Abstract
High postprandial triglycerides are a strong cardiovascular disease risk factor. However, the extent triglycerides rise during daily living due to multiple meals remains poorly defined, especially in at-risk individuals. The aim of this case study was to quantify 24-h triglycerides in an individual with normal-weight obesity (NWO), metabolic syndrome (MetS), and type 2 diabetes (T2D) consuming a Western-style diet. In the morning, an indwelling catheter was inserted into a forearm vein and metabolic markers were measured hourly for 18-h and every 3-h for the last 6-h. The participants with NWO and T2D both experienced peak triglycerides ~280 mg/dL, which is considered an adverse response. The participant MetS had a similar relative change in triglycerides, but only reached 214 mg/dL. Interestingly, The participant with NWO displayed the greatest triglyceride area under the curve. Overall, we report that all three participants’ triglycerides nearly tripled with Western diet consumption, despite considerably different risk-profiles.
1. Introduction
Postprandial/non-fasting triglycerides are an independent risk factor for cardiovascular disease (CVD) and may be more closely linked to CVD than fasting triglycerides [1]. Mechanistically, this relationship appears to be due to the strong correlation between postprandial triglycerides and triglyceride-rich lipoprotein remnant cholesterol, which is an increasingly appreciated source of residual CVD risk [2]. Postprandial triglycerides may be especially of interest in countries consuming Westernized diets rich in total and saturated fat, as fat from multiple meals has an additive effect on circulating triglycerides throughout the day [3]. However, despite the reality that individuals spend the majority of the day in the postprandial state, studies evaluating the summative effect of high-fat meals on postprandial triglycerides have thus far not examined a full 24-h period or had other limitations. For example, available reports have only included two meals, may not have reflected realistic dietary behaviours (e.g., consuming the same meal twice, utilized meals formulated for laboratory use), or have not allowed individuals the option to eat freely (i.e., overeat if they choose) [3,4,5]. Additionally, studies have largely focused on healthy individuals, as opposed to those who may be at higher CVD risk. In this preliminary case report, we measured triglycerides, as well as glucose, over 24 h in three phenotypes that are linked to increased CVD risk: normal-weight obesity (NWO), metabolic syndrome (MetS), and type 2 diabetes (T2D) [6,7,8].
2. Materials and Methods
2.1. Participants
One middle-aged male was recruited for each at-risk phenotype (NWO, MetS and T2D). NWO was defined as body mass index (BMI) < 25 kg/m2 and ≥30% total body fat [9]. The International Diabetes Federation (IDF) criteria [10] was used to identify MetS and this participant was free of T2D. The individual with T2D had a formal medical diagnosis. MetS used tobacco products (i.e., cigarettes). T2D was taking medications relevant to the study outcomes (20 mg Atorvastin daily, 750 mg Metformin twice daily). Given that fasting and non-fasting triglycerides and glucose have established reference ranges, and we have previously observed that potential controls for this study (i.e., normal-weight and metabolically healthy, metabolically healthy obesity) do not always have a significant postprandial triglyceride response after a high-fat meal [11], such participants were not recruited for this study.
2.2. Study Design
Participants reported to the lab between 0700 and 0800 having fasted for 10–12 h. After measuring height, mass, blood pressure, and assessing body composition via bioimpedance (Seca; Hamburg, Germany), a 24-guage indwelling catheter was inserted into a forearm vein and a 0.9% NaCl drip initiated. Blood was drawn and metabolic parameters (i.e., triglycerides and glucose) were measured hourly for 18 h. (Alere Cholestech; Hayward, CA, USA). During the last 6 h of the 24 h cycle, triglycerides and glucose were measured every 3 h. Participants were required to remain sedentary in the laboratory. Participants were also provided a bed to sleep on and slept 6.5–9 h. The study protocol was approved by the Oklahoma State Institutional Review Board and in accordance with the Declaration of Helsinki. Participants provided written consent.
2.3. Meal Intervention
During recruitment, participants were screened to ensure that the minimum intake for the dietary intervention approximated what they might reasonably consume. From there, the diet was consumed in a semi-ad libitum manner to allow participants to reach their normal satiety level. In this way, we aimed to capture a “true-to-life” 24 h period of participants’ dietary intake. To simulate a typical WD, popular commercial and fast foods were chosen (Table 1). Specifically, participants were given 1.5–2 Jimmy Dean’s breakfast bowls (immediately following baseline blood draw), a McDonald’s double quarter pounder with cheese combo with French fries with the option for additional French fries for lunch (4 h after breakfast), 4–6 slices of Little Caesar’s pizza for dinner (5 h after lunch), and 2 Blue Bell Moo Bars (3 h after dinner). Participants were allowed up to 3 calorie-free, caffeinated beverages (i.e., black coffee, unsweetened tea, diet soda) until 1200. We have previously observed that minor caffeine intake does not influence fasting and postprandial triglycerides in the context of a single high-fat meal [12].

