Abstract
A totally green process based on reactive extrusion was used for the production of cassava starch hydrogels through reaction with two organic crosslinking agents, citric (CA) and tartaric (TA) acids. CA and TA were used at different concentrations (0, 2.5, 5.0, 10.0, 15.0, and 20.0%). Degree of substitution (DS) of hydrogels ranged from 0.023 to 0.365. Fourier transform infrared spectroscopy results showed a new band appearing at 1730 cm−1 associated with ester carbonyl groups. X-ray diffraction indicated that reactive extrusion resulted in the disappearance of diffraction peaks of native starch and samples with lower crystallinity indices ranging from 37% (native starch) to 8–11% in starch hydrogels. Morphology analysis showed that the original granular structure of starch was lost and replaced by a rougher and irregular structure. Water holding capacity values of starch hydrogels obtained by reactive extrusion were superior to those of native starch and the control sample (extruded without the crosslinking agents). Hydrogels obtained with the highest CA or TA concentration (20.0%) resulted in the higher DS and swelling capacities, resulting in samples with 870 and 810% of water retention, respectively. Reactive extrusion was effective in obtaining starch hydrogels by reaction with organic acids.
1. Introduction
Hydrogels are materials that can be produced from natural or synthetic polymers; these polymers are crosslinked to result in three-dimensional polymeric matrices that have the ability of retain water or biological fluids without dissolving. Hydrogels can be obtained in different formats, including films, membranes, powders, and micro or nanogels [1,2,3].
Starch is a biodegradable, nontoxic, and inexpensive biopolymer available worldwide with readily free hydroxyl groups with potential for functionalization [4], including crosslinking reactions [2,5]. The crosslinking for chemical modification of starch through reaction with bi- or polyfunctional agents is largely reported in the literature [6,7,8,9,10,11,12,13]. The crosslinking agents can form ether or ester intermolecular bonds between hydroxyl groups on starch molecules, resulting in a reinforced polymer matrix. Depending on the level of substitution, the resulting materials can present changes in solubility and swelling power, which are important properties for hydrogels [11,14,15].
Starch hydrogels have several potential applications; however, to obtain food-grade, biodegradable and biocompatible hydrogels, the high cost of raw materials, and the generation of toxic solvents can be considered significant disadvantages. Epichlorohydrin and glutaraldehyde have been widely used to crosslink polysaccharide materials; however, they suffer from certain toxicity [2,16,17]. In contrast, polycarboxylic acids such as CA and TA are inexpensive and nontoxic reagents that can be safely used to obtain crosslinked starches [4,18,19,20]. CA and other polycarboxylic acids had been described as efficient esterification and crosslinking agents of starch, and they also present a hydrolytic action depending on the processing conditions [4,15,20].
In the last few years, the use of CA and other organic acids as esterification and crosslinking agents to obtain modified starches from several sources has been reported in literature, emphasizing the efficiency, and lower environmental impact of their use [11,13,18,19,20,21,22,23,24,25,26].
Starch modification has been traditionally carried out in tank reactors with several reagents and long processing time, resulting in negative impacts from effluent generation; thus, the use of physical methods can be an interesting approach to minimize the excessive use of reagents [13,16,22,27]. Lipatova and Yusova [28] reported the effectiveness of mechanical activation in a rotor–stator device on starch crosslinking with CA to obtain biodegradable hydrogels.
Alternatively, the reactive extrusion process is an efficient and versatile method for modifying starch and it is considered a green technological solution since the extruder is used as a reactor, where chemical reactions such as esterification, hydroxypropylation, and crosslinking can be performed [27,28,29,30]. Reactive extrusion combines the thermomechanical energy necessary to disintegrate the native structure of the starch granule, favoring the reaction between starch and organic acids in a single process without using other reagents. Additionally, reactive extrusion is a continuous process that has commercial viability, and it is easy to adapt to industrial scales, offering short reaction time (2–3 min) [12,16,31,32,33].
The use of reactive extrusion to obtain esterified and crosslinked starches through reaction with organic acids is an interesting research topic that could be further investigated to contribute to knowledge in the area. Recently, Farhat et al. [22], Hong et al. [23], and Ye et al. [13] reported the use of reactive extrusion to obtain starch citrates from native corn, waxy corn, and rice starches, and they stressed that this process can be considered a promising alternative to obtain esterified and crosslinked starches.
There are few reports in the literature about the production of starch citrates by reactive extrusion [13,22,23], while starch hydrogels obtained through reaction with TA by reactive extrusion have not yet been reported in the literature. Reactive extrusion can be considered an ecofriendly process to obtain esterified and crosslinked starches, and this study intended to explore the potential of this technology for the obtainment of new biobased materials, such as starch hydrogels, contributing to the generation of knowledge in this area. Therefore, this study aimed to obtain cassava starch hydrogels by reactive extrusion using CA and TA as crosslinking and esterifying agents and to study their physicochemical and microstructural properties.
2. Materials and Methods
2.1. Materials
Cassava starch (20% amylose and 80% amylopectin) was purchased from Pinduca Co., Ltd. (Araruna, Brazil). CA and TA of analytical grade were purchased from Synthlab (Synthlab, Diadema, Brazil), similar to all other chemicals and solvents employed in this study.
