against HPV in Europe [66].
A study published in 2017 analyzed the cost-effectiveness of the nine-valent vaccine in Italy. In comparison with the four-valent vaccine, the nine-valent vaccine, when implemented in a program inclusive of both females and males, shows an incremental cost-effectiveness ratio (ICER) of 10,463 € per quality-adjusted life year (QALY) when considering vaccination for both male and female populations. The ICER is 4483 € when considering only the female population [67].
8. Conclusions
It is crucial to emphasize that cervical cancer is a preventable and curable disease if detected early and treated adequately. Ensuring all women have access to affordable and effective cervical cancer prevention and management services remains a priority. Additionally, it is important to focus on other types of preventable cancers through vaccination, paying attention to gender-neutral preventive approach. A robust health system is imperative to achieve universal health coverage. Despite the presence of numerous barriers to HPV vaccination, highlighted particularly during the COVID-19 pandemic, efforts are underway to resume and strengthen HPV vaccination and cervical cancer screening programs. To support all these claims, 17 November has been established as the Cervical Cancer Elimination Day of Action and the anniversary of the launch of the World Health Organization’s (WHO) Global Strategy to accelerate the elimination of cervical cancer as a public health problem.
Author Contributions
G.G. and M.O. equally contributed to this work: conceptualization, writing—original draft preparation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
G.G. declares no conflicts of interest directly related to this review. Outside this work, G.G. declares that, in the last two years, he has had financing relationships with entities with commercial interests in the healthcare field: GSK, MSD, Pfizer, Novavax, Moderna, Seqirus, Emergent Biosolutions, Viatris, and Sanofi Pasteur. M.O. declares no conflicts of interest.
References
- Myers, D.J.; Kwan, E.; Fillman, E.P. Epidermodysplasia Verruciformis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534198/ (accessed on 8 November 2023).
- Kirnbauer, R.; Booy, F.; Cheng, N.; Lowy, D.R.; Schiller, J.T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 1992, 89, 12180–12184. [Google Scholar] [CrossRef]
- Wierzbicka, M.; San Giorgi, M.R.M.; Dikkers, F.G. Transmission and clearance of human papillomavirus infection in the oral cavity and its role in oropharyngeal carcinoma—A review. Rev. Med. Virol. 2023, 33, e2337. [Google Scholar] [CrossRef]
- Mammas, I.N.; Sourvinos, G.; Spandidos, D.A. The paediatric story of human papillomavirus (Review). Oncol. Lett. 2014, 8, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Dunne, E.F.; Park, I.U. HPV and HPV-associated diseases. Infect. Dis. Clin. N. Am. 2013, 27, 765–778. [Google Scholar] [CrossRef]
- Moscicki, A.-B.; Schiffman, M.; Burchell, A.; Albero, G.; Giuliano, A.R.; Goodman, M.T.; Kjaer, S.K.; Palefsky, J. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine 2012, 30, F24–F33. [Google Scholar] [CrossRef] [PubMed]
- Winer, R.L.; Hughes, J.P.; Feng, Q.; Xi, L.F.; Cherne, S.; O’Reilly, S.; Kiviat, N.B.; Koutsky, L.A. Early natural history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol. Biomark. Prev. 2011, 20, 699–707. [Google Scholar] [CrossRef]
- Zur Hausen, H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr. Top. Microbiol. Immunol. 1994, 186, 131–156. [Google Scholar] [PubMed]
- Alemany, L.; de Sanjosé, S.; Tous, S.; Quint, W.; Vallejos, C.; Shin, H.; Bravo, L.E.; Alonso, P.; Lima, M.A.; Guimerà, N.; et al. Time trends of human papillomavirus types in invasive cervical cancer, from 1940 to 2007. Int. J. Cancer 2014, 135, 88–95. [Google Scholar] [CrossRef]
- Bosch, F.X.; Lorincz, A.; Munoz, N.; Meijer, C.J.L.M.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer world wide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Dykens, J.A.; Peterson, C.E.; Holt, H.K.; Harper, D.M. Gender neutral HPV vaccination programs: Reconsidering policies to expand cancer prevention globally. Front. Public Health 2023, 11, 1067299. [Google Scholar] [CrossRef]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S.; ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 10 March 2023. Available online: https://hpvcentre.net/statistics/reports/XWX.pdf (accessed on 8 November 2023).