Table 1.
Participant and Meal Characteristics.
2.4. Data Analysis
Triglyceride and glucose area under the curve (AUC) and incremental AUC were calculated using GraphPad Prism 8.0.3 (GraphPad Prism Inc.; La Jolla, CA, USA).
3. Results
3.1. Participant Characteristics
NWO presented with high body fat (≥30%), but largely normal metabolic risk factors, aside from elevated total and LDL cholesterol and diastolic blood pressure (Table 1). MetS displayed high systolic/diastolic blood pressure, low HDL-cholesterol, also had high LDL-cholesterol. T2D displayed elevated fasting glucose (147 mg/dL) and diastolic blood pressure.
3.2. Postprandial Metabolic Outcomes
All participants’ triglycerides remained above fasting ≥ 75% of the 24-h period and all exhibited peak triglycerides between lunch and dinner. Both NWO and T2D had an adverse postprandial triglyceride response per current guidelines (i.e., ≥220 mg/dL) [13], evidenced by peak triglycerides of 278 mg/dL and 281 mg/dL, respectively (Figure 1A). MetS had a similar 3-fold increase from fasting to peak triglycerides, but only reached 214 mg/dL. Interestingly, NWO had the greatest triglyceride 24-h AUC (3393 mg/dL × 24 h), which was slightly higher than T2D (3165 mg/dL × 24 h), and MetS had the lowest (2772 mg/dL × 24 h; Figure 1C). NWO also displayed the greatest 24-h triglyceride incremental AUC (1414 mg/dL × 24 h), and MetS was just below NWO (1392 mg/dL × 24 h), which was driven by lower fasting triglycerides (Figure 1D). T2D had the lowest 24-h triglyceride incremental AUC (1166 mg/dL × 24 h).
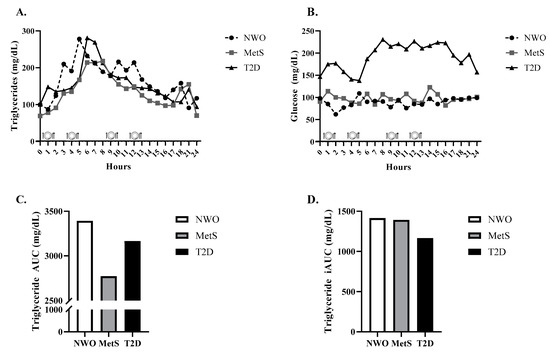
Figure 1.
24-h Triglycerides, Glucose, Triglyceride AUC, and Triglyceride incremental AUC. 24-h triglycerides (A), glucose (B), triglyceride AUC (C), and triglyceride iAUC (D) in an individual with NWO, MetS, and T2D. Abbreviations: NWO normal-weight obese; MetS metabolic syndrome; T2D type 2 diabetes; AUC area under the curve; iAUC incremental area under the curve.
With respect to 24-h glucose, T2D peaked at 231 mg/dL and experienced an AUC of 3383 mg/dL × 24 h (Figure 1B) and incremental AUC of 962 mg/dL × 24 h. MetS glucose parameters were much lower (peak = 123 mg/dL, AUC = 1958 mg/dL × 24 h, incremental AUC = 164 mg/dL) and NWO had similar response to MetS (i.e., peak glucose = 109 mg/dL, AUC = 1773 mg/dL × 24 h, incremental AUC = 201 mg/dL).
4. Discussion
In the present case study, we observed that all three at-risk participants presented with fasting triglycerides within the normal range (<150 mg/dL), but their triglycerides nearly tripled from baseline when consuming a WD, despite marked differences in body composition, risk factors, and health status. NWO and T2D had an adverse postprandial response per the commonly utilized Expert Panel Conesus Statement (i.e., ≥220 mg/dL) [13], and MetS was just below this mark. Surprisingly, NWO had a similar postprandial triglyceride response to T2D and the greatest AUC, although the statin likely mitigated postprandial triglycerides in T2D. Participants displayed dramatic differences in glucose control, which led to a greater 24-h glucose in T2D, while MetS and NWO displayed similar glucose profiles. The large metabolic response in T2D was notable given their generally healthy fasting values, likely due to medication.
