Control of Precipitation of Cellulose Solutions in N-Methylmorpholine-N-oxide by Introducing Polyacrylonitrile Additives
Abstract
1. Introduction
2. Materials and Methods
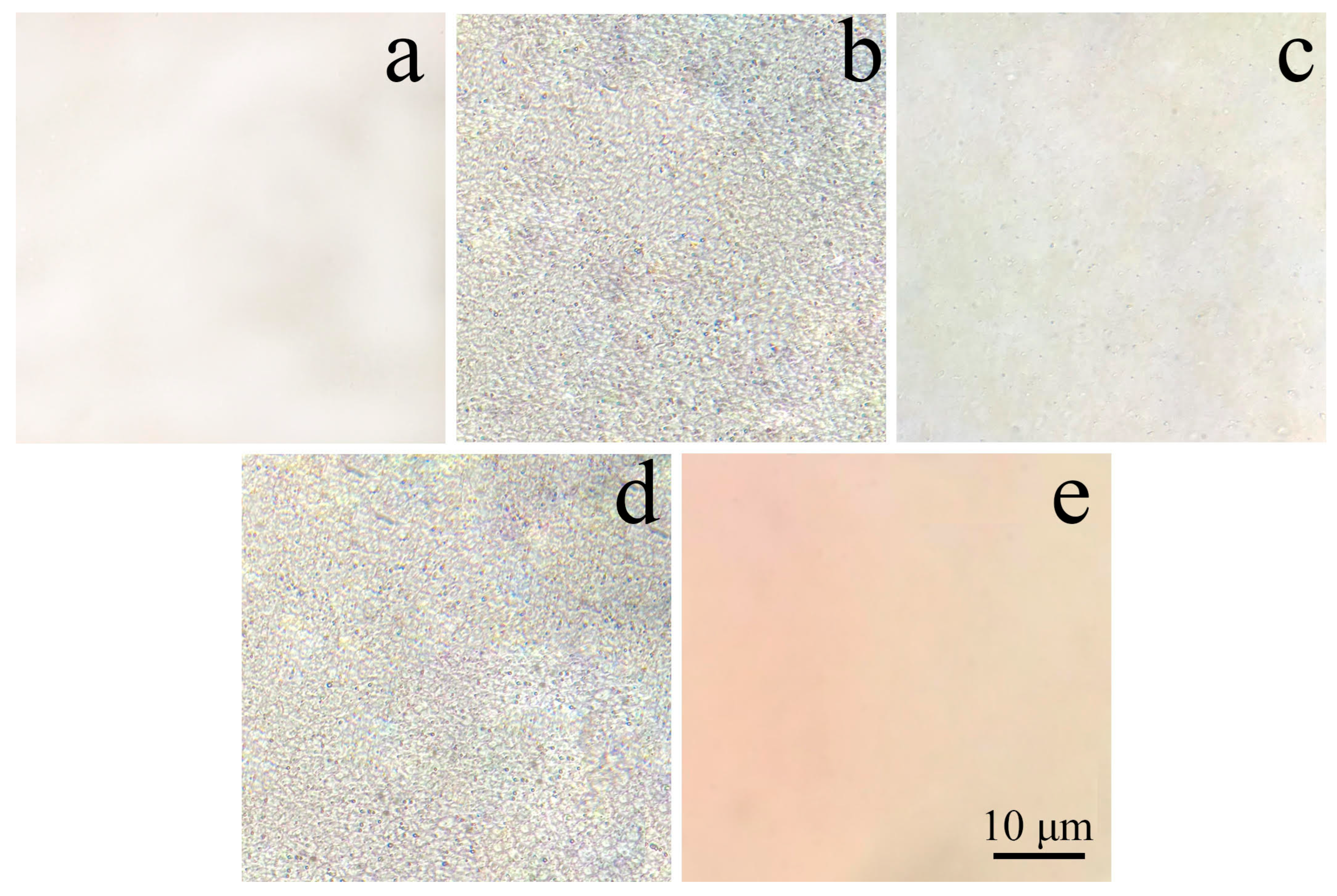
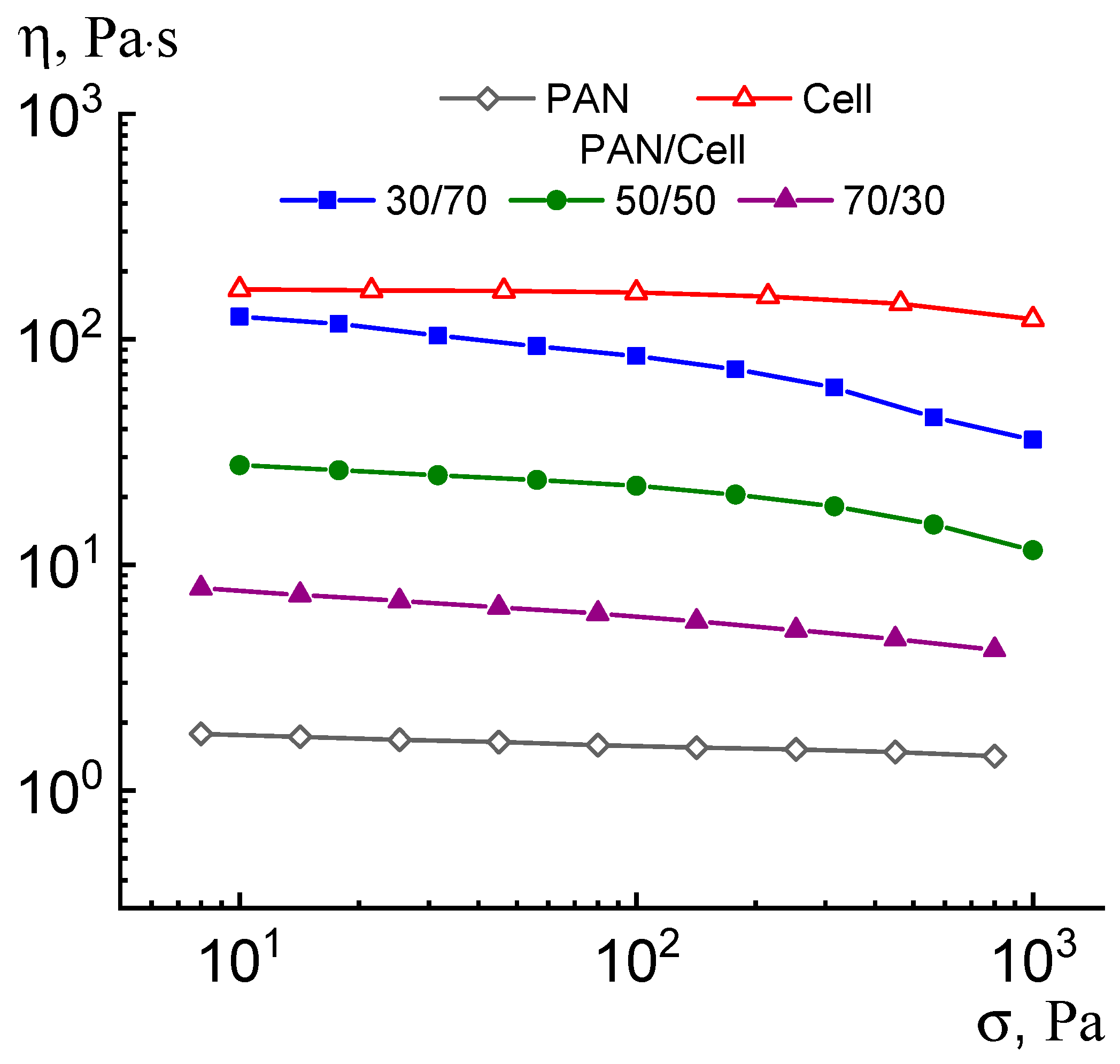
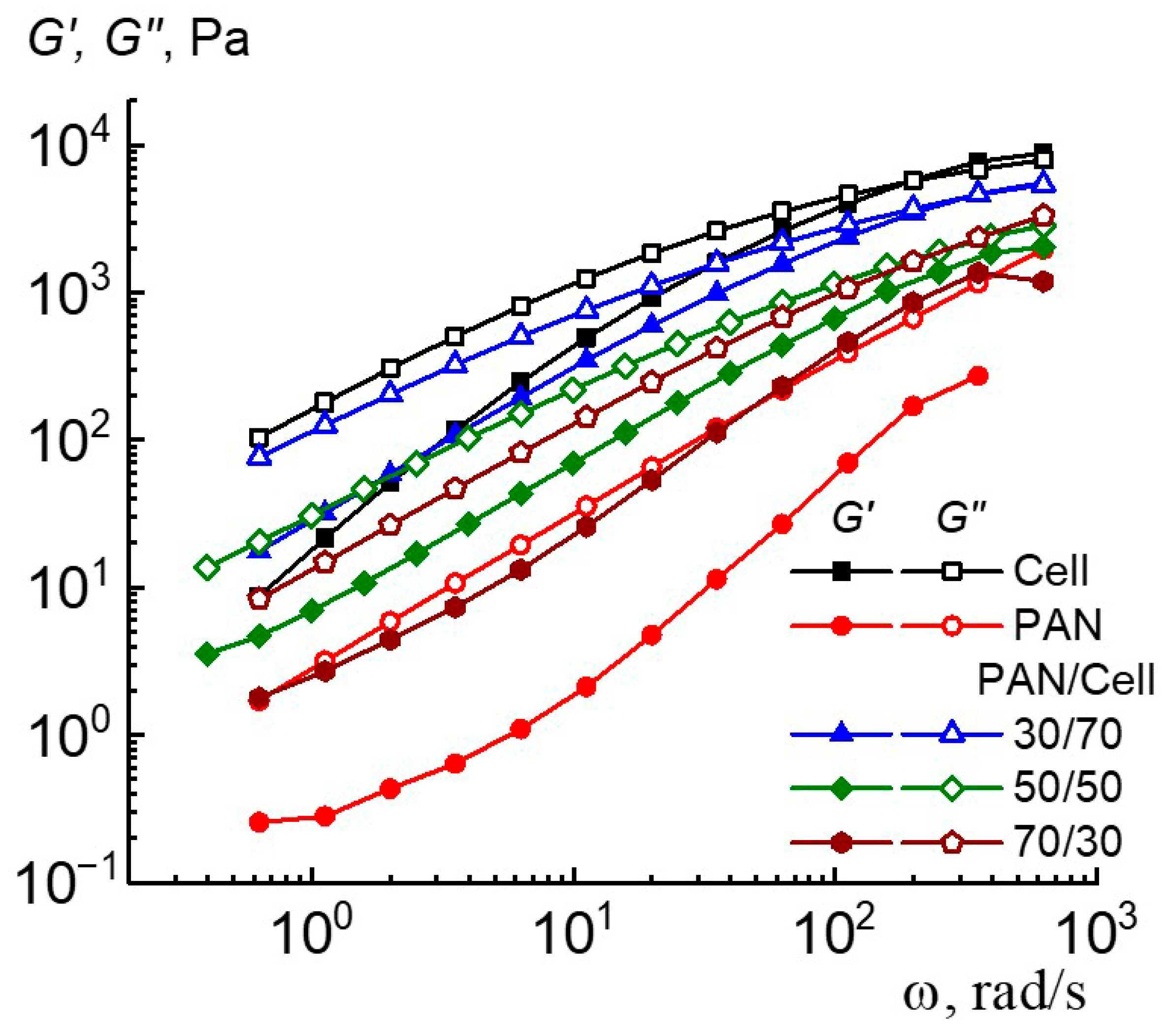
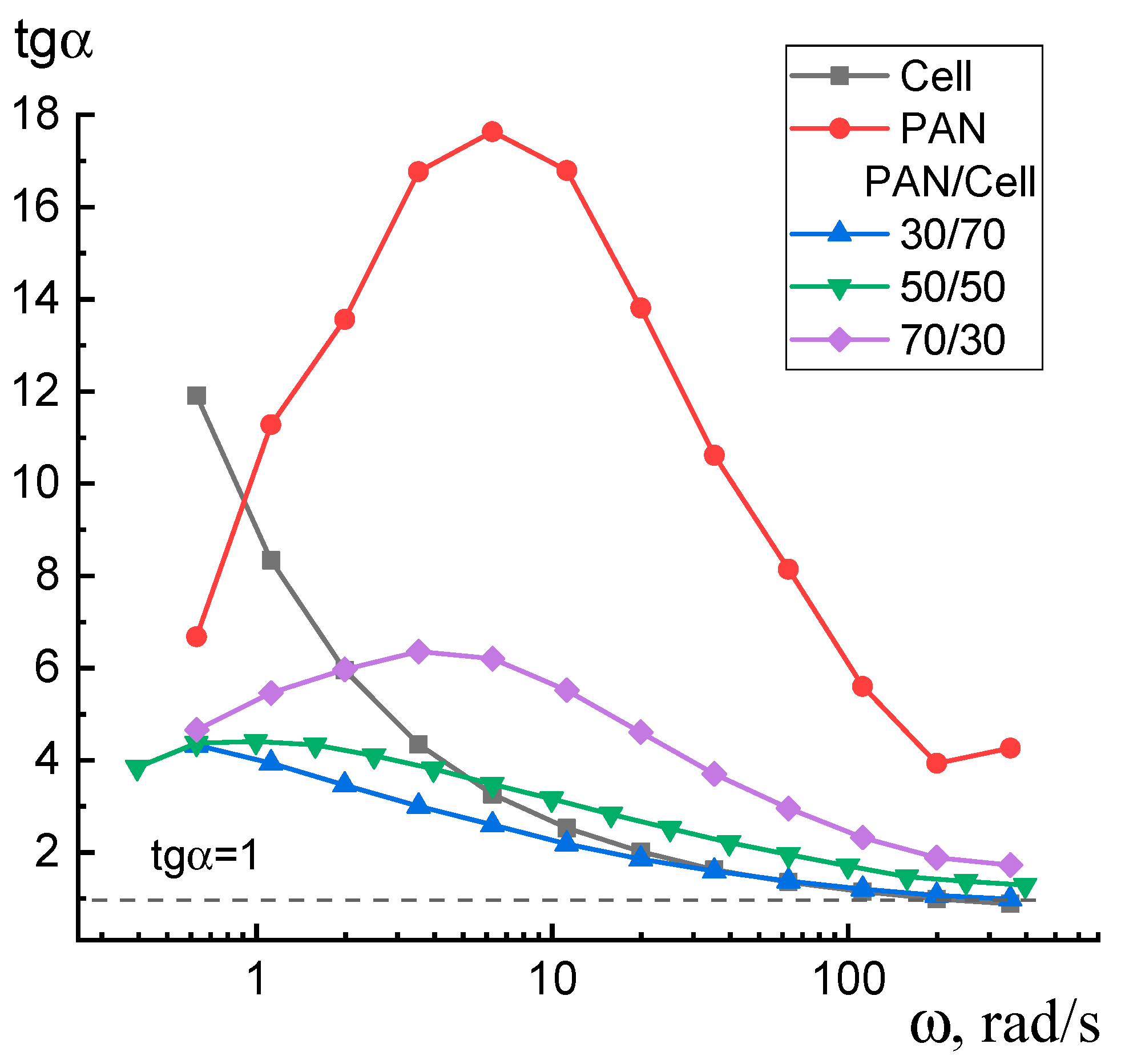
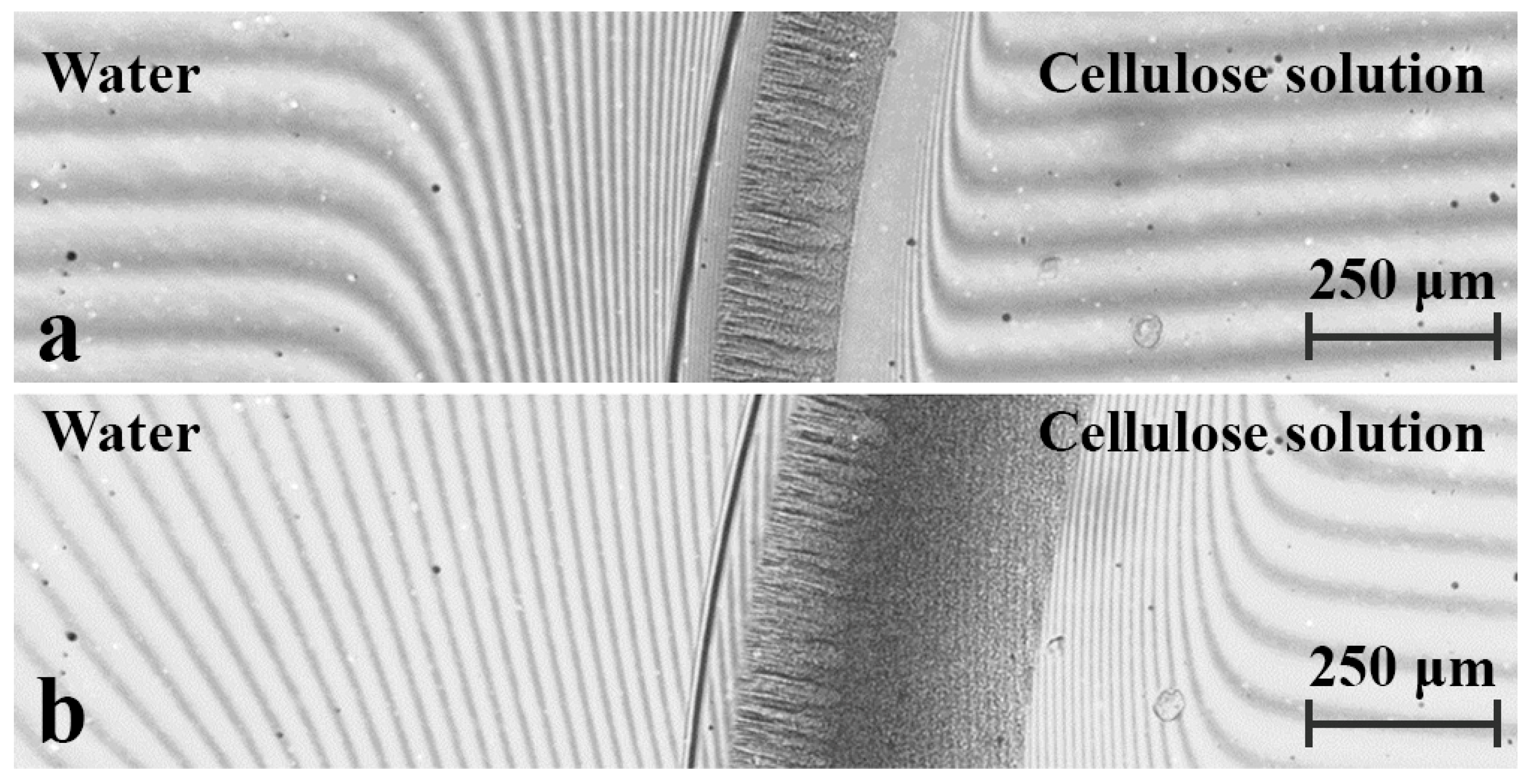
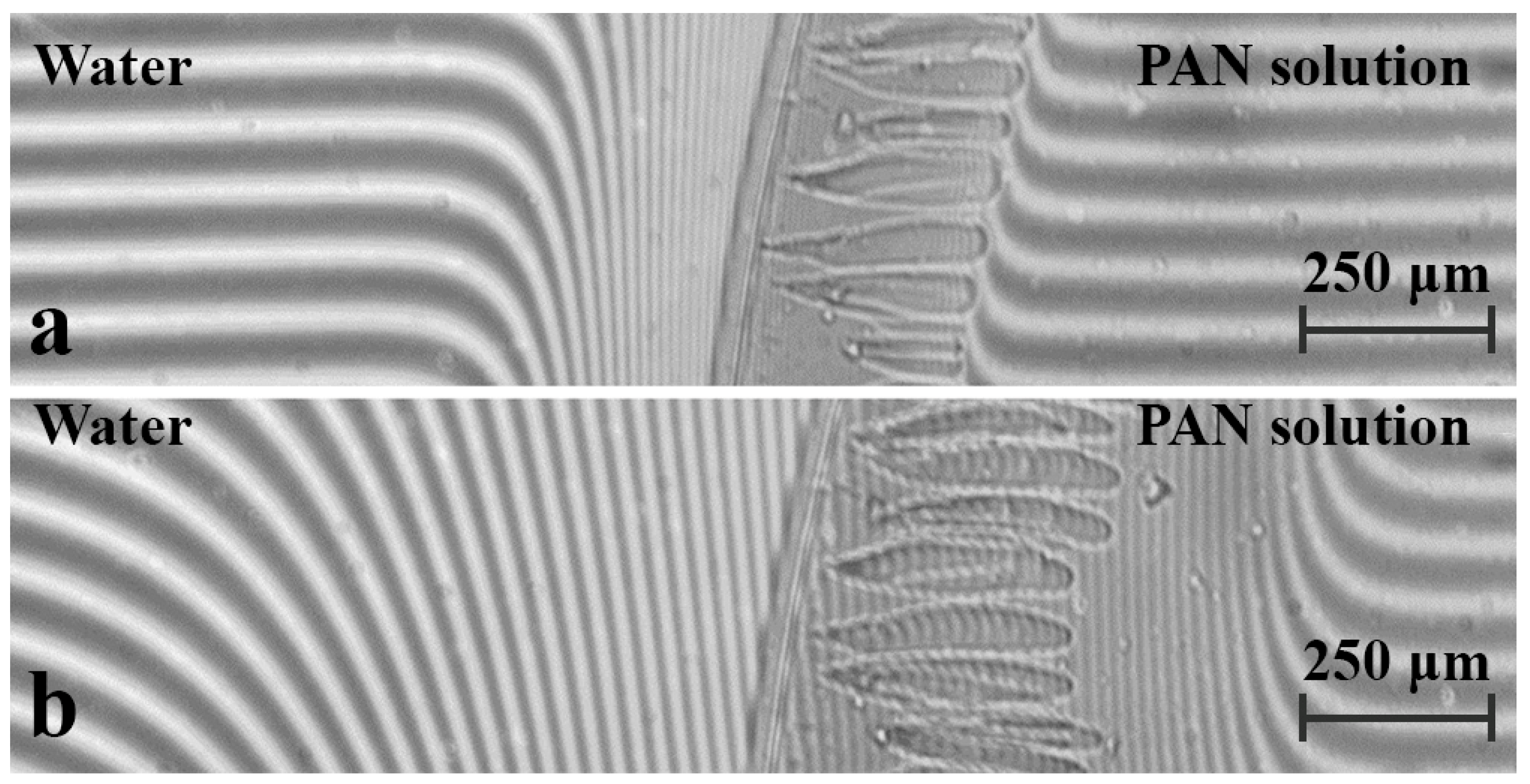
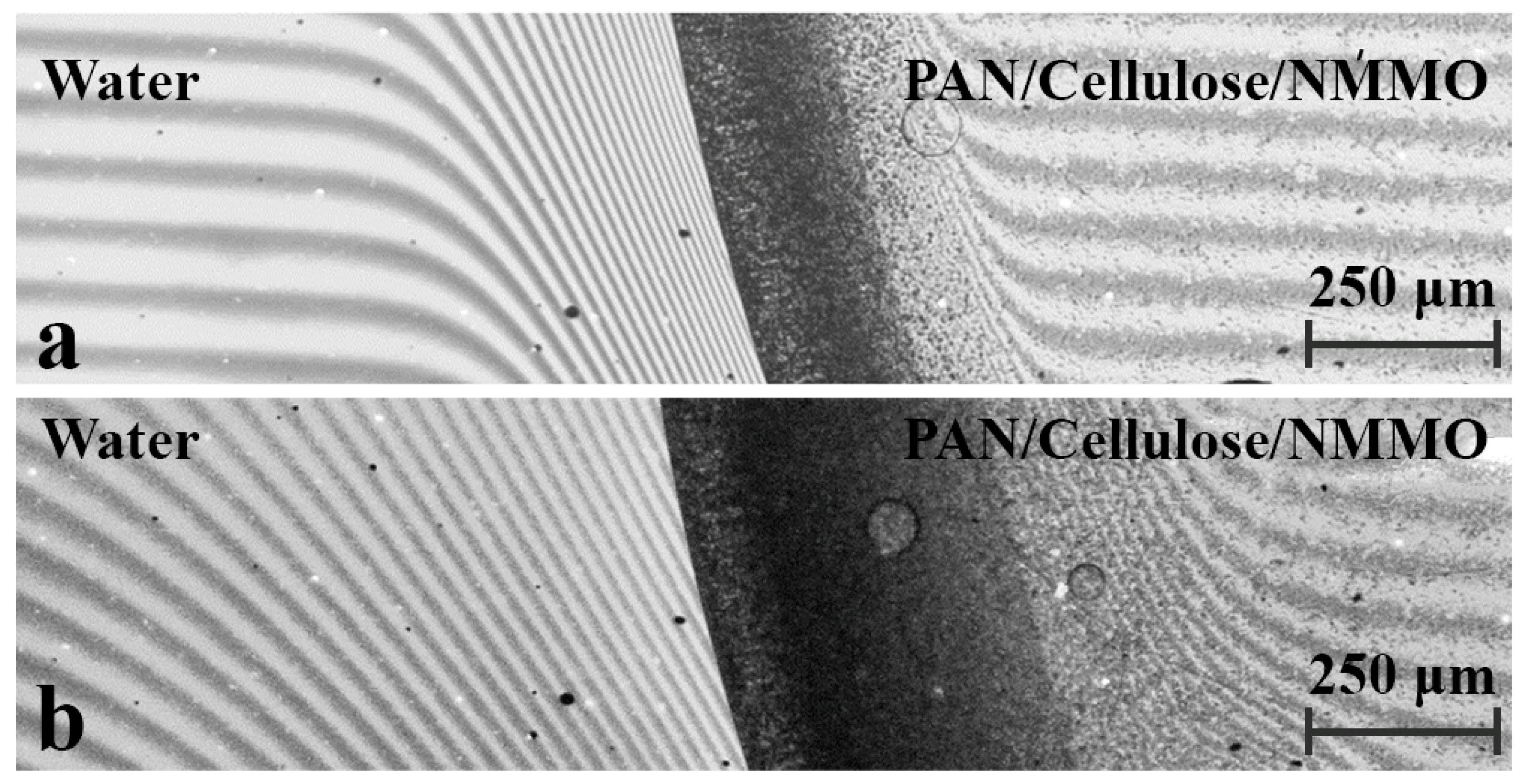
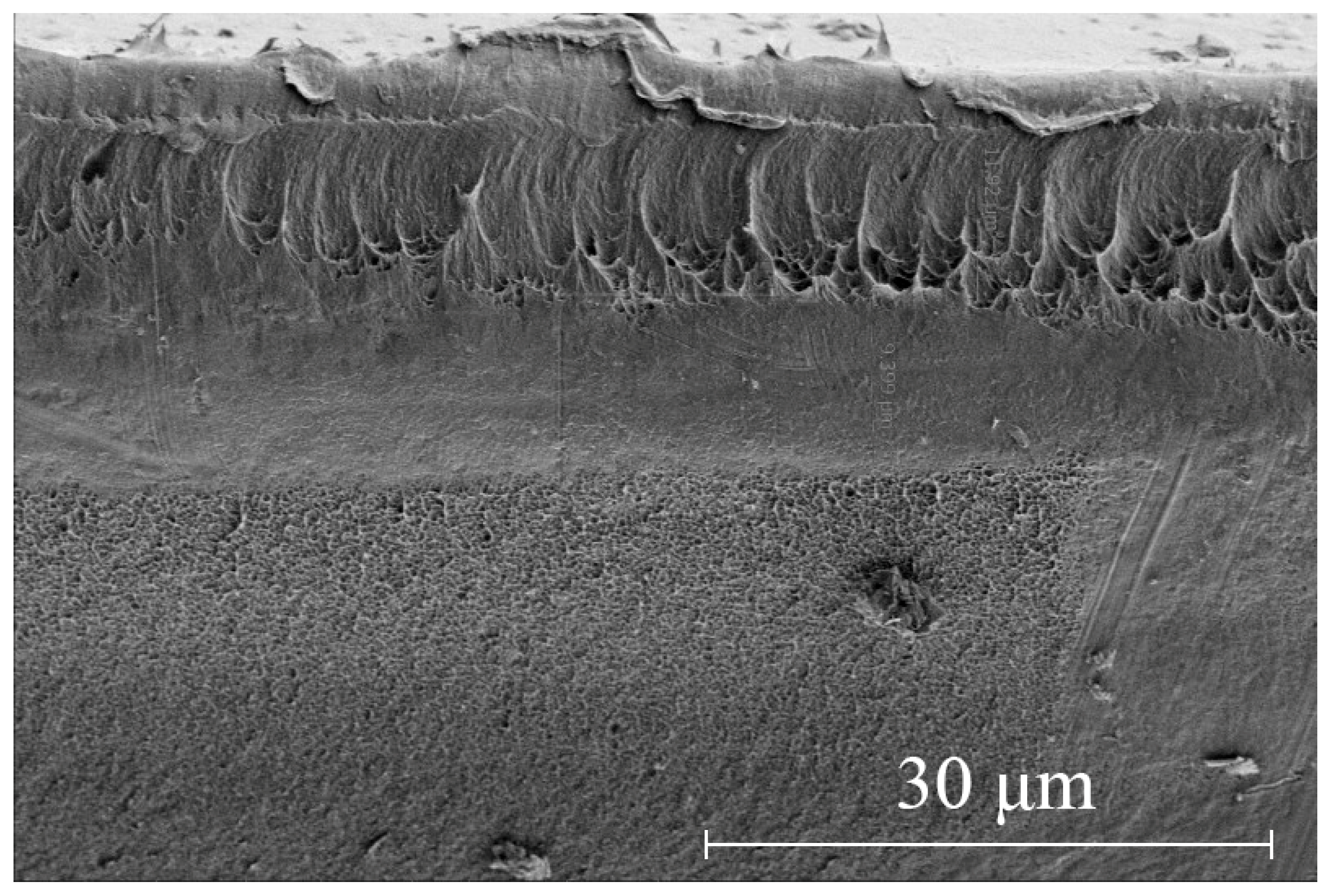
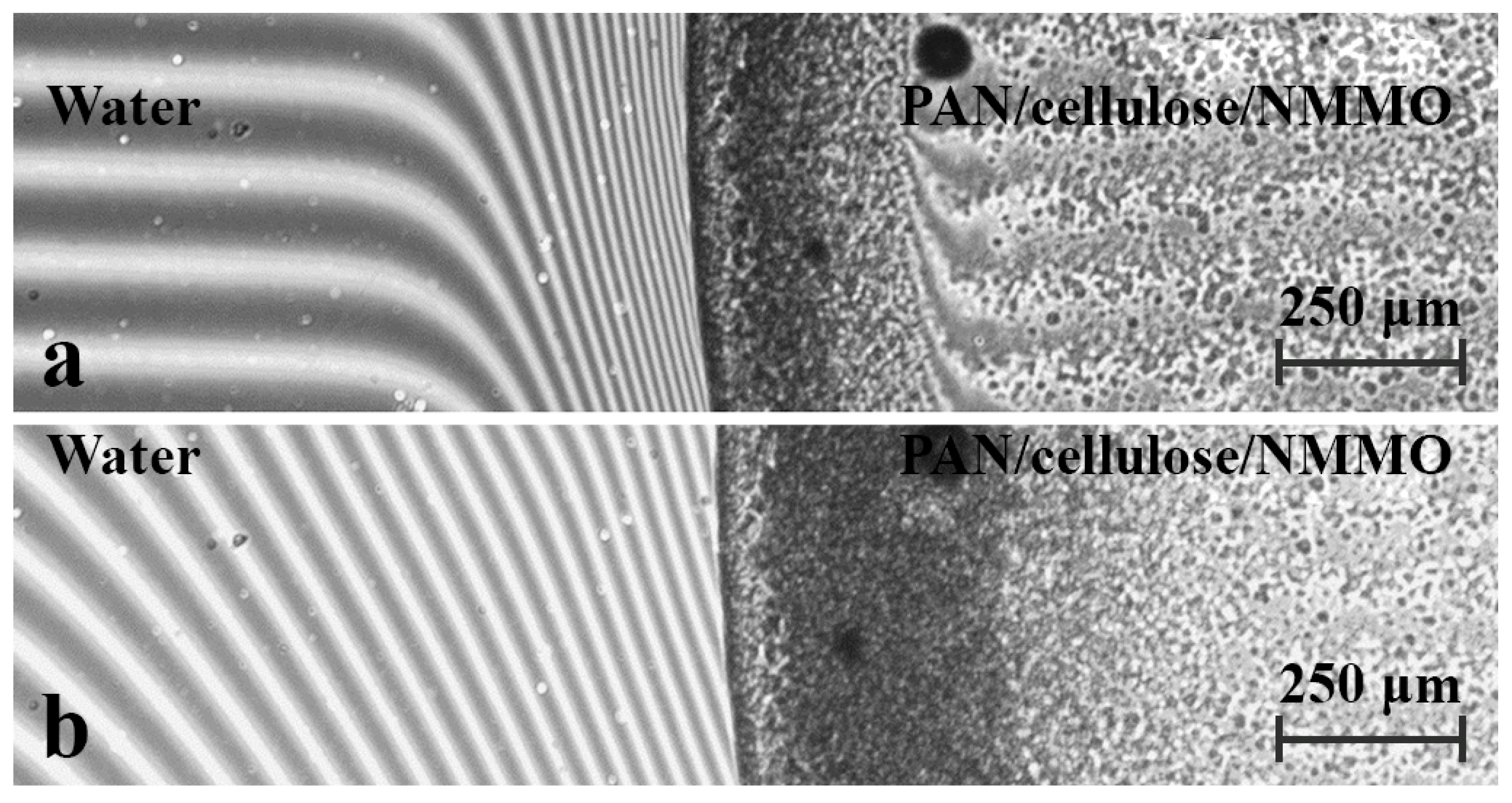
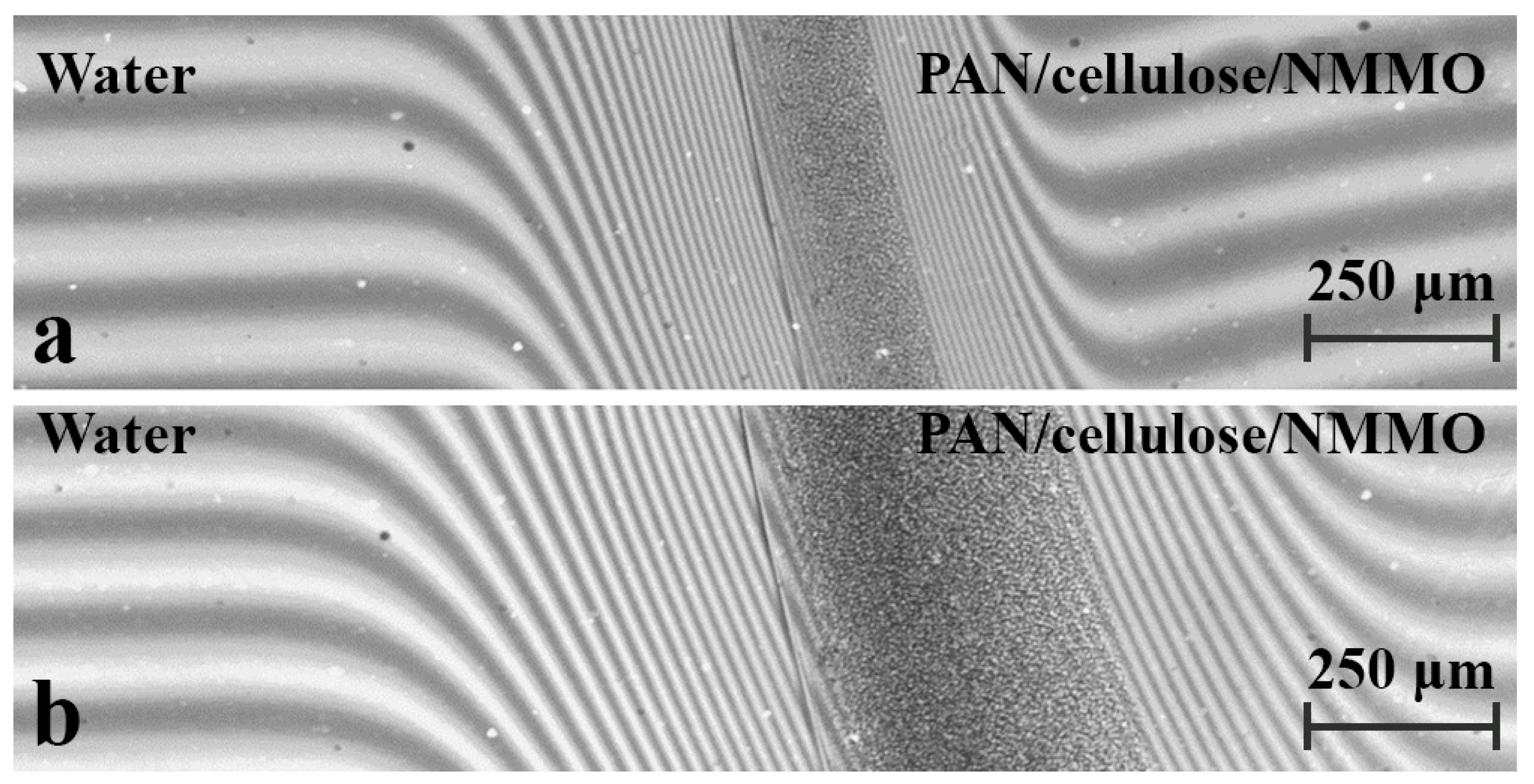
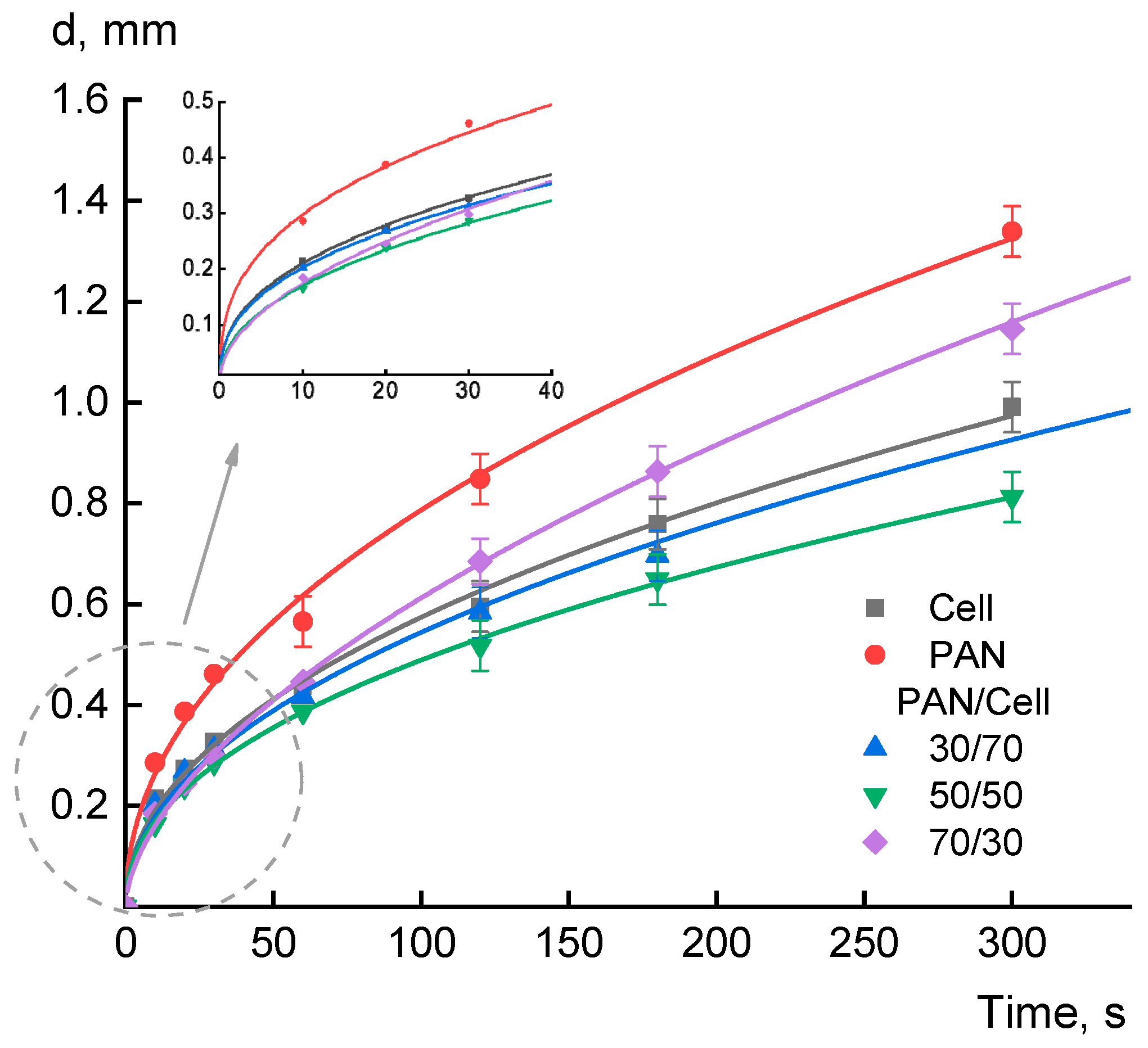
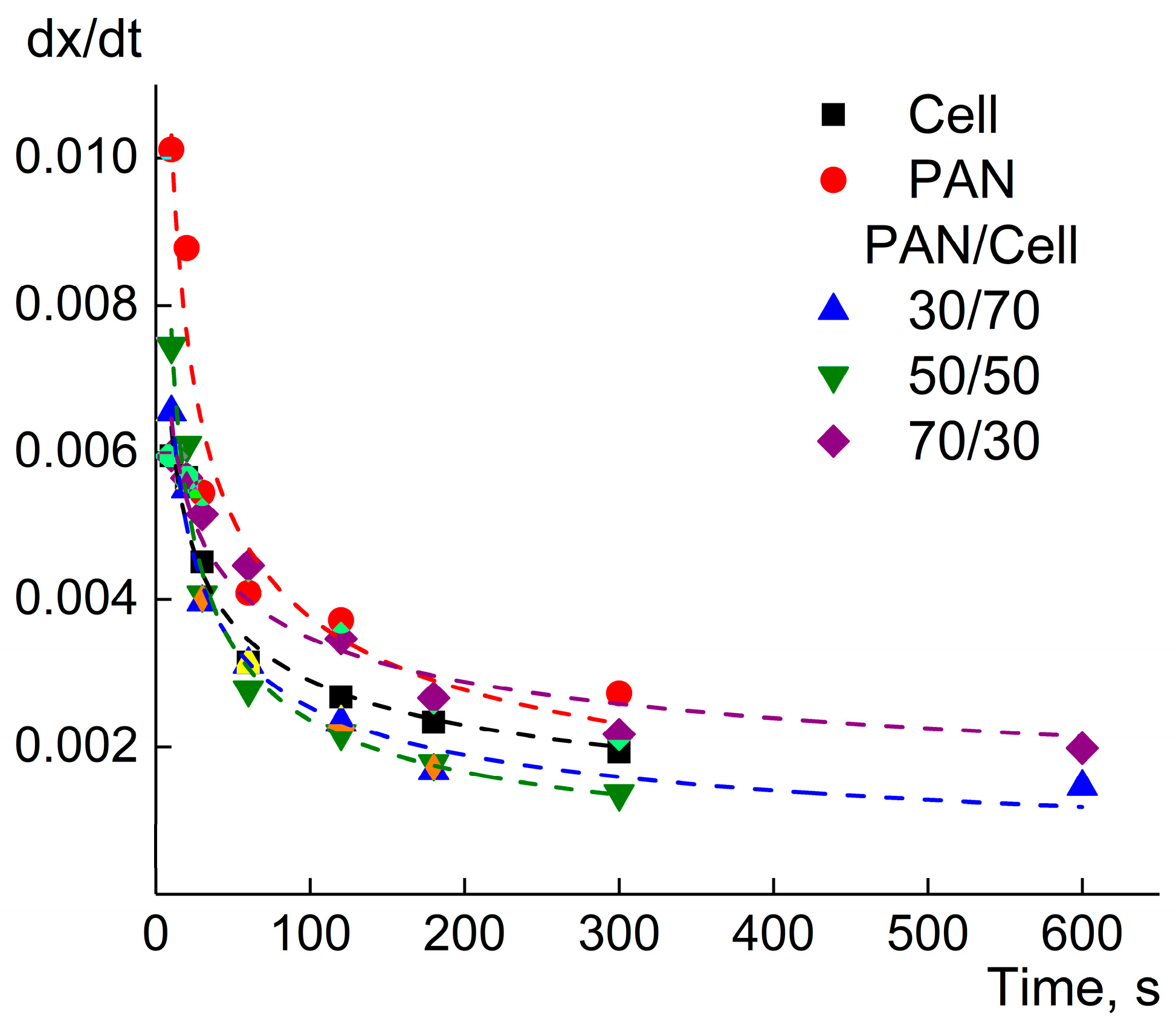
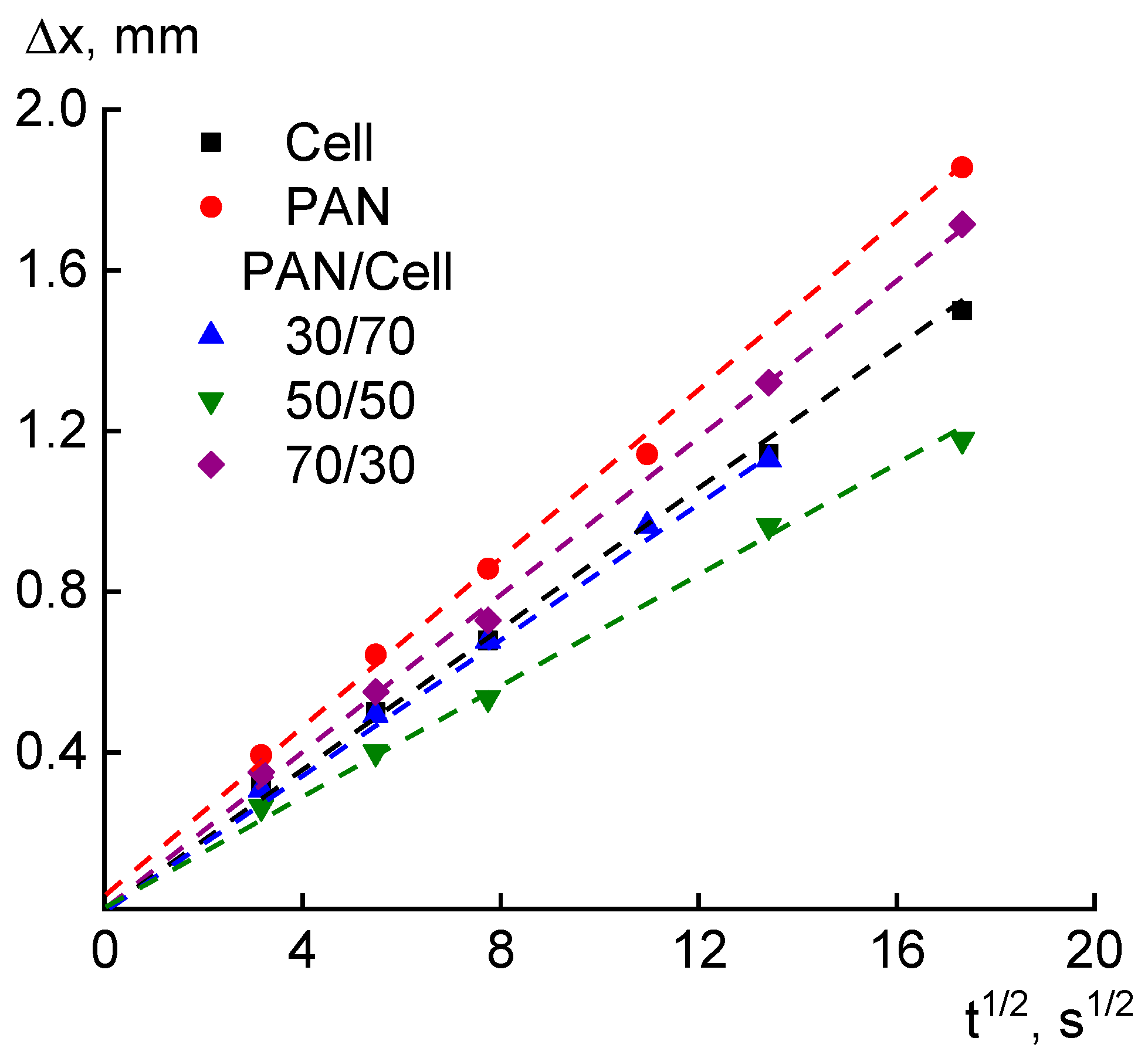
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shcherbina, L.A.; Chikunskaya, V.M.; Ogorodnikov, V.A.; Budkute, I.A. Synthesis of a Fiber-Forming Copolymer of Acrylonitrile in Dimethylsulfoxide. Fibre Chem. 2023, 54, 288–295. [Google Scholar] [CrossRef]
- Kolobkov, A.S.; Malakhovskii, S.S. Development of Carbon Fiber Production Technologies. A Review. Fibre Chem. 2020, 52, 1–5. [Google Scholar] [CrossRef]
- Pradere, C.; Sauder, C. Transverse and longitudinal coefficient of thermal expansion of carbon fibers at high temperatures (300–2500 K). Carbon 2008, 46, 1874–1884. [Google Scholar] [CrossRef]
- Chae, H.G.; Newcomb, B.A.; Gulgunje, P.V.; Liu, Y.; Gupta, K.K.; Kamath, M.G.; Lyons, K.M.; Ghoshal, S.; Pramanik, C.; Giannuzzi, L.; et al. High strength and high modulus carbon fibers. Carbon 2015, 93, 81–88. [Google Scholar] [CrossRef]