2.2. Reactive Extrusion Process
CA and TA were employed at different concentrations (0, 2.5, 5.0, 10.0, 15.0, and 20.0%—g acid/100 g starch) as esterifying/crosslinking agents. Crosslinked starch hydrogels were prepared by dissolving the different concentrations of CA or TA in distilled water, which were mixed with starch to obtain samples with a moisture content of 32%. Each sample was stored in plastic sealed bags for 1 h before extrusion. A control sample (S0) was also extruded in the presence of water (without Ca or TA), resulting in a moisture content of 32%. The reactive extrusion parameters were based on previous study of Gil-Giraldo et al. [34]. The extrusion process was performed in a single screw extruder (AX Plastics, Diadema, Brazil) with a screw length/diameter ratio (L/D) of 40 and a screw diameter of 1.6 cm, and a cylindrical matrix of 0.8 cm in diameter. The temperature in all four heating zones was 100 °C, and the screw speed was 60 rpm. Each sample was collected from the extruder and air-dried (45 °C) to a constant weight and ground. Then, each sample was washed three times with absolute ethanol to remove the unreacted CA or TA, as described by Ye et al. [13] and Gil-Giraldo et al. [34]. Finally, samples were air-dried at 45 °C to a constant weight, ground, and sieved through an 80-mesh sieve to obtain powders that were employed for characterization. Starch hydrogel samples prepared by reaction with CA were labeled as SC2.5, SC5, SC10, SC15, and SC20 throughout the study. Starch hydrogel samples prepared with TA were labeled as ST2.5, ST5, ST10, ST15, and ST20 throughout the study.
2.3. Degree of Substitution (DS)
The DS of each sample was determined in triplicate employing the method described by Volkert et al. [12] and Ye et al. [13] by titration, with some modifications. Each sample (1 g) was placed in a 250 mL Erlenmeyer flask, 20 mL of deionized water and two drops of phenolphthalein were added. The solution was titrated with a sodium hydroxide solution (0.1 mol/L) until the endpoint was reached, indicating that all the free Ca or TA had been neutralized. After that, 25 mL of aqueous sodium hydroxide solution (0.5 mol/L) was added, and the sample was agitated for 60 min at room temperature. The excess alkali was back-titrated with a standard aqueous hydrochloric acid solution (0.5 mol/L) until the endpoint. A blank titration using extruded starch without CA or TA was carried out. Esterified carboxyl groups (EG, %) and DS were calculated as follows: EG = [((v0 − v1) × c × M × 100))/m], and DS = (162 × EG)/(100M − (M − 1)EG; where EG is the content of esterified carboxyl groups (%); v0 is the volume of aqueous hydrochloric acid solution consumed by the blank (mL); v1 is the volume of aqueous hydrochloric acid solution consumed by the esterified starch sample (mL); c is the concentration of aqueous hydrochloric acid solution (mol/L); M is the molar mass of the substituent (citrate or tartarate); and m is the mass of the samples (mg).
2.4. Fourier Transform-Infrared Spectroscopy (FTIR)
Pulverized hydrogels samples were mixed with potassium bromide and compressed into pellets. The FTIR analyses were performed using a spectral resolution of 4 cm−1 and a spectral range of 4000–500 cm−1 in a Shimadzu FTIR-8300 (Kyoto, Japan) equipment.
2.5. X-ray Diffraction (XRD)
XRD analysis was carried out in a Panalytical X’Pert PRO MPD diffractometer (Almelo, The Netherlands) with copper Kα radiation (λ = 1.5418 Ǻ) under operational conditions of 40 kV and 30 mA, with a with a ramp rate of 1°/min. The relative crystallinity index (CI) was calculated using the method described by Cheetham and Tao [35].
2.6. Scanning Electron Microscopy (SEM)
SEM analyses were performed with an FEI Quanta 200 microscope (Hillsboro, OR, USA) using an accelerating voltage of 20 kV. Each hydrogel sample was mounted for surface visualization on bronze stub, and then its surface was coated with a thin gold layer (40–50 nm).
2.7. Water Holding Capacity (WHC)
WHC was determined according to the methodology described by Butt et al. [36], with minor modifications. Approximately 1 g of each sample was weighed, and 10 mL of water was added to a pre-weighed centrifuge tube (W1). The samples were shaken for 30 min on a shaker at 25 °C and 200 rpm (Quimis Q 225M, Diadema, Brazil) and then centrifuged for 30 min at 2200 rpm (Hettich Centrifuge, Model 320R, Germany). After centrifugation, the supernatant was decanted, and tubes with residue were weighed (W2). The WHC was calculated as WHC (g/g) = ((W2 − W1) − (initial mass of sample))/(initial mass of sample).
2.8. Swelling Power at Different Times and Temperatures
The swelling power at different times was determined according to the procedure described by Yoshimura et al. [37]. Dried samples (0.5 g) were left to swell within permeable nylon tea bags (Japanese Industrial Standard, JIS K 7223). The tea bags were immersed in water at 25 °C for 1, 24, and 48 h, and then each tea bag was removed from the water, and excess water was drained for 1 min. The weight of the tea bag and samples was then measured (Wt), and the swelling was calculated according to the following equation: Swelling (g/g) = ((Wt − Wb) − Wp)/(Wp)), where Wb is the weight of a blank tea bag after water treatment, and Wp is the weight of the dry sample. Swelling power was determined in the same way at different temperatures (10, 25, and 55 °C) for 24 h.