- Bruni, L.; Albero, G.; Rowley, J.; Alemany, L.; Arbyn, M.; Giuliano, A.R.; E Markowitz, L.; Broutet, N.; Taylor, M. Global and regional estimates of genital human papillomavirus prevalence among men: A systematic review and meta-analysis. Lancet Glob. Health 2023, 11, e1345–e1362. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; WHO: Geneva, Switzerland, 2020; Available online: https://iris.who.int/bitstream/handle/10665/336583/9789240014107-eng.pdf?sequence=1 (accessed on 8 November 2023).
- IARC; World Health Organization. Estimated Age-Standardized Incidence Rates (World) in 2020, Worldwide, Both Sexes, All Ages. Available online: https://gco.iarc.fr/today/home (accessed on 8 November 2023).
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018, a worldwide incidence analysis. Lancet Glob. Health. 2019, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human Papillomavirus (HPV) and Cervical Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 8 November 2023).
- de Sanjose, S.; Brotons, M.; Pavon, M.A. The natural history of human papillomavirus infection. Best Prac. Res. Clin. Obs. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers 2016, 2, 16086. [Google Scholar] [CrossRef]
- Baseman, J.G.; Koutsky, L.A. The epidemiology of human papillomavirus infections. J. Clin. Virol. 2005, 32, S16–S24. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Burchell, A.N.; Schiffman, M.; Giuliano, A.R.; Jde Sanjose, S.; Bruni, L.; Tortolero-Luna, G.; Kjaer, S.K.; Muñoz, N. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 2008, 26, K1–K16. [Google Scholar] [CrossRef]
- Chesson, H.W.; Dunne, E.F.; Hariri, S.; Markowitz, L.E. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm. Dis. 2014, 41, 660–664. [Google Scholar] [CrossRef]
- Burger, E.A.; Kim, J.J.; Sy, S.; Castle, P.E. Age of acquiring causal human papillomavirus (HPV) infections: Leveraging simulation models to explore the natural history of HPV-induced cervical cancer. Clin. Infect. Dis. 2017, 65, 893–899. [Google Scholar] [CrossRef]
- Smith, J.S.; Melendy, A.; Rana, R.K.; Pimenta, J.M. Age-specific prevalence of infection with human papillomavirus in females: A global review. J. Adolesc. Health 2008, 43, e21–e41. [Google Scholar] [CrossRef]
- Bruni, L.; Diaz, M.; Castellsagué, M.; Ferrer, E.; Bosch, F.X.; de Sanjosé, S. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010, 202, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Lee, J.-H.; Fulp, W.; Villa, L.L.; Lazcano, E.; Papenfuss, M.R.; Abrahamsen, M.; Salmeron, J.; Anic, G.M.; E Rollison, D.; et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): A cohort study. Lancet 2011, 377, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Sichero, L.; Giuliano, A.R.; Villa, L.L. Human papillomavirus and genital disease in men: What we have learned from the HIM study. Acta Cytol. 2019, 63, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Lieblong, B.J.; Montgomery, B.E.E.; Su, L.J.; Nakagawa, M. Natural history of human papillomavirus and vaccinations in men: A literature review. Health Sci. Rep. 2019, 2, e118. [Google Scholar] [CrossRef]
- Zou, K.; Huang, Y.; Li, Z. Prevention and treatment of human papillomavirus in men benefits both men and women. Front. Cell. Infect. Microbiol. 2022, 12, 1077651. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Unger, E.R.; Liu, G.; Steinau, M.; Meites, E.; Dunne, E.; Markowitz, L.E. Prevalence of genital human papillomavirus in males, United States, 2013–2014. J. Infect. Dis. 2017, 215, 1070–1079. [Google Scholar] [CrossRef]
- ICO/IARC Information Centre on HPV and Cancer. Italy. Human Papillomavirus and Related Cancers, Fact Sheet 2023. Available online: https://hpvcentre.net/statistics/reports/ITA_FS.pdf?t=1699608665888 (accessed on 10 November 2023).