- Iovleva, M.M.; Smirnova, V.N.; Budnitskii, G.A. The Solubility of Polyacrylonitrile. Fibre Chem. 2001, 33, 262–264. [Google Scholar] [CrossRef]
- Bochek, A.M. Effect of Hydrogen Bonding on Cellulose Solubility in Aqueous and Nonaqueous Solvents. Russ. J. Appl. Chem. 2003, 76, 1711–1719. [Google Scholar] [CrossRef]
- Skvortsov, I.Y.; Malkin, A.Y.; Kuzin, M.S.; Bondarenko, G.N.; Gerasimenko, P.S.; Litmanovich, E.A. Rheology and molecular interactions in polyacrylonitrile solutions: Role of a solvent. J. Mol. Liq. 2022, 364, 119938. [Google Scholar] [CrossRef]
- Golova, L.K.; Borodina, O.E.; Kuznetsova, L.K.; Krylova, T.B. The Solid-Phase MMO Process. Fibre Chem. 2000, 32, 243–251. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Levin, I.S.; Sorokin, S.E. Structure of Polyacrylonitrile Fibers Produced from N-Methylmorpholine-N-Oxide Solutions. Fibre Chem. 2019, 50, 508–513. [Google Scholar] [CrossRef]
- Vinogradov, M.I.; Makarov, I.S.; Golova, L.K.; Bondarenko, G.N.; Kulichikhin, V.G. Structural–Morphological and Rheological Features of Joint Solutions of Cellulose and PAN Copolymer in N-Methylmorpholine-N-Oxide. Polym. Sci. Ser. A 2023, 65, 280–291. [Google Scholar] [CrossRef]
- Sayyed, J.; Mohite, L.V.; Deshmukh, N.A.; Pinjari, D.V. Intensification of lyocell dissolution process and dope characteristics using pre-swelled cellulosic pulp. Chem. Eng. Process. Process Intensif. 2020, 148, 107826. [Google Scholar] [CrossRef]
