Hydrogen-Rich Gaseous Mixture for Enhanced Combustion in a Flex-Fuel Engine: An Experimental Analysis
Abstract
1. Introduction
2. Materials and Methods
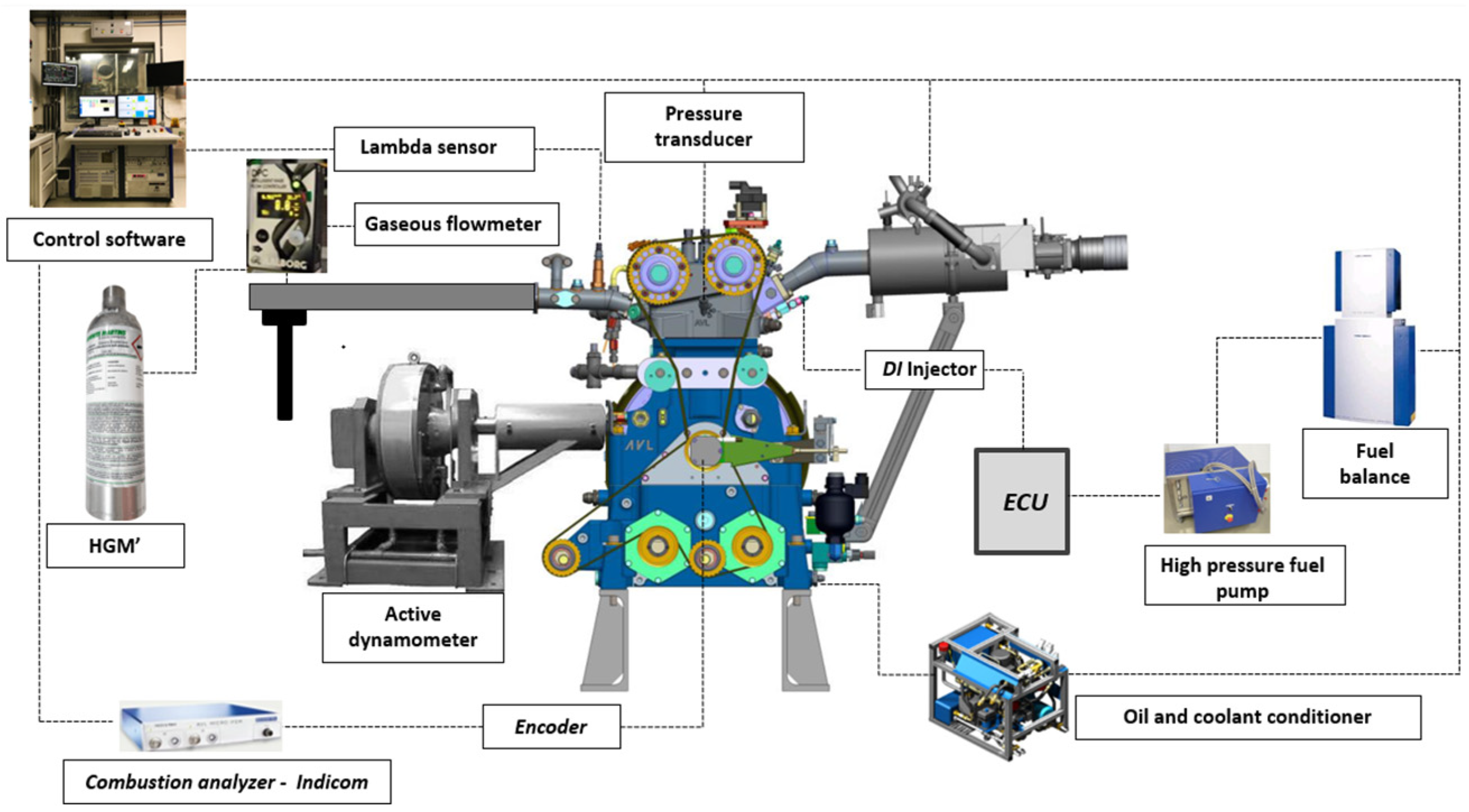