- Screening per IL Cancro Del Collo Dell’Utero—Ministero Della Salute. Available online: https://www.salute.gov.it/portale/tumori/dettaglioContenutiTumori.jsp?lingua=italiano&id=5543&area=tumori&menu=screening (accessed on 8 November 2023).
- Azzari, C.; Ricci, S.; Canessa, C.; Ghiori, F.; Lippi, F. anni di protezione anti-HPV: Verso nuove frontiere. RIAP. Riv. Immunol. Allergol. Pediatr. 2016, 3, 38–45. [Google Scholar]
- Molet, L.; Girlich, D.; Bonnin, R.A.; Proust, A.; Bouligand, J.; Bachelerie, F.; Hantz, S.; Deback, C. Identification by high-throughput sequencing of HPV variants and quasispecies that are untypeable by linear reverse blotting assay in cervical specimens. Papillomavirus Res. 2019, 8, 100169. [Google Scholar] [CrossRef]
- Calabrò, G.E.; Carini, E.; Favaretti, C.; Bonanni, P.; De Vincenzo, R.; Ghelardi, A.; Tafuri, S. Report di approfondimento e valutazione, con metodologia HTA (Health Technology Assessment), della vaccinazione anti-HPV nelle donne trattate per lesioni HPV-correlate. QIJPH 2019, 8, 7. Available online: https://www.ijph.it/pdf/2019-v8-n7.pdf (accessed on 8 November 2023).
- European Centre for Disease Prevention and Control. Prevention and Control Measures for Human Papillomavirus. Available online: https://www.ecdc.europa.eu/en/human-papillomavirus/prevention-control (accessed on 8 November 2023).
- European Centre for Disease Prevention and Control. Guidance on HPV Vaccination in EU Countries: Focus on Boys, People Living with HIV and 9-Valent HPV Vaccine Introduction, 2020; ECDC: Stockholm, Sweden, 2020. [Google Scholar]
- Calendario Vaccinale per la Vita, 4° Edizione 2019. Available online: http://www.igienistionline.it/docs/2019/21cvplv.pdf (accessed on 10 November 2023).
- SCP Gardasil 9. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000737_044268_RCP.pdf&retry=0&sys=m0b1l3 (accessed on 8 November 2023).
- Presidenza del Consiglio dei Ministri. Conferenza Permanente per i Rapporti tra lo Stato le Regioni e le Province Autonome. Piano Nazionale dI Prevenzione Vaccinale (PNPV) 2023–2025 e Calendario Nazionale Vaccinale. Available online: https://www.trovanorme.salute.gov.it/norme/dettaglioAtto.spring?id=95963&page=newsett (accessed on 15 November 2023).
- Ministero della Salute. Commento alle Coperture Vaccinali al 31 Dicembre 2021 per HPV. Available online: https://www.salute.gov.it/imgs/C_17_tavole_27_1_6_file.pdf (accessed on 17 November 2023).