- Wang, K.; Li, K.; Gu, Y.; Yang, G.; Yao, X.; Zhang, Y. Dissolving-grade pulp and lyocell fibers prepared from cotton stalks. Cellulose 2025, 32, 2227–2243. [Google Scholar] [CrossRef]
- Hedlund, A.; Theliander, H.; Köhnke, T. Mass transport during coagulation of cellulose-ionic liquid solutions in different non-solvents. Cellulose 2019, 26, 8525–8541. [Google Scholar] [CrossRef]
- Varfolomeeva, L.A.; Skvortsov, I.Y.; Levin, I.S.; Shandryuk, G.A.; Patsaev, T.D.; Kulichikhin, V.G. Polyacrylonitrile Fibers with a Gradient Silica Distribution as Precursors of Carbon-Silicon-Carbide Fibers. Polymers 2023, 15, 2579. [Google Scholar] [CrossRef] [PubMed]
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Mironova, M.V.; Levin, I.S.; Bondarenko, G.N.; Kulichikhin, V.G. The Role of Isobutanol as a Precipitant of Cellulose Films Formed from N-Methylmorpholine N-Oxide Solutions: Phase State and Structural and Morphological Features. Polym. Sci. Ser. A 2019, 61, 598–609. [Google Scholar] [CrossRef]
- Skvortsov, I.Y.; Kulichikhin, V.G.; Ponomarev, I.I.; Varfolomeeva, L.A.; Kuzin, M.S.; Skupov, K.M.; Serenko, O.A. Solubility, Rheology, and Coagulation Kinetics of Poly-(O-Aminophenylene) Naphthoylenimide Solutions. Polymers 2020, 12, 2454. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, C.; Duan, C.; Hu, H.; Li, H.; Li, J.; Liu, Y.; Ma, X.; Stavik, J.; Ni, Y. Regenerated cellulose by the Lyocell process, a brief review of the process and properties. BioResources 2018, 13, 4577–4592. [Google Scholar] [CrossRef]