2.9. Statistical Analysis
Statistical analysis was carried out employing the Statistica software version 7.0 (Statsoft, OK, USA) and Tukey’s test was employed for mean comparison (p ≤ 0.05).
3. Results
3.1. Degree of Substitution (DS)
Starch is composed of two polymers of D-glucopyranose, amylose, and amylopectin. Amylose is the linear fraction consisting of α-D-glucopyranose linked through α (1 → 4) linkages, and amylopectin is the branched fraction containing linear fractions linked through α (1 → 4) linkages, and ramifications at α (1 → 6); each glucopyranosyl unit in linear chains presents three reactive hydroxyl groups at position C2, C3, and C6 that have potential for functionalization [6,38]. DS is a useful parameter in the study of starch modification, and it can be defined as average number of hydroxyl groups substituted per D-glucose unit [38].
DS values of modified samples are presented in Table 1. For samples modified with CA and TA, the increase in the acid concentration resulted in higher DS values, while comparing CA- and TA-modified samples, CA-modified samples had higher DS values (Table 1). According to Seidel et al. [39], the use of different polyfunctional carboxylic acids as crosslinking and esterifying agents results in different materials, which is related to their structures. CA (C6H8O7) is a tricarboxylic acid formed by two carboxylic groups spaced by three CH2 groups with one OH group and one COOH group linked at the central carbon, while TA (C4H6O6) is a dicarboxylic acid spaced by two CH2 groups, each bearing one OH group. The difference in length of the spacer between CA and TA molecules and the kind and number of functional groups possibly resulted in a more efficient CA reaction with starch. Shen et al. [10] also stressed that carboxylic acids that have more than two carboxyl groups act more efficiently as crosslink agents than dicarboxylic acids. These authors reported that CA (tricarboxylic) was more efficient as a crosslinking agent than succinic acid (dicarboxylic).

Table 1.
Degree of substitution (DS) and water holding capacity (WHC) of native starch, control sample (S0) and starches hydrogels obtained by reactive extrusion.
A higher DS value was obtained for the SC20 sample (DS = 0.365), and a lower DS value was obtained for the ST2.5 sample (DS = 0.023) (Table 1). The values observed in this study were close to the values reported by Ye et al. [13] who chemically modified rice starch by reactive extrusion with CA; they reported values ranging from 0.037 to 0.138 using CA levels between 10 and 40% (g CA/100 g starch). Farhat et al. [22] reported DS values of 0.52 to 0.99 in starch citrates obtained from corn starch by reactive extrusion with CA concentrations of 40 and 100% (g CA/100 g starch). According to Ye et al. [13], reactive extrusion can be efficiently used for the obtainment of starch citrates.
The DS values of modified starch samples obtained in this study were slightly higher than those obtained for starch citrate prepared by high-temperature/long-term reactions in semidry conditions, as reported by other authors [14,25]. Mei et al. [14] reported that when CA concentration was increased from 10 to 30%, DS values increased from 0.058 to 0.178 in cassava starch citrates obtained after 8 h of reaction at room temperature.
3.2. Fourier Transform-Infrared Spectroscopy (FTIR)
The FTIR spectra of starch samples are shown in Figure 1. It was possible to identify a new important band at 1730 cm−1 in all hydrogel samples that indicates the stretching vibration of the carbonyl ester group, which provides clear evidence that the ester bonds were formed successfully. These results agreed with other authors’ results [11,13,15,19,22,26,28] who used CA and/or TA as crosslinking and esterifying agents for starch modification. It is important to highlight that all modified samples were washed with ethanol before analysis to prevent free CA or TA from remaining in the samples; thus, the band at 1730 cm−1 observed in all modified samples resulted from the formation of a covalent ester bond between the CA or TA and starch.
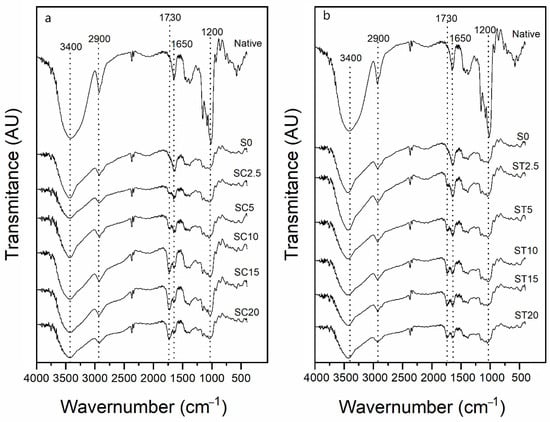
Figure 1.
FTIR spectra of native starch, the control sample (extruded without reagent—S0) and starch hydrogels obtained by reactive extrusion through reaction with CA (a) and TA (b).
Other bands are also observed in the FTIR spectra of all samples: a broad band at 3400–3500 cm−1 that was associated with O-H elongation and hydrogen bond vibration; a band at 2900 cm−1 associated with C-H asymmetric elongation and vibration; a band at 1650 cm−1 associated with water adsorbed in amorphous regions of starch, and a band at 1200 cm−1 that was attributed to the stretching vibration of C-O in C-OH groups [13,15,20,22,26,28,40].