While some studies have evaluated the additive effect of realistic, Western-style foods on serum triglycerides over two meals (e.g., [3]), this is the first study to our knowledge to measure triglycerides throughout a 24-h period while simulating a “true-to-life” WD. Adding to the realistic nature of the study, participants were informally screened prior to the study to determine whether their eating habits were similar to our planned intervention, and if participants ate beyond the minimum requirement it was by their own volition. Potential limitations include limited sample, likely disrupted sleep, and the lack of refined sugar included in our WD (due to concerns with glucose control and T2D).
Overall, we report that the participants with NWO and T2D had pronounced, and nearly identical, daily postprandial triglycerides when consuming a WD, and the participant with MetS approximated an adverse response. Given the inherent limitations that accompany a case study, future, larger studies should seek to confirm our observation that a range of at-risk individuals experience a deleterious postprandial TG response during daily living.
Author Contributions
B.H.K. and S.R.E. conceived and designed research; B.H.K., C.M.S. and K.L.P. collected data. B.H.K. prepared figures and drafted manuscript; B.H.K., C.M.S., K.L.P. and S.R.E. edited and revised manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Internal sources at Oklahoma State University.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Oklahoma State University (IRB-20-424-STW, approved 10/27/2020).
Informed Consent Statement
Written informed consent was obtained from all participants involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bansal, S.; Buring, J.E.; Rifai, N.; Mora, S.; Sacks, F.M.; Ridker, P.M. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007, 298, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varbo, A.; Nordestgaard, B.G. Remnant cholesterol and triglyceride-rich lipoproteins in atherosclerosis progression and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerson, S.R.; Kurti, S.P.; Teeman, C.S.; Emerson, E.M.; Cull, B.J.; Haub, M.D.; Rosenkranz, S.K. Realistic Test-Meal Protocols Lead to Blunted Postprandial Lipemia but Similar Inflammatory Responses Compared with a Standard High-Fat Meal. Curr. Dev. Nutr. 2017, 1, e000232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, R.B.; Karpe, F.; Milne, R.W.; Burdge, G.C.; Wootton, S.A.; Frayn, K.N. Dietary fatty acids make a rapid and substantial contribution to VLDL-triacylglycerol in the fed state. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E732–E739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Oostrom, A.; Castro Cabezas, M.; Ribalta, J.; Masana, L.; Twickler, T.B.; Remijnse, T.; Erkelens, D. Diurnal triglyceride profiles in healthy normolipidemic male subjects are associated to insulin sensitivity, body composition and diet. Eur. J. Clin. Investig. 2000, 30, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Hinnouho, G.-M.; Czernichow, S.; Dugravot, A.; Nabi, H.; Brunner, E.J.; Kivimaki, M.; Singh-Manoux, A. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: The Whitehall II cohort study. Eur. Heart J. 2015, 36, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S. Cardiovascular disease risk factors, type 2 diabetes mellitus, and the Framingham Heart Study. Trends Cardiovasc. Med. 2010, 20, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Korenfeld, Y.; Boarin, S.; Korinek, J.; Jensen, M.D.; Parati, G.; Lopez-Jimenez, F. Normal weight obesity: A risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur. Heart J. 2010, 31, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, E.; Somers, V.K.; Sochor, O.; Goel, K.; Lopez-Jimenez, F. The concept of normal weight obesity. Prog. Cardiovasc. Dis. 2014, 56, 426–433. [Google Scholar] [CrossRef] [PubMed]
- The IDF Consensus Wordwide Definition of the Metabolic Syndrome. Available online: https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome (accessed on 15 February 2021).
- Keirns, B.H.; Hart, S.M.; Sciarrillo, C.M.; Poindexter, K.L.; Clarke, S.L.; Emerson, S.R. Postprandial Triglycerides, Flow-Mediated Dilation, and the Inflammatory Cytokine Milieu in Metabolically Healthy Obesity: A Cross-Sectional Pilot Study. Obesities 2021, 1, 58–71. [Google Scholar] [CrossRef]
- Sciarrillo, C.M.; Keirns, B.H.; Elliott, D.C.; Emerson, S.R. The effect of black coffee on fasting metabolic markers and an abbreviated fat tolerance test. Clin. Nutr. ESPEN 2021, 41, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.D.; Mikhailidis, D.P.; Kovar, J.; Lairon, D.; Nordestgaard, B.G.; Chye Ooi, T.; Perez-Martinez, P.; Bilianou, H.; Anagnostopoulou, K.; Panotopoulos, G. Assessment and clinical relevance of non-fasting and postprandial triglycerides: An expert panel statement. Curr. Vasc. Pharmacol. 2011, 9, 258–270. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).