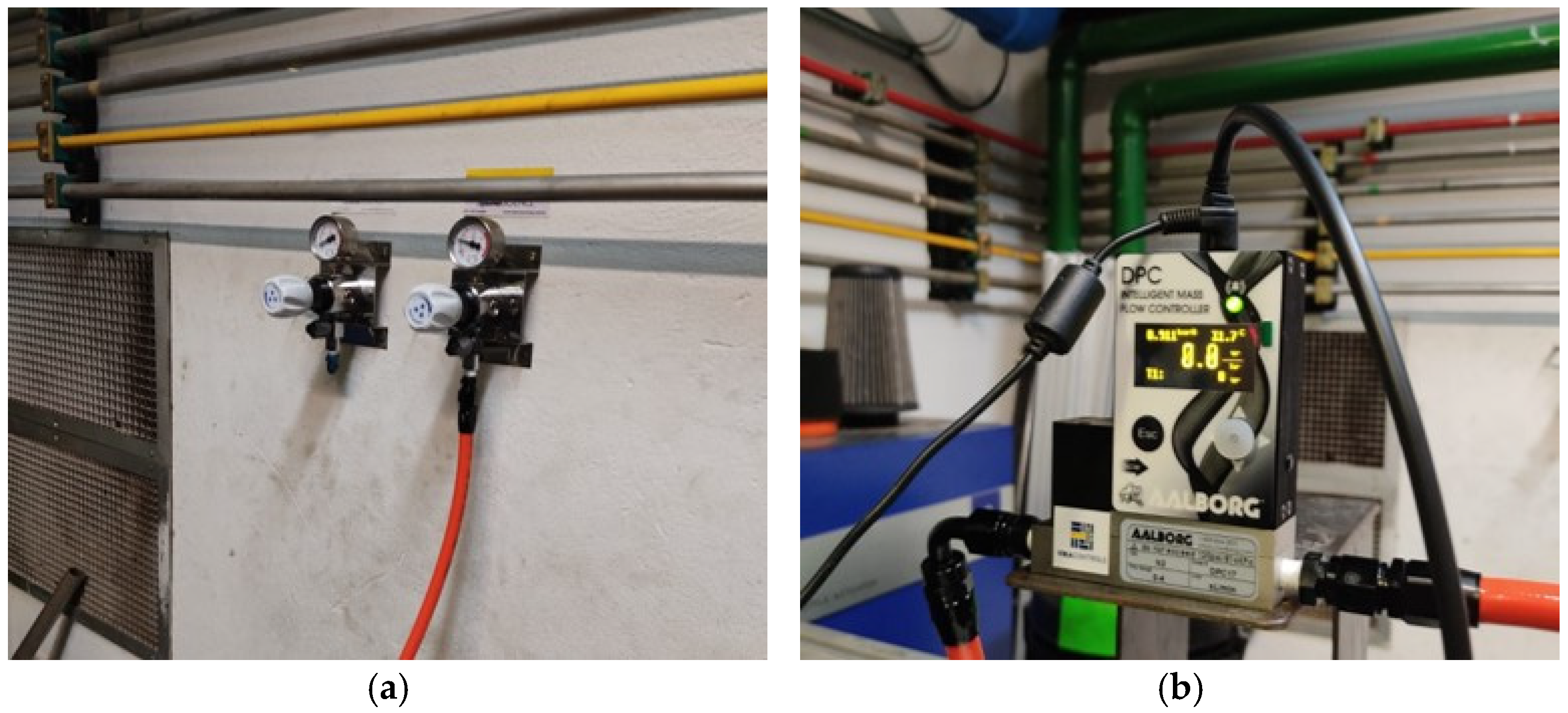
2.1. Experimental Setup
2.2. Engine Calibration Procedure
2.3. Statistical Analysis