3.3. X-ray Diffraction (XRD)
The diffractograms and relative crystallinity are shown in Figure 2. Native cassava starch presented diffraction peaks at 2θ = 15.03°, 17.03°, 17.93°, and 23.02° (Figure 2), which are typical of an A-type crystallinity [41,42].
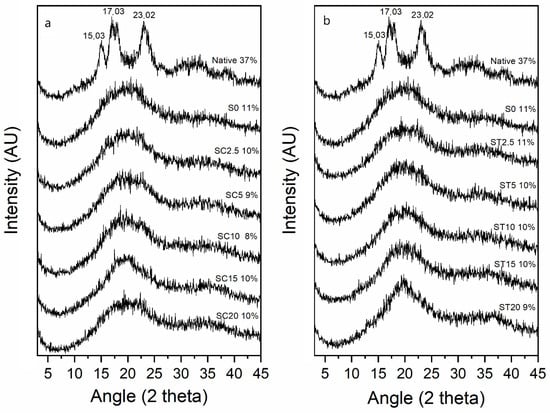
Figure 2.
X-ray diffraction (XRD) patterns and relative crystallinity index (CI) of native starch, the control sample (extruded without reagent—S0) and starch hydrogels obtained by reactive extrusion through reaction with CA (a) and TA (b).
The crystallinity index (CI) of native starch was 37%, which is typical of a semicrystalline material, and a similar value (37.64%) was reported by Mei et al. [14] for native cassava starch. The CI for all extruded samples (with and without reagent) decreased, and the CI values ranged from 8 to 11% (Figure 2). During the reactive extrusion process, the starch granules were exposed to high temperatures and high shear forces, strongly affecting their crystalline structure, resulting in the disappearance of diffraction peaks and samples with low crystallinity indices (Figure 2). These results agreed with other authors that used reactive extrusion to modify starches [13,27]. Cai et al. [27] reported that rice starch crosslinked by reactive extrusion with sodium trimetaphosphate and propylene oxide had its crystalline structure almost completely disrupted, with CI values very close to those obtained in this study, ranging from 11 to 14%.
Relative crystallinity affects the physicochemical properties of starch [43]. Some authors claim that the lower crystallinity of starch hydrogels can be important for their application as carriers of various compounds, such as in a drug and chemical element delivery system and in agricultural applications [41,44,45].
3.4. Scanning Electron Microscopy (SEM)
As observed in the SEM micrograph (Figure 3), native cassava starch granules exhibited an elliptical or truncated elliptical shape, with a smooth surface without disruption. These results agreed with those reported by other authors [41,42].

Figure 3.
SEM images of native starch, the control sample (extruded without reagent—S0), and starch hydrogels obtained by reactive extrusion through reaction with CA (SC2.5 and SC20) and TA (ST2.5 and ST20).
After being subjected to extrusion, all extruded samples showed structural morphological differences in relation to native cassava starch. During reactive extrusion, the starch granules undergo changes in their structure, disintegrating totally or partially, depending on the input energy level, forming rougher structures with irregular and fragmented aggregates. SEM micrographs also demonstrated that the sample surfaces did not contain residual starch granules, which indicated that the granules were destroyed due to the thermomechanical and chemical modification effects, which agreed with the XRD results. Other authors also reported the disintegration of the granular structure of starch subjected to reactive extrusion [29,46,47].
In addition to the extrusion process, the hydrolytic action of CA and TA can also favor the breakdown of starch granules [15,20]. All samples presented several pores on their surfaces (Figure 3); however, the SC20 and ST20 samples presented more compact structures, which can occur because the higher acid concentration possibly resulted in a lower viscosity of these samples during extrusion, yielding more homogeneous structures. The presence of pores can be interesting in the case of superabsorbent hydrogels for use in agriculture because they can facilitate the entry of water into their structure. According to Duquette et al. [20], starch hydrogels prepared by reaction with itaconic acid and CA in aqueous medium present highly irregular surfaces with the presence of pores, which can favor the diffusion of solvent and ions inside the hydrogel matrix, and additionally can favor trap particles by physical entrapment.
3.5. Water Holding Capacity (WHC)
WHC is the capacity of the sample to absorb and hold water in its polymeric network [48]. Native cassava starch presented the lowest WHC (1.84 g/g) value (Table 1). The S0 sample (extruded without reagent) and all crosslinked starch hydrogels presented significant improvements in WHC values (Table 1).
WHC depends on the presence of hydrophilic groups that can absorb and bind water molecules, leading to more swollen materials. Hydrophilic functional groups on the starch, CA and TA chains can form hydrogen bonds with water, allowing the hydrogels to hold water without dissolving. During reactive extrusion, the intramolecular bonds and the crystalline structure of starch granules were broken, resulting in the weakening of associative forces between starch chains, increasing the retention of water at low temperature by the hydrophilic groups that were exposed, which was also reported by other authors [13,33].
The sample with the higher WHC was prepared with 20.0% CA (SC20 sample, Table 1); also the sample with the higher DS, which is indicative that the starch chains were crosslinked, resulted in a more reinforced polymeric matrix capable to retain water. According to Lemos et al. [2], modified starches are reinforced by crosslinking the polymeric chains because of the covalent bonds formed, which improves their capacity to maintain water in their matrix without dissolving. Butt et al. [36] reported that the introduction of hydrophilic groups from CA resulted in increased water binding capacity of citrates due to the insertion of more hydrophilic substituent groups.