- Gabutti, G.; D’Anchera, E.; De Motoli, F.; Savio, M.; Stefanati, A. Human Papilloma Virus Vaccination: Focus on the Italian Situation. Vaccines 2021, 9, 1374. [Google Scholar] [CrossRef]
- Reuschenbach, M.; Doorbar, J.; del Pino, M.; Joura, E.A.; Walker, C.; Drury, R.; Rauscher, A.; Saah, A.J. Prophylactic HPV vaccines in patients with HPV-associated diseases and cancer. Vaccine 2023, 41, 6194–6205. [Google Scholar] [CrossRef] [PubMed]
- Joura, E.A.; Giuliano, A.R.; Iversen, O.-E.; Bouchard, C.; Mao, C.; Mehlsen, J.; Moreira, E.D.; Ngan, Y.; Petersen, L.K.; Lazcano-Ponce, E.; et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015, 372, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Olsson, S.E.; Block, S.; Castellsague, X.; Gray, G.E.; Herrera, T.; Huang, L.-M.; Kim, D.S.; Pitisuttithum, P.; Chen, J.; et al. Immunogenicity and Safety of a 9-Valent HPV Vaccine. Pediatrics 2015, 136, e28–e39. [Google Scholar] [CrossRef] [PubMed]
- Castellsagué, X.; Giuliano, A.; Goldstone, S.; Guevara, A.; Mogensen, O.; Palefsky, J.; Group, T.; Shields, C.; Liu, K.; Maansson, R.; et al. Immunogenicity and safety of the 9-valent HPV vaccine in men. Vaccine 2015, 33, 6892–6901. [Google Scholar] [CrossRef] [PubMed]
- Joura, E.A.; Ulied, A.; Vandermeulen, C.; Figueroa, M.R.; Seppä, I.; Aguado, J.J.H.; Ahonen, A.; Reich, O.; Virta, M.; Perino, A.; et al. Immunogenicity and safety of a nine-valent human papillomavirus vaccine in women 27–45 years of age compared to women 16–26 years of age: An open-label phase 3 study. Vaccine 2021, 39, 2800–2809. [Google Scholar] [CrossRef] [PubMed]
- Schilling, A.; Parra, M.M.; Gutierrez, M.; Restrepo, J.; Ucros, S.; Herrera, T.; Engel, E.; Huicho, L.; Shew, M.; Maansson, R.; et al. Coadministration of a 9-Valent Human Papillomavirus Vaccine with Meningococcal and Tdap Vaccines. Pediatrics 2015, 136, e563–e572. [Google Scholar] [CrossRef] [PubMed]
- Iversen, O.E.; Miranda, M.J.; Ulied, A.; Soerdal, T.; Lazarus, E.; Chokephaibulkit, K.; Block, S.L.; Skrivanek, A.; Nur Azurah, A.G.; Fong, S.M.; et al. Immunogenicity of the 9-Valent HPV Vaccine Using 2-Dose Regimens in Females and Males vs a 3-Dose Regimen in Women. JAMA 2016, 316, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, S.K.; Nygård, M.; Sundström, K.; Munk, C.; Berger, S.; Dzabic, M.; Fridrich, K.E.; Waldstrøm, M.; Sørbye, S.W.; Bautista, O.; et al. Long-term effectiveness of the nine-valent human papillomavirus vaccine in Scandinavian women: Interim analysis after 8 years of follow-up. Hum. Vaccines Immunother. 2021, 17, 943–949. [Google Scholar] [CrossRef]
- Olsson, S.E.; Restrepo, J.A.; Reina, J.C.; Pitisuttithum, P.; Ulied, A.; Varman, M.; Van Damme, P.; Moreira, E.D., Jr.; Ferris, D.; Block, S.; et al. Long-term immunogenicity, effectiveness, and safety of nine-valent human papillomavirus vaccine in females and males 9 to 15 years of age: Interim analysis after 8 years of follow-up. Papillomavirus Res. 2020, 10, 100203. [Google Scholar] [CrossRef]
- Restrepo, J.; Herrera, T.; Samakoses, R.; Reina, J.C.; Pitisuttithum, P.; Ulied, A.; Bekker, L.-G.; Moreira, E.D.; Olsson, S.-E.; Block, S.L.; et al. Ten-Year Follow-up of 9-Valent Human Papillomavirus Vaccine: Immunogenicity, Effectiveness, and Safety. Pediatrics 2023, 152, e2022060993. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Joura, E.A.; Garland, S.M.; Huh, W.K.; Iversen, O.-E.; Kjaer, S.K.; Ferenczy, A.; Kurman, R.J.; Ronnett, B.M.; Stoler, M.H.; et al. Nine-valent HPV vaccine efficacy against related diseases and definitive therapy: Comparison with historic placebo population. Gynecol. Oncol. 2019, 154, 110–117. [Google Scholar] [CrossRef]
- Gardasil 9. Highlights of Prescribing Information. Available online: https://www.merck.com/product/usa/pi_circulars/g/gardasil_9/gardasil_9_pi.pdf (accessed on 8 November 2023).
