Extraction, Quantification, and Characterization of Chitin from Marine Biofouling Organisms Amphipods (Jassa sp.) and Hydroids (Coryne sp.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chitin Extraction and Quantification
2.2.1. Pre-Treatment
2.2.2. Demineralization
2.2.3. Deproteinization
2.2.4. Quantification
2.3. Degree of Acetylation (DA) Determination
2.4. Crystallinity Index (CrI) Determination
2.5. Molecular Weight (MW) Determination of Chitin
2.5.1. Chitin Solution Preparation
2.5.2. Viscosity Measurements and MW Calculations
2.6. Thermogravimetric Analysis (TGA)
2.7. Surface Morphology Determination
3. Results and Discussion
3.1. Chitin Quantification
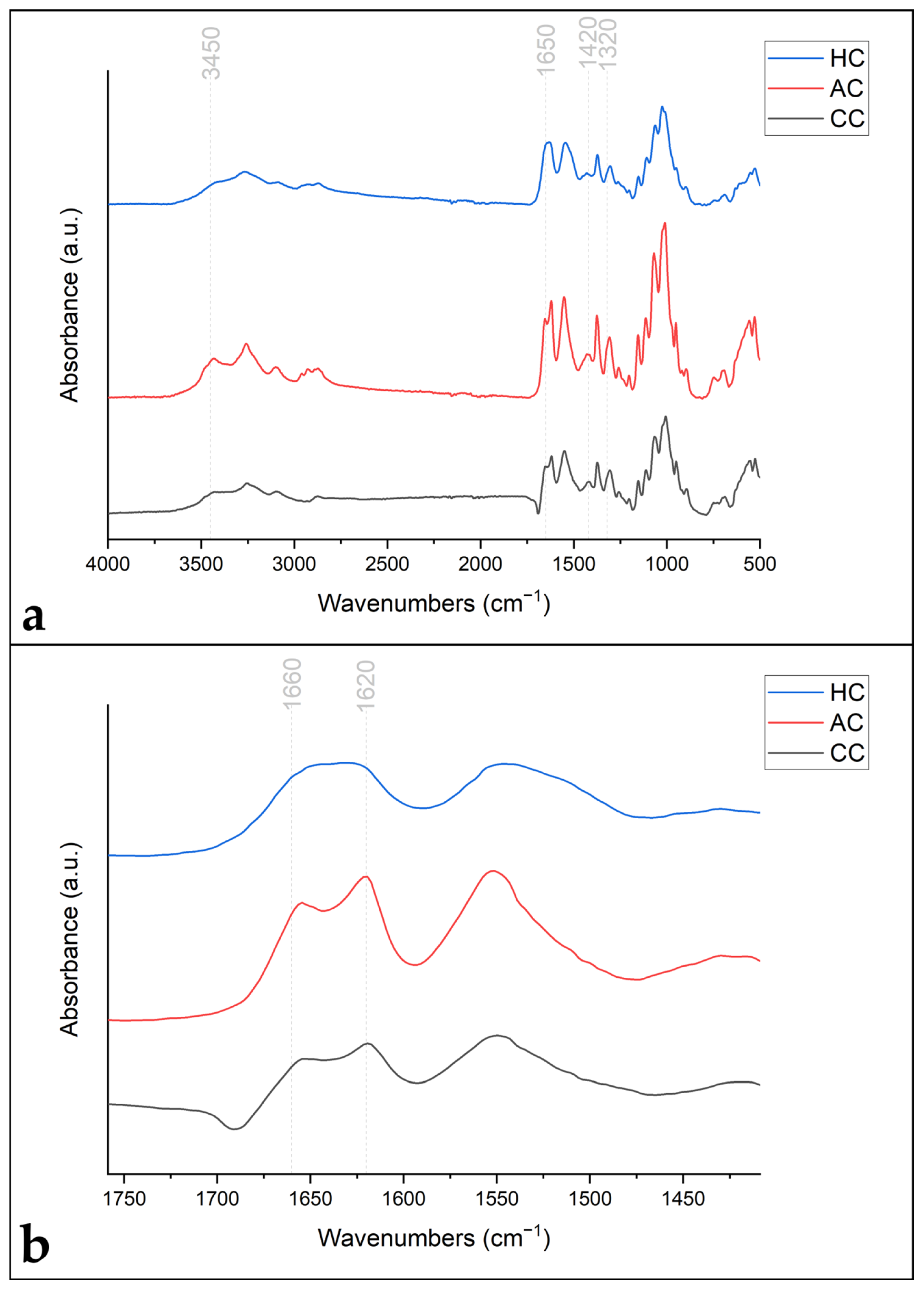
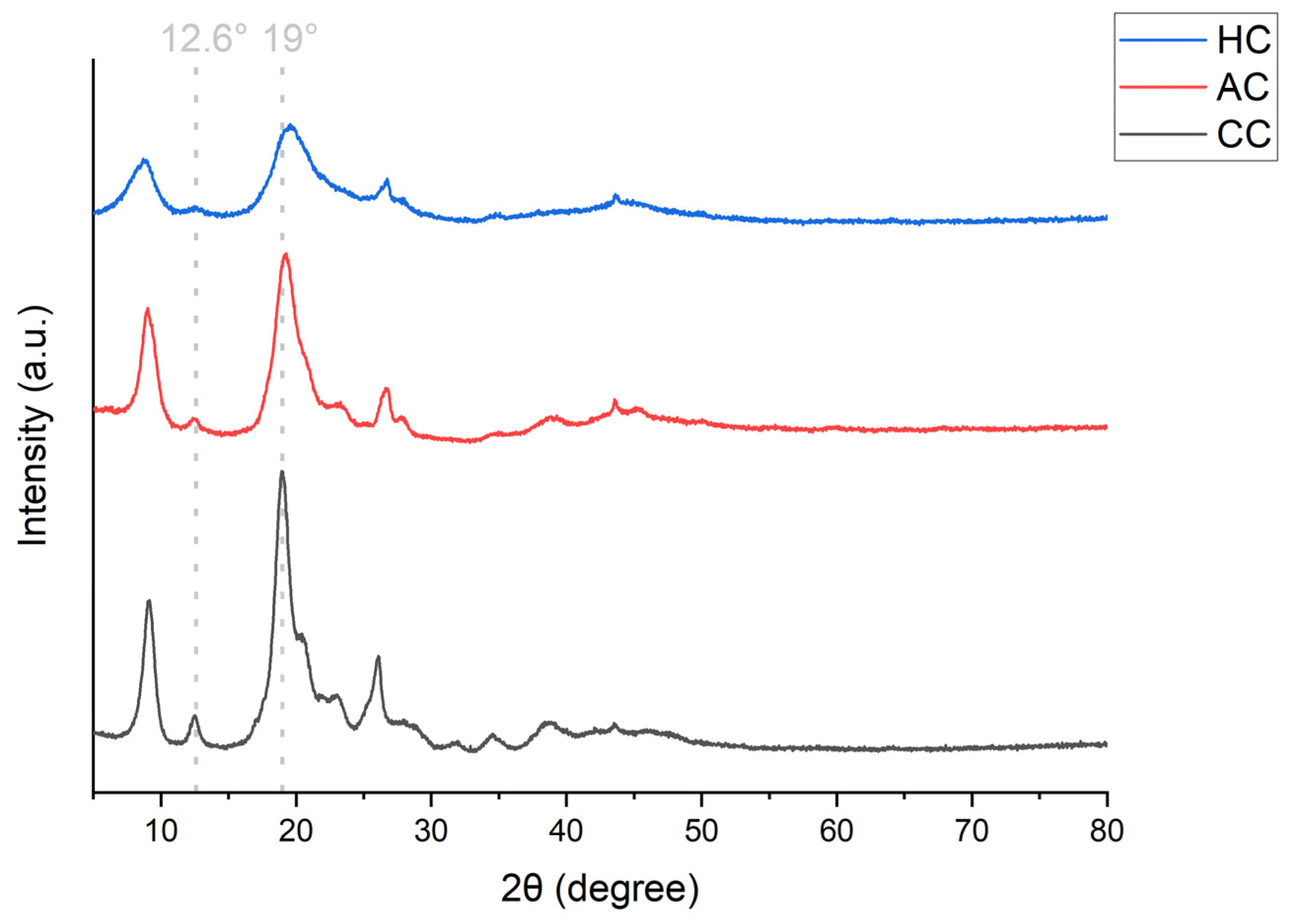
3.2. Chitin Characterization
3.2.1. Degree of Acetylation (DA)
3.2.2. Structural Conformation
3.2.3. Crystallinity Index (CrI)
3.2.4. Molecular Weight (MW)
3.2.5. Thermal Stability
3.2.6. Surface Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CrI | Crystallinity Index |
| DA | Degree of Acetylation |
| MW | Molecular Weight |
| TGA | Thermogravimetric Analysis |
| AC | Amphipods Chitin |
| HC | Hydroids Chitin |
| CC | Commercial Chitin |
References
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Lv, J.; Lv, X.; Ma, M.; Oh, D.-H.; Jiang, Z.; Fu, X. Chitin and chitin-based biomaterials: A review of advances in processing and food applications. Carbohydr. Polym. 2023, 299, 120142. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Jang, M.; Kong, B.; Jeong, Y.; Lee, C.H.; Nah, J. Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Moreno-Tovar, R.; Bucio, L.; Thions, C.; Tehuacanero-Cuapa, S. Thermal degradation and lifetime of β-chitin from Dosidicus gigas squid pen: Effect of impact at 9.7 GPa and a comparative study with α-chitin. Carbohydr. Polym. 2021, 251, 116987. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Poerio, A.; Petit, C.; Jehl, J.-P.; Arab-Tehrany, E.; Mano, J.F.; Cleymand, F. Extraction and Physicochemical Characterization of Chitin from Cicada orni Sloughs of the South-Eastern French Mediterranean Basin. Molecules 2020, 25, 2543. [Google Scholar] [CrossRef] [PubMed]