Other authors reported a contrary trend, with reductions in water holding capacity in crosslinked starch samples obtained by reactive extrusion with CA [13] and they reported that at a sufficiently high DS, it is difficult for water to penetrate into the starch citrate. Probably, in this study, the DS were not high enough to prevent the water to penetrate into the hydrogel internal structure.
The S0 sample (extruded without reagents) presented a WHC value of 4.99 g/g, a value that was higher than that presented by the crosslinked sample with CA 2.5, and similar to those presented by SC5, ST2.5 and ST5 (Table 1). During extrusion, the granular and semicrystalline structure of starch was destroyed, as observed by XRD and SEM, resulting in materials with lower crystallinity, which certainly contributed to the increase in the WHC of this sample. Heebthong and Ruttarattanamongkol [33] reported that the thermomechanical energy from the extrusion process results in disruption of the starch molecular arrangement and that water can be transferred easily into its internal structure.
3.6. Swelling Power at Different Times and Temperatures
The swelling power of samples is shown in Figure 4. It can be observed that the swelling of native starch was significantly lower than those of the S0 and samples crosslinked with CA and TA at all swelling times. The swelling of hydrogels crosslinked with CA after 1 h ranged from 3.1 (SC2.5) to 4.2 g/g (SC20), while the hydrogels crosslinked with TA ranged from 2.9 g/g (ST2.5) to 3.7 (g/g) (ST20). For all samples, the increase in time from 1 to 24 h resulted in samples with significantly higher swelling values (Figure 4), and between 24 and 48 h the swelling degree stabilized.
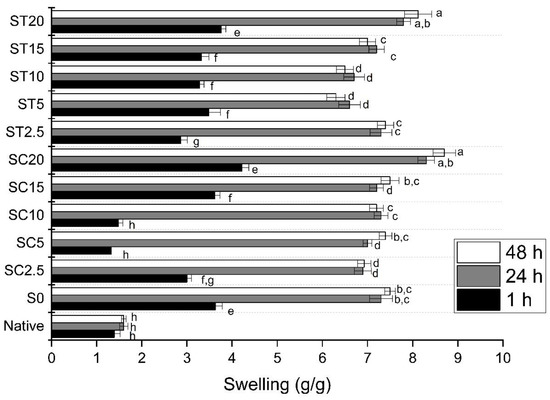
Figure 4.
Effect of time on swelling of native starch, the control sample (extruded without reagent) and starch hydrogels obtained by reactive extrusion through reaction with CA and TA. Different letters indicate significant difference between means by Tukey’s test (p ≤ 0.05).
After 48 h of immersion in water, the higher values were 8.7 and 8.1 g/g for SC20 and ST20 samples, resulting in 870 and 810% of water retention, respectively, suggesting that crosslinked samples can swell and retain water at room temperature. Batista et al. [49] reported that superabsorbent hydrogels can absorb over 100% and up to thousands of times their dry weight in water, and they can be driven for several applications, including agriculture, drug delivery, food packaging, or as adsorbent materials to decontaminate soil and water, between others [3,18,20,49].
The high concentrations of CA and TA improved granule swelling due to better starch hydration, and samples SCA.20 and STA.20 also showed higher DS values (0.36 and 2.85, respectively). During reaction with 20.0% CA and TA, the starch acquires a three-dimensional matrix, which possibly results in individual chains that repel each other due to steric hindrance, as crosslinked starch derivatives try to exist in the lowest and most stable state. The repulsion between the chains can increase the capacity for expansion without dissolving, preserving the structure of the material [11,46]. Duquette et al. [20] reported that the amount of CA in starch hydrogels affects the swelling by acting as a crosslinking agent ensuring the stability of their swollen structure, and also by acting as a source of hydrophilic functional groups.
When the temperature was evaluated, all the samples presented significant increases in their swelling with increases in temperature from 10 to 55 °C (Figure 5). Hung et al. [50] reported the increase in swelling of CA-crosslinked rice starches with increasing temperature. Samples obtained through reaction with 20.0% CA and TA presented the higher swelling values at all temperatures.
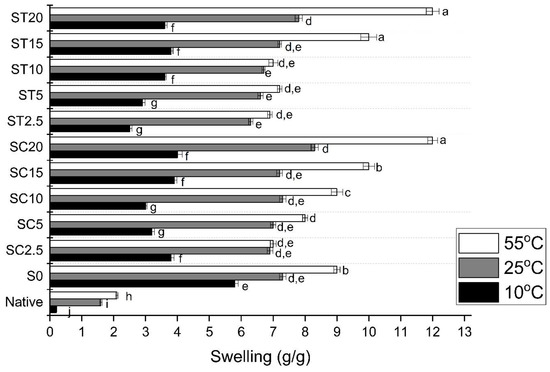
Figure 5.
Effect of temperature on swelling of native starch, the control sample (extruded without reagent) and starch hydrogels obtained by reactive extrusion through reaction with CA and TA. Different letters indicate significant difference between means by Tukey’s test (p ≤ 0.05).