- Romanov, V.V.; Sokira, A.N.; Lunina, O.B.; Iovleva, M.M. Morphological features of the structure of fibres prepared from solutions of cellulose in methylmorpholine oxide. Fibre Chem. 1988, 20, 38–39. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Mironova, M.V.; Arkharova, N.A.; Klechkovskaya, V.V.; Kulichikhin, V.G. Morphological transformations in the process of coagulation of cellulose solution in N-methylmorpholine N-oxide with isobutanol. Polym. Sci. Ser. C 2021, 63, 161–169. [Google Scholar] [CrossRef]
- Hedlund, A.; Könke, T.; Theliander, H. Diffusion of ionic liquid–cellulose solutions during coagulation in water: Mass transport and coagulation rate measurements. Macromolecules 2017, 50, 8707–8719. [Google Scholar] [CrossRef]
- Biganska, O.; Navard, P. Kinetics of precipitation of cellulose from cellulose–NMMO-water solutions. Biomacromolecules 2005, 6, 1948–1953. [Google Scholar] [CrossRef]
- Makarova, V.V.; Antonov, S.V.; Brantseva, T.V.; Kulichikhin, V.G.; Anokhina, T.S. Phase-equilibrium and cellulose-coagulation kinetics for cellulose solutions in N-methylmorpholine-N-oxide. Polym. Sci. Ser. A 2016, 58, 732–743. [Google Scholar] [CrossRef]
- Laity, P.R.; Glover, P.M.; Hay, J.N. Composition and phase changes observed by magnetic resonance imaging during non-solvent induced coagulation of cellulose. Polymer 2002, 43, 5827–5837. [Google Scholar] [CrossRef]