- FAO. The state of world fisheries and aquaculture 2024. In Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Bannister, J.; Sievers, M.; Bush, F.; Bloecher, N. Biofouling in marine aquaculture: A review of recent research and developments. Biofouling 2019, 35, 631–648. [Google Scholar] [CrossRef]
- Fletcher, L.M.; Davidson, I.C.; Bucknall, B.G.; Atalah, J. Salmon farm biofouling and potential health impacts to fish from stinging cnidarians. Aquaculture 2023, 568, 739315. [Google Scholar] [CrossRef]
- Collaborative Effort Looks into Biofouling, IntraFish.Com|Latest Seafood, Aquaculture and Fisheries News. 2008. Available online: https://www.intrafish.com/aquaculture/collaborative-effort-looks-into-biofouling/1-1-569282 (accessed on 22 July 2025).
- Dürr, S.; Watson, D.I. Biofouling and Antifouling in Aquaculture. In Biofouling; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 267–287. [Google Scholar] [CrossRef]
- Floerl, O.; Sunde, L.; Bloecher, N. Potential environmental risks associated with biofouling management in salmon aquaculture. Aquac. Environ. Interact. 2016, 8, 407–417. [Google Scholar] [CrossRef]
- Bloecher, N.; Broch, O.J.; Davies, E.J.; Pedersen, M.O.; Floerl, O. Catch my drift? Between-farm dispersal of biofouling waste from salmon pen net cleaning: Potential risks for fish health. Sci. Total Environ. 2024, 928, 172464. [Google Scholar] [CrossRef] [PubMed]
- Fitridge, I.; Dempster, T.; Guenther, J.; de Nys, R. The impact and control of biofouling in marine aquaculture: A review. Biofouling 2012, 28, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalez, V.; Sanchez-Jerez, P. Fouling assemblages associated with off-coast aquaculture facilities: An overall assessment of the Mediterranean Sea. Mediterr. Mar. Sci. 2017, 18, 87–96. [Google Scholar] [CrossRef]
- Ulaski, B.P.; Konar, B. Seasonal and site-specific differences in biofouling communities on Pacific oyster Mariculture farms. J. Exp. Mar. Biol. Ecol. 2024, 578, 152031. [Google Scholar] [CrossRef]
- Tretenichenko, E.M.; Datsun, V.M.; Ignatyuk, L.N.; Nud’ga, L.A. Preparation and properties of chitin and chitosan from a hydroid polyp. Russ. J. Appl. Chem. 2006, 79, 1341–1346. [Google Scholar] [CrossRef]