After 48 h of immersion in water at 25 °C, the S0 sample presented a swelling value of 7.5 g/g, a value that was higher than those presented by the crosslinked samples with CA and TA at 2.5 and 5.0% (Figure 4), which had the lowest swelling capacity values. During extrusion, the starch granules are subjected to high temperature and pressure, and the hydrogen bonds and the crystal structure are broken, resulting in disruption of their granular structure. The free hydroxyl groups of starch chains when in contact with water can form hydrogen bonds and swell [35,51]. Similar results have also been described by Monroy et al. [42], who observed that ultrasound-modified starches lose their crystallinity and present increased swelling values.
4. Conclusions
Reactive extrusion was effectively used for the production of cassava starch hydrogels through reaction with two crosslinking agents, CA and TA. Evidence for the occurrence of chemical modification was verified by FTIR spectroscopy with the appearance of a new band at 1730 cm−1, resulting in materials with different degrees of substitution, which were higher for hydrogels obtained by crosslinking with CA. XDR showed that the starch crystalline structure was broken during reactive extrusion, and morphology analysis showed that the original granular structure of starch was lost and replaced with a rougher and more irregular structure, which resulted in hydrogels with higher water holding capacity than native starch and the control sample (extruded without the crosslinking agents). Hydrogel samples obtained with the highest CA or TA concentrations (15.0 and 20.0%) resulted in the higher DS values and water holding and swelling capacities.
Reactive extrusion was effective in obtaining starch hydrogels by reaction with organic acids, with the advantages of reduced processing time and low effluent generation when compared to conventional processes, with great possibilities to be adapted to an industrial scale, expanding the potential of starch hydrogels for several applications, including the obtainment of food-grade products.
Author Contributions
B.M.M.: experiment, writing—review and editing. J.M., G.A.G.-G., J.F.P. and B.M.S.: experiment, validation. F.Y.: resources, writing—review and editing. S.M.: conceptualization, project administration, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by CAPES-Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for the grant to the postgraduate students.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Laboratory of Spectroscopy (ESPEC), Laboratory of Electronic Microscopy and Microanalysis (LMEM) and the Laboratory of X-ray Analysis (LARX) of the State University of Londrina for the analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Erdagi, S.I.; Ngwabebhoh, F.A.; Yildiz, U. Genipin crosslinked gelatin-diosgenin-nanocellulose hydrogels for potential wound dressing and healing applications. Int. J. Biol. Macromol. 2020, 149, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Lemos, P.V.F.; Marcelino, H.R.; Cardoso, L.G.; Souza, C.O.; Druzian, J.I. Starch chemical modifications applied to drug delivery systems: From fundamentals to FDA-approved raw materials. Int. J. Biol. Macromol. 2021, 184, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, Y.Y.; Zha, X.Q.; Li, Q.M.; Pan, L.H.; Luo, J.P. Research progress on polysaccharide/protein hydrogels: Preparation method, functional property and application as delivery systems for bioactive ingredients. Food Res. Int. 2021, 147, 110542. [Google Scholar] [CrossRef] [PubMed]
- Golachowski, A.; Drożdz, W.; Golachowska, M.; Kapelko-Zeberska, M.; Raszewski, B. Production and properties of starch citrates—Current research. Foods 2020, 9, 1311. [Google Scholar] [CrossRef] [PubMed]
- Elgaied-Lamouchi, D.; Descamps, N.; Lefevre, P.; Rambur, I.; Pierquin, J.-Y.; Siepmann, F.; Siepmann, J.; Muschert, S. Starch-based controlled release matrix tablets: Impact of the type of starch. J. Drug Deliv. Sci. Technol. 2021, 61, 102152. [Google Scholar] [CrossRef]
- Dastidar, T.G.; Netravali, A.N. ‘Green’ crosslinking of native starches with malonic acid and their properties. Carbohydr. Polym. 2012, 90, 1620–1628. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Lin, L.; Zhang, J.; Liu, W.; Xie, J.; Ding, Y. Functional, physicochemical properties and structure of cross-linked oxidized maize starch. Food Hydrocoll. 2014, 36, 45–52. [Google Scholar] [CrossRef]
- Olsson, E.; Hedenqvist, M.S.; Johansson, C.; Järnström, L. Influence of citric acid and curing on moisture sorption, diffusion and permeability of starch films. Carbohydr. Polym. 2013, 94, 765–772. [Google Scholar] [CrossRef]
- Otache, M.A.; Duru, R.U.; Achugasim, O.; Abayeh, O.J. Advances in the modification of starch via esterification for enhanced properties. J. Polym. Environ. 2020, 29, 1365–1379. [Google Scholar] [CrossRef]