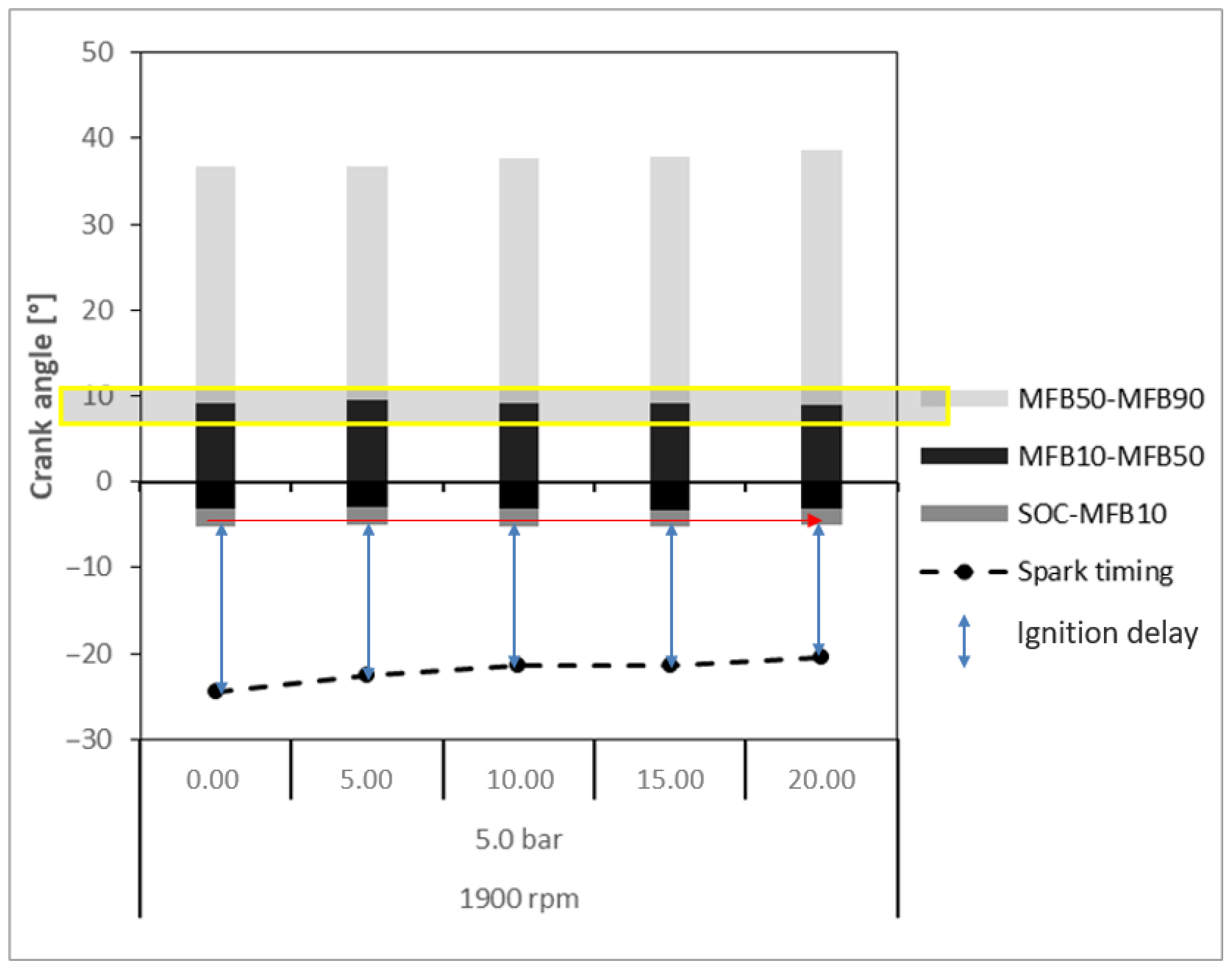
3. Results and Discussion
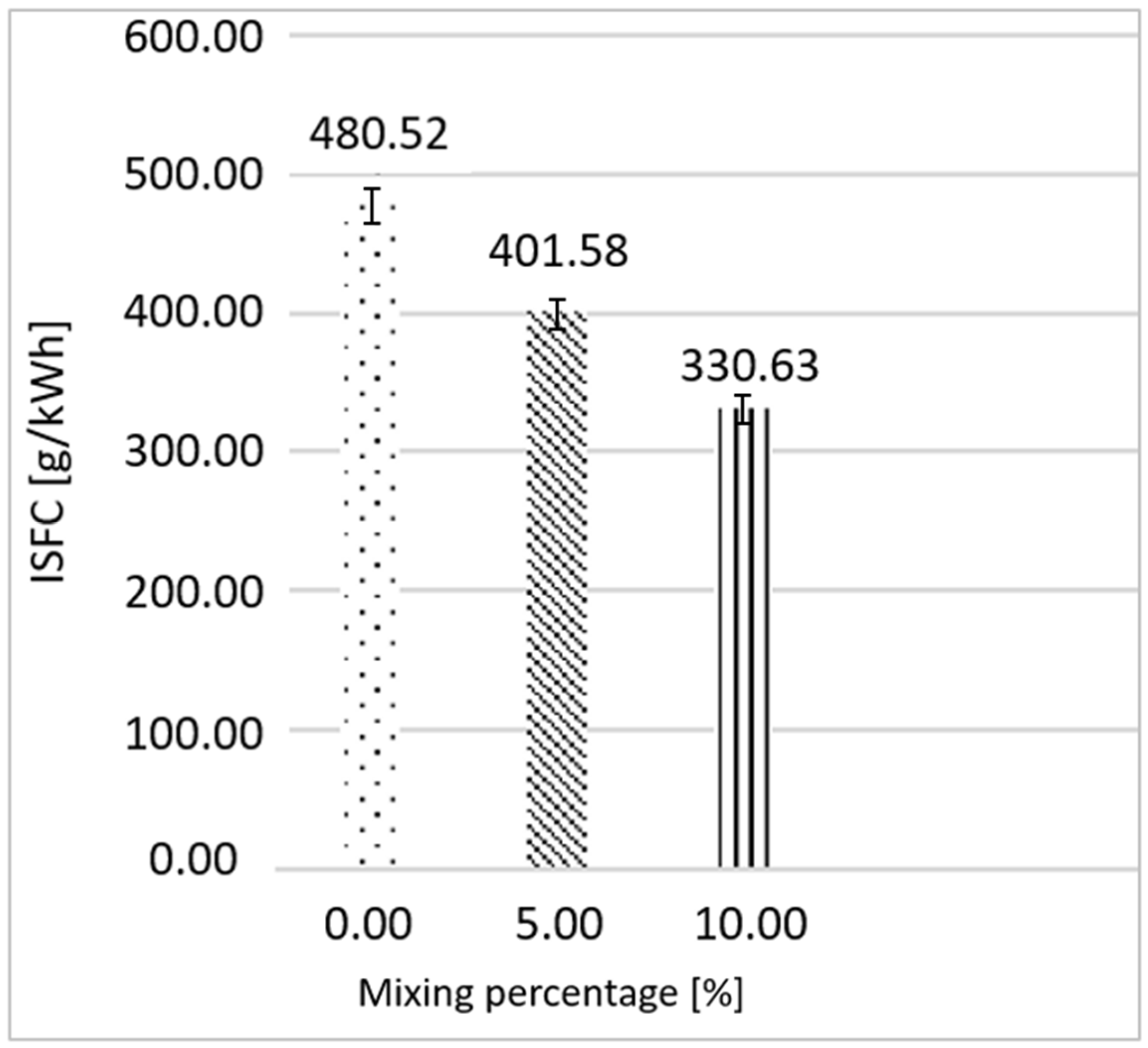