- Ritter, C.J.; Bourne, D.G. Marine amphipods as integral members of global ocean ecosystems. J. Exp. Mar. Biol. Ecol. 2024, 572, 151985. [Google Scholar] [CrossRef]
- Achinivu, E.C.; Shamshina, J.L.; Rogers, R.D. Chitin extracted from various biomass sources: It’s not the same. Fluid Phase Equilibria 2022, 552, 113286. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Gharibzadeh, M.; Osfouri, S.; Jamekhorshid, A.; Jafari, S.A. Microbial chitin extraction and characterization from green tiger shrimp waste: A comparative study of culture mediums along with bioprocess optimization. Int. J. Biol. Macromol. 2023, 242, 125213. [Google Scholar] [CrossRef]
- Focher, B.; Beltrame, P.; Naggi, A.; Torri, G. Alkaline N-deacetylation of chitin enhanced by flash treatments. Reaction kinetics and structure modifications. Carbohydr. Polym. 1990, 12, 405–418. [Google Scholar] [CrossRef]
- Li, G.; Du, Y.; Tao, Y.; Liu, Y.; Li, S.; Hu, X.; Yang, J. Dilute solution properties of four natural chitin in NaOH/urea aqueous system. Carbohydr. Polym. 2010, 80, 970–976. [Google Scholar] [CrossRef]
- Mohan, K.; Ravichandran, S.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Rajeevgandhi, C.; Rajan, D.K.; Seedevi, P. Extraction and characterization of chitin from sea snail Conus inscriptus (Reeve, 1843). Int. J. Biol. Macromol. 2019, 126, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Rasti, H.; Parivar, K.; Baharara, J.; Iranshahi, M.; Namvar, F. Chitin from the Mollusc Chiton: Extraction, Characterization and Chitosan Preparation. Iran. J. Pharm. Res. 2017, 16, 366–379. [Google Scholar] [PubMed]
- Abdulkarim, A.; Isa, M.T.; Abdulsalam, S.; Muhammad, A.J.; Ameh, A.O. Extraction and Characterisation of Chitin and Chitosan from Mussel Shell. Civ. Environ. Eng. 2013, 3, 108–114. [Google Scholar]
- Abdou, E.S.; Nagy, K.S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef]
- Tang, D.; Qian, J.; Wang, N.; Shu, J. Determining the degree of acetylation of chitin/chitosan using a SSNMR 13C method on the basis of cross polarization reciprocity relation. Carbohydr. Res. 2020, 498, 108168. [Google Scholar] [CrossRef]
- Alabaraoye, E.; Achilonu, M.; Hester, R. Biopolymer (Chitin) from Various Marine Seashell Wastes: Isolation and Characterization. J. Polym. Environ. 2018, 26, 2207–2218. [Google Scholar] [CrossRef]