- Shen, T.C.; Cabasso, I. Macromolecular Solutions, Solvent-Property Relationship in Polymers; Seymour, R.B., Stahl, G.A., Eds.; Pergamon Press: New York, NY, USA, 1982. [Google Scholar]
- Makarov, I.S.; Golova, L.K.; Kuznetsova, L.K.; Antonov, S.V.; Kotsyuk, A.V.; Ignatenko, V.Y.; Kulichikhin, V.G. Influence of Precipitation and Conditioning Baths on the Structure, Morphology, and Properties of Cellulose Films. Fibre Chem. 2016, 48, 298–305. [Google Scholar] [CrossRef]
- Fink, H.P.; Weigel, P.; Purz, H.J.; Johannes, G. Structure formation of regenerated cellulose materials from NMMO-Solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Skvortsov, I.Y.; Kalugina, A.D.; Litvinova, E.G.; Malkin, A.Y.; Khotimskiy, V.S.; Kulichikhin, V.G. Fibers spinning from poly (trimethylsilylpropyne) solutions. J. Appl. Polym. Sci. 2020, 137, 48511. [Google Scholar] [CrossRef]
- Banduryan, S.I.; Iovleva, M.M.; Belousov, Y.Y.; Ivanova, N.A. Structure formation in solutions of cellulose in N-methylmorpholine N-oxide and during its precipitation. Fibre Chem. 1985, 16, 323–325. [Google Scholar] [CrossRef]
- Uddin, A.J.; Yamamoto, A.; Gotoh, Y.; Nagura, M.; Iwata, M. Regenerated cellulose fibers from waste bagasse using ionic liquid. Text. Res. J. 2010, 80, 1949–1958. [Google Scholar] [CrossRef]
- Papkov, S.P. Theory of the preparation of superstrong polymer fibres. Fibre Chem. 1981, 13, 212–216. [Google Scholar] [CrossRef]