- Shen, L.; Xu, H.; Kong, L.; Yang, Y. Non-Toxic crosslinking of starch using polycarboxylic acids: Kinetic study and quantitative correlation of mechanical properties and crosslinking degrees. J. Polym. Environ. 2015, 23, 588–594. [Google Scholar] [CrossRef]
- Tupa, M.V.; Altuna, L.; Herrera, M.L.; Foresti, M.L. Preparation and characterization of modified starches obtained in acetic anhydride/tartaric acid medium. Starch-Staerke 2020, 72, 1900300. [Google Scholar] [CrossRef]
- Volkert, B.; Lehmann, A.; Greco, T.; Nejad, M.H. A comparison of different synthesis routes for starch acetates and the resulting mechanical properties. Carbohydr. Polym. 2010, 79, 571–577. [Google Scholar] [CrossRef]
- Ye, J.; Luo, S.; Huang, A.; Chen, J.; Liu, C.; McClements, D.J. Synthesis and characterization of citric acid esterified rice starch by reactive extrusion: A new method of producing resistant starch. Food Hydrocoll. 2019, 92, 135–142. [Google Scholar] [CrossRef]
- Mei, J.Q.; Zhou, D.N.; Jin, Z.Y.; Xu, X.M.; Chen, H.Q. Effects of citric acid esterification on digestibility, structural and physicochemical properties of cassava starch. Food Chem. 2015, 187, 378–384. [Google Scholar] [CrossRef]
- Simões, B.M.; Cagnin, C.; Yamashita, F.; Olivato, J.B.; Garcia, P.S.; Mali, S.; Grossmann, M.V.E. Citric acid as crosslinking agent in starch/xanthan gum hydrogels produced by extrusion and thermopressing. LWT 2020, 125, 108950. [Google Scholar] [CrossRef]
- Moad, G. Chemical modification of starch by reactive extrusion. Prog. Polym. Sci. 2011, 36, 218–237. [Google Scholar] [CrossRef]
- Uliniuc, A.; Hamaide, T.; Popa, M.; Bǎcǎițǎ, S. Modified starch-based hydrogels cross-linked with citric acid and their use as drug delivery systems for levofloxacin. Soft Mater. 2013, 11, 83–493. [Google Scholar] [CrossRef]
- Ačkar, D.; Babić, J.; Jozinović, A.; Miličević, B.; Jokić, S.; Miličević, R.; Rajič, M.; Šubarić, D. Starch modification by organic acids and their derivatives: A review. Molecules 2015, 20, 19554–19570. [Google Scholar] [CrossRef]
- Miskeen, S.; Hong, J.S.; Choi, H.D.; Kim, J.Y. Fabrication of citric acid-modified starch nanoparticles to improve their thermal stability and hydrophobicity. Carbohydr. Polym. 2021, 253, 117242. [Google Scholar] [CrossRef]
- Duquette, D.; Nzediegwu, C.; Portillo-Perez, G.; Dumont, G.M.; Prasher, S. Eco-Friendly synthesis of hydrogels from starch, citric acid, and itaconic acid: Swelling capacity and metal chelation properties. Starch-Staerke 2020, 72, 1900008. [Google Scholar] [CrossRef]
- Alimi, B.A.; Workneh, T.S. Structural and physicochemical properties of heat moisture treated and citric acid modified acha and iburu starches. Food Hydrocoll. 2018, 81, 449–455. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.; Mignard, N.; Taha, M.; Becquart, F.; Ayoub, A. Polysaccharides and lignin based hydrogels with potential pharmaceutical use as a drug delivery system produced by a reactive extrusion process. Int. J. Biol. Macromol. 2017, 104, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.S.; Chung, H.J.; Lee, B.H.; Kim, H.S. Impact of static and dynamic modes of semi-dry heat reaction on the characteristics of starch citrates. Carbohydr. Polym. 2020, 233, 115853. [Google Scholar] [CrossRef] [PubMed]
- Kapelko-Zeberska, M.; Buksa, K.; Szumny, A.; Zieba, T.; Gryszkin, A. Analysis of molecular structure of starch citrate obtained by a well-stablished method. LWT. 2016, 69, 334–341. [Google Scholar] [CrossRef]
- Srikaeo, K.; Hao, P.T.; Lerdluksamee, C. Effects of heating temperatures and acid concentrations on physicochemical properties and starch digestibility of citric acid esterified tapioca starches. Starch-Staerke 2019, 71, 1800065. [Google Scholar] [CrossRef]
- Zhou, J.; Tong, J.; Su, X.; Ren, L. Hydrophobic starch nanocrystals preparations through crosslinking modification using citric acid. Int. J. Biol. Macromol. 2016, 91, 1186–1193. [Google Scholar] [CrossRef]
- Cai, C.; Wei, B.; Tian, Y.; Ma, R.; Chen, L.; Qiu, L.; Jin, Z. Structural changes of chemically modified rice starch by one-step reactive extrusion. Food Chem. 2019, 288, 354–360. [Google Scholar] [CrossRef]
- Lipatova, I.M.; Yusova, A.A. Effect of mechanical activation on starch crosslinking with citric acid. Int. J. Biol. Macromol. 2021, 185, 688–695. [Google Scholar] [CrossRef]
- Cai, C.; Tian, Y.; Yu, Z.; Sun, C.; Jin, Z. In vitro digestibility and predicted glycemic index of chemically modified rice starch by one-step reactive extrusion. Starch-Staerke 2019, 72, 1900012. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Valencia, G.A. Reactive extrusion-processed native and phosphated starch-based food packaging films governed by the hierarchical structure. Int. J. Biol. Macromol. 2021, 172, 439–451. [Google Scholar] [CrossRef]