- Lavall, R.; Assis, O.; Campanafilho, S. β-Chitin from the pens of Loligo sp.: Extraction and characterization. Bioresour. Technol. 2007, 98, 2465–2472. [Google Scholar] [CrossRef]
- Smets, R.; Verbinnen, B.; Van De Voorde, I.; Aerts, G.; Claes, J.; Van Der Borght, M. Sequential Extraction and Characterisation of Lipids, Proteins, and Chitin from Black Soldier Fly (Hermetia illucens) Larvae, Prepupae, and Pupae. Waste Biomass Valorization 2020, 11, 6455–6466. [Google Scholar] [CrossRef]
- Kaya, M.; Lelešius, E.; Nagrockaitė, R.; Sargin, I.; Arslan, G.; Mol, A.; Baran, T.; Can, E.; Bitim, B. Differentiations of Chitin Content and Surface Morphologies of Chitins Extracted from Male and Female Grasshopper Species. PLoS ONE 2015, 10, e0115531. [Google Scholar] [CrossRef]
- Berton, P.; Shen, X.; Rogers, R.D.; Shamshina, J.L. 110th Anniversary: High-Molecular-Weight Chitin and Cellulose Hydrogels from Biomass in Ionic Liquids without Chemical Crosslinking. Ind. Eng. Chem. Res. 2019, 58, 19862–19876. [Google Scholar] [CrossRef]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Sun, Z.; Ali, M.A. Bio-scaffolds produced from irradiated squid pen and crab chitosan with hydroxyapatite/β-tricalcium phosphate for bone-tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.S.; da Silva Agostini, D.L.; Job, A.E.; González, E.R.P. Thermal studies of chitin–chitosan derivatives. J. Therm. Anal. Calorim. 2013, 114, 321–327. [Google Scholar] [CrossRef]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A.; Chen, G.; Guan, J. Solubility of Chitin: Solvents, Solution Behaviours and Their Related Mechanisms. In Solubility of Polysaccharides; Xu, Z., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Stawski, D.; Rabiej, S.; Herczyńska, L.; Draczyński, Z. Thermogravimetric analysis of chitins of different origin. J. Therm. Anal. Calorim. 2008, 93, 489–494. [Google Scholar] [CrossRef]
- Chandran, R.; Williams, L.; Hung, A.; Nowlin, K.; LaJeunesse, D. SEM characterization of anatomical variation in chitin organization in insect and arthropod cuticles. Micron 2016, 82, 74–85. [Google Scholar] [CrossRef]



| Source | Chitin Content (%) | Starting Material | Reference |