- Makarov, I.S.; Vinogradov, M.I.; Golova, L.K.; Arkharova, N.A.; Shambilova, G.K.; Makhatova, V.E.; Naukenov, M.Z. Design and Fabrication of Membranes Based on PAN Copolymer Obtained from Solutions in N-methylmorpholine-N-oxide. Polymers 2022, 14, 2861. [Google Scholar] [CrossRef]
- Pochivalov, K.V.; Basko, A.V.; Ilyasova, A.N.; Lebedeva, T.N.; Yurov, M.Y.; Bronnikov, S.V. Experimental phase diagram for the PVDF–DMAc– water ternary system with new topology: Method of construction, thermodynamics, and structure formation of membranes. Polymer 2023, 282, 126152. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, Y.; Pan, F.; Zhou, L.; Ji, X.; Kang, Z.; Zhang, S. Study on ionic liquid/cellulose/coagulator phase diagram and its application in green spinning process. J. Mol. Liq. 2019, 289, 111127. [Google Scholar] [CrossRef]
- Eckelt, J.; Eich, T.; Röder, T.; Rüf, H.; Sixta, H.; Wolf, B.A. Phase diagram of the ternary system NMMO/water/cellulose. Cellulose 2009, 16, 373–379. [Google Scholar] [CrossRef]
- Golova, L.K. New cellulose fiber lyocell. Russ. J. Gen. Chem. 2002, XLVI, 49–57. [Google Scholar]
- Palchikova, E.E.; Makarov, I.S.; Mironova, M.V.; Vinogradov, M.I.; Golova, L.K.; Kulichikhin, V.G. Phase Transforations in a PAN–N-Methylmorpholine-N-Oxide–Water System. Colloid J. 2022, 84, 730–740. [Google Scholar] [CrossRef]
- Golova, L.K.; Borodina, O.E.; Rudinskaya, G.Y.; Papkov, S.P. Optical Properties and Structure of Highly Concentrated Solutions of Cellulose in N-Methylmorpholine N-Oxide. Fibre Chem. 2001, 33, 140–144. [Google Scholar] [CrossRef]