- González-Seligra, P.; Goyanes, S.; Famá, L. Effect of the Incorporation of Rich-Amylopectin Starch Nano/Micro Particles on the Physicochemical Properties of Starch-Based Nanocomposites Developed by Flat-Die Extrusion. Starch-Stärke 2022, 74, 2100080. [Google Scholar] [CrossRef]
- Formela, K.; Zedler, L.; Hejna, A.; Tercjak, A. Reactive extrusion of bio-based polymer blends and composites—Current trends and future developments. Express Polym. Lett. 2018, 12, 24–57. [Google Scholar] [CrossRef]
- Heebthong, K.; Ruttarattanamongkol, K. Physicochemical properties of cross-linked cassava starch prepared using a pilot-scale reactive twin-screw extrusion process (REX). Starch-Staerke 2016, 68, 528–540. [Google Scholar] [CrossRef]
- Gil-Giraldo, G.A.; Mantovan, J.; Marim, B.M.; Kishima, J.O.F.; Mali, S. Surface modification of cellulose from oat hull with citric acid using ultrasonication and reactive extrusion assisted processes. Polysaccharides 2021, 2, 218–233. [Google Scholar] [CrossRef]
- Cheetham, N.W.H.; Tao, L. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Butt, N.A.; Ali, T.M.; Hasnain, A. Rice starch citrates and lactates: A comparative study on hot water and cold water swelling starches. Int. J. Biol. Macromol. 2019, 127, 107–117. [Google Scholar] [CrossRef]
- Yoshimura, T.; Matsuo, K.; Fujioka, R. Novel biodegradable superabsorbent hydrogels derived from cotton cellulose and succinic anhydride: Synthesis and characterization. J. Appl. Polym. Sci. 2006, 99, 3251–3256. [Google Scholar] [CrossRef]
- Namazi, H.; Dadkhah, A. Convenient method for preparation of hydrophobically modified starch nanocrystals with using fatty acids. Carbohydr. Polym. 2010, 79, 731–737. [Google Scholar] [CrossRef]
- Seidel, C.; Kulicke, W.M.; Heß, C.; Hartmann, B.; Lechner, M.D.; Lazik, W. Influence of the cross-linking agent on the gel structure of starch derivatives. Starch-Staerke 2001, 53, 305–310. [Google Scholar] [CrossRef]
- Hasanin, M.S. Simple, economic, ecofriendly method to extract starch nanoparticles from potato peel waste for biological applications. Starch-Staerke 2021, 73, 2100055. [Google Scholar] [CrossRef]
- Minakawa, A.F.K.; Faria-Tischer, P.C.; Mali, S. Simple ultrasound method to obtain starch micro- and nanoparticles from cassava, corn and yam starches. Food Chem. 2019, 283, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Monroy, Y.; Rivero, S.; García, M.A. Microstructural and techno-functional properties of cassava starch modified by ultrasound. Ultrason. Sonochem. 2018, 42, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Zobel, H.F. Molecules to Granules: A Comprehensive starch review. Starch-Stärke 1998, 40, 44–50. [Google Scholar] [CrossRef]
- Biduski, B.; Silva, W.M.F.; Colussi, R.; Halal, S.L.; El, M.; Lim, L.T.; Dias, A.R.G.; Zavareze, E. Starch hydrogels: The influence of the amylose content and gelatinization method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.F.; Silva, C.; Coelho, J.F.J.; Simões, S. Oral films: Current status and future perspectives: I—Galenical development and quality attributes. J. Control. Release. 2015, 206, 108–121. [Google Scholar] [CrossRef]
- Fonseca-Florido, H.A.; Soriano-Corral, F.; Yañez-Macías, R.; González-Morones, P.; Hernández-Rodríguez, F.; Aguirre-Zurita, J.; Ávila-Orta, C.; Rodríguez-Velázquez, J. Effects of multiphase transitions and reactive extrusion on in situ thermoplasticization/succination of cassava starch. Carbohydr. Polym. 2019, 225, 115250. [Google Scholar] [CrossRef]
- Murúa-Pagola, B.; Beristain-Guevara, C.I.; Martínez-Bustos, F. Preparation of starch derivatives using reactive extrusion and evaluation of modified starches as shell materials for encapsulation of flavoring agents by spray drying. J. Food Eng. 2009, 91, 380–386. [Google Scholar] [CrossRef]
- Mehfooz, T.; Ali, T.M.; Ahsan, M.; Abdullah, S.; Hasnain, A. Morphological, functional and thermal characteristics of hydroxypropylated-crosslinked barley starches. J. Food Meas. Charact. 2021, 15, 237–246. [Google Scholar] [CrossRef]
- Batista, R.A.; Espitia, P.J.P.; Quintans, J.S.S.; Freitas, M.M.; Cerqueira, M.A.; Teixeira, J.A.; Cardoso, J.C. Hydrogel as an alternative structure for food packaging systems. Carbohydr. Polym. 2019, 205, 106–116. [Google Scholar] [CrossRef]
- Hung, P.; Van, V.; Vien, N.L.; Lan, N.T. Resistant starch improvement of rice starches under a combination of acid and heat-moisture treatments. Food Chem. 2016, 191, 67–73. [Google Scholar] [CrossRef]
- De Graaf, R.A.; Broekroelofs, A.; Janssen, L.P.B.M. The acetylation of starch by reactive extrusion. Starch-Staerke 1998, 50, 198–205. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).