- Wang, W.; Kothari, S.; Skufca, J.; Giuliano, A.R.; Sundström, K.; Nygård, M.; Koro, C.; Baay, M.; Verstraeten, T.; Luxembourg, A.; et al. Real-world impact and effectiveness of the quadrivalent HPV vaccine: An updated systematic literature review. Expert Rev. Vaccines 2022, 21, 1799–1817. [Google Scholar] [CrossRef]
- Hansen, J.; Yee, A.; Lewis, N.; Li, S.; Velicer, C.; Saddier, P.; Klein, N.P. Safety of 9-valent human papillomavirus vaccine administered to males and females in routine use. Vaccine 2023, 41, 1819–1825. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Safety Update of HPV Vaccines. Extract from Report of GACVS: Meeting of 7–8 June 2017, Weekly Epidemiological Record; 14 July 2017. Available online: https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/human-papillomavirus-vaccines/safety#cms (accessed on 8 November 2023).
- Regione Lazio. Trasmssione Documento: “Raccomandazioni Riguardanti la Co-Somministrazione dei Vaccini”. Available online: https://www.vaccinarsinlazio.org/assets/uploads/files/7/rl-documento-co-somministrazione-vaccini-384782-28apr20-1.pdf (accessed on 8 November 2023).
- American Association for Cancer Research (ACCR). 2023, April. Available online: https://www.aacr.org/about-the-aacr/newsroom/news-releases/awareness-of-the-link-between-hpv-and-cervical-cancer-has-declined/#:~:text=%E2%80%93%20Americans%20have%20become%20less%20aware,%2C%20oral%2C%20and%20penile%20cancer (accessed on 8 November 2023).
- Joshi, S.; Anantharaman, D.; Muwonge, R.; Bhatla, N.; Panicker, G.; Butt, J.; Poli, U.R.R.; Malvi, S.G.; Esmy, P.O.; Lucas, E.; et al. Evaluation of immune response to single dose of quadrivalent HPV vaccine at 10-year post-vaccination. Vaccine 2023, 41, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Baisley, K.; Kemp, T.J.; Kreimer, A.R.; Basu, P.; Changalucha, J.; Hildesheim, A.; Porras, C.; Whitworth, H.; Herrero, R.; Lacey, C.J.; et al. Comparing one dose of HPV vaccine in girls aged 9–14 years in Tanzania (DoRIS) with one dose of HPV vaccine in historical cohorts: An immunobridging analysis of a randomised controlled trial. Lancet Glob. Health 2022, 10, e1485–e1493. [Google Scholar] [CrossRef] [PubMed]
- Union for International Cancer Control (UICC). Cervical Cancre Elimination. Available online: https://www.uicc.org/what-we-do/areas-focus/cervical-cancer-elimination (accessed on 8 November 2023).
- Camera dei Deputati. I nuovi Livelli Essenziali di Assistenza (LEA). Available online: https://www.camera.it/temiap/documentazione/temi/pdf/1105044.pdf (accessed on 8 November 2023).
- EURACTIV. France Prepares for HPV Vaccination Campaign in Schools. Available online: https://www.euractiv.com/section/politics/news/france-prepares-for-hpv-vaccination-campaign-in-schools (accessed on 8 November 2023).
- Mennini, F.S.; Bonanni, P.; Bianic, F.; de Waure, C.; Baio, G.; Plazzotta, G.; Uhart, M.; Rinaldi, A.; Largeron, N. Cost-effectiveness analysis of the nine-valent HPV vaccine in Italy. Cost Eff. Resour. Alloc. 2017, 15, 11. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).