- Skvortsov, I.Y.; Varfolomeeva, L.A.; Kulichikhin, V.G. The effect of tetraethoxysilane on the phase state, rheological properties, and coagulation features of polyacrylonitrile solutions. Colloid J. 2019, 81, 165–175. [Google Scholar] [CrossRef]
- Mironova, M.V.; Tarasov, A.E.; Kuzin, M.S.; Skvortsov, I.Y.; Arkharova, N.A.; Podval’naya, Y.V.; Grishchuk, A.A.; Badamshin, E.R.; Kulichikhin, V.G. Rheological and Relaxational Properties of Mixed Solutions Based on Linear and Highly Branched Polyacrylonitrile. Polym. Sci. Ser. A 2022, 64, 354–365. [Google Scholar] [CrossRef]
- Makarova, V.; Kulichikhin, V. Application of Interferometry to Analysis of Polymer-Polymer and Polymer-Solvent Interactions. In Interferometry–Research and Applications in Science and Technology; Padron, I., Ed.; InTechOpen: London, UK, 2012; pp. 395–436. [Google Scholar] [CrossRef][Green Version]
- Makarova, V.V.; Antonov, S.V.; Anokhina, T.A.; Volkov, V.V. Optical microinterferometry method for evaluation of phase state and diffusion in ternary systems: Phase separation in cellulose/N-Methylmorpholine-N-oxide/non-solvent mixtures. J. Phys. Conf. Ser. 2016, 751, 012045. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Asaadi, S.; Ahvenainen, P.; Sixta, H. Water-induced crystallization and nano-scale spinodal decomposition of cellulose in NMMO and ionic liquid dope. Cellulose 2019, 26, 281–289. [Google Scholar] [CrossRef]