|---|---|---|---|
| Amphipod (Jassa sp.) | 12.34 ± 1.10 | Whole organism | (this study) |
| Hydroid (Coryne sp.) | 32.75 ± 0.49 | Whole organism | (this study) |
| Brown shrimp shell | 21.53 | Skeletal-only | [29] |
| Squid pen | 49.00 | Skeletal-only | [29] |
| Crab shell | 16.73 | Skeletal-only | [29] |
| Crayfish shell | 20.60 | Skeletal-only | [29] |
| Conus shell | 21.65 | Skeletal-only | [26] |
| Mussel shell | 21.32 | Skeletal-only | [28] |
| Source | Degree of Acetylation A1320/A1420 (DA, %) | Degree of Acetylation A1650/A3450 (DA, %) | Structural Conformation | Crystallinity Index (CrI, %) | Molecular Weight (MW, kDa) |
|---|---|---|---|---|---|
| Commercial chitin (CC) | 95.6 ± 5.3 | 94.4 ± 3.7 | α | 76.11 | 336 ± 31 |
| Amphipod chitin (AC) | 58.4 ± 1.4 | 59.2 ± 1.7 | α | 75.80 | 33 ± 3 |
| Hydroid chitin (HC) | 66.7 ± 1.2 | 64.8 ± 0.5 | β | 57.43 | 101 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvoski, C.; Lobarbio, C.F.; Plowman-Holmes, M.; Bell, P.; Chambers, B.; Cumming, M. Extraction, Quantification, and Characterization of Chitin from Marine Biofouling Organisms Amphipods (Jassa sp.) and Hydroids (Coryne sp.). Polysaccharides 2025, 6, 87. https://doi.org/10.3390/polysaccharides6040087
Selvoski C, Lobarbio CF, Plowman-Holmes M, Bell P, Chambers B, Cumming M. Extraction, Quantification, and Characterization of Chitin from Marine Biofouling Organisms Amphipods (Jassa sp.) and Hydroids (Coryne sp.). Polysaccharides. 2025; 6(4):87. https://doi.org/10.3390/polysaccharides6040087
Chicago/Turabian StyleSelvoski, Christopher, Camila Flor Lobarbio, Matthew Plowman-Holmes, Peter Bell, Benie Chambers, and Mathew Cumming. 2025. "Extraction, Quantification, and Characterization of Chitin from Marine Biofouling Organisms Amphipods (Jassa sp.) and Hydroids (Coryne sp.)" Polysaccharides 6, no. 4: 87. https://doi.org/10.3390/polysaccharides6040087
APA StyleSelvoski, C., Lobarbio, C. F., Plowman-Holmes, M., Bell, P., Chambers, B., & Cumming, M. (2025). Extraction, Quantification, and Characterization of Chitin from Marine Biofouling Organisms Amphipods (Jassa sp.) and Hydroids (Coryne sp.). Polysaccharides, 6(4), 87. https://doi.org/10.3390/polysaccharides6040087