- Nikulova, U.V.; Chalykh, A.E. Phase Equilibrium and Interdiffusion in Blends of Polystyrene with Polyacrylates. Polymers 2021, 13, 2283. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mironova, M.; Makarov, I.; Palchikova, E.; Makarov, G.; Vinogradov, M.; Orlov, M.; Komarov, I. Control of Precipitation of Cellulose Solutions in N-Methylmorpholine-N-oxide by Introducing Polyacrylonitrile Additives. Polysaccharides 2025, 6, 88. https://doi.org/10.3390/polysaccharides6040088
Mironova M, Makarov I, Palchikova E, Makarov G, Vinogradov M, Orlov M, Komarov I. Control of Precipitation of Cellulose Solutions in N-Methylmorpholine-N-oxide by Introducing Polyacrylonitrile Additives. Polysaccharides. 2025; 6(4):88. https://doi.org/10.3390/polysaccharides6040088
Chicago/Turabian StyleMironova, Maria, Igor Makarov, Ekaterina Palchikova, Georgy Makarov, Markel Vinogradov, Maxim Orlov, and Ivan Komarov. 2025. "Control of Precipitation of Cellulose Solutions in N-Methylmorpholine-N-oxide by Introducing Polyacrylonitrile Additives" Polysaccharides 6, no. 4: 88. https://doi.org/10.3390/polysaccharides6040088
APA StyleMironova, M., Makarov, I., Palchikova, E., Makarov, G., Vinogradov, M., Orlov, M., & Komarov, I. (2025). Control of Precipitation of Cellulose Solutions in N-Methylmorpholine-N-oxide by Introducing Polyacrylonitrile Additives. Polysaccharides, 6(4), 88. https://doi.org/10.3390/polysaccharides6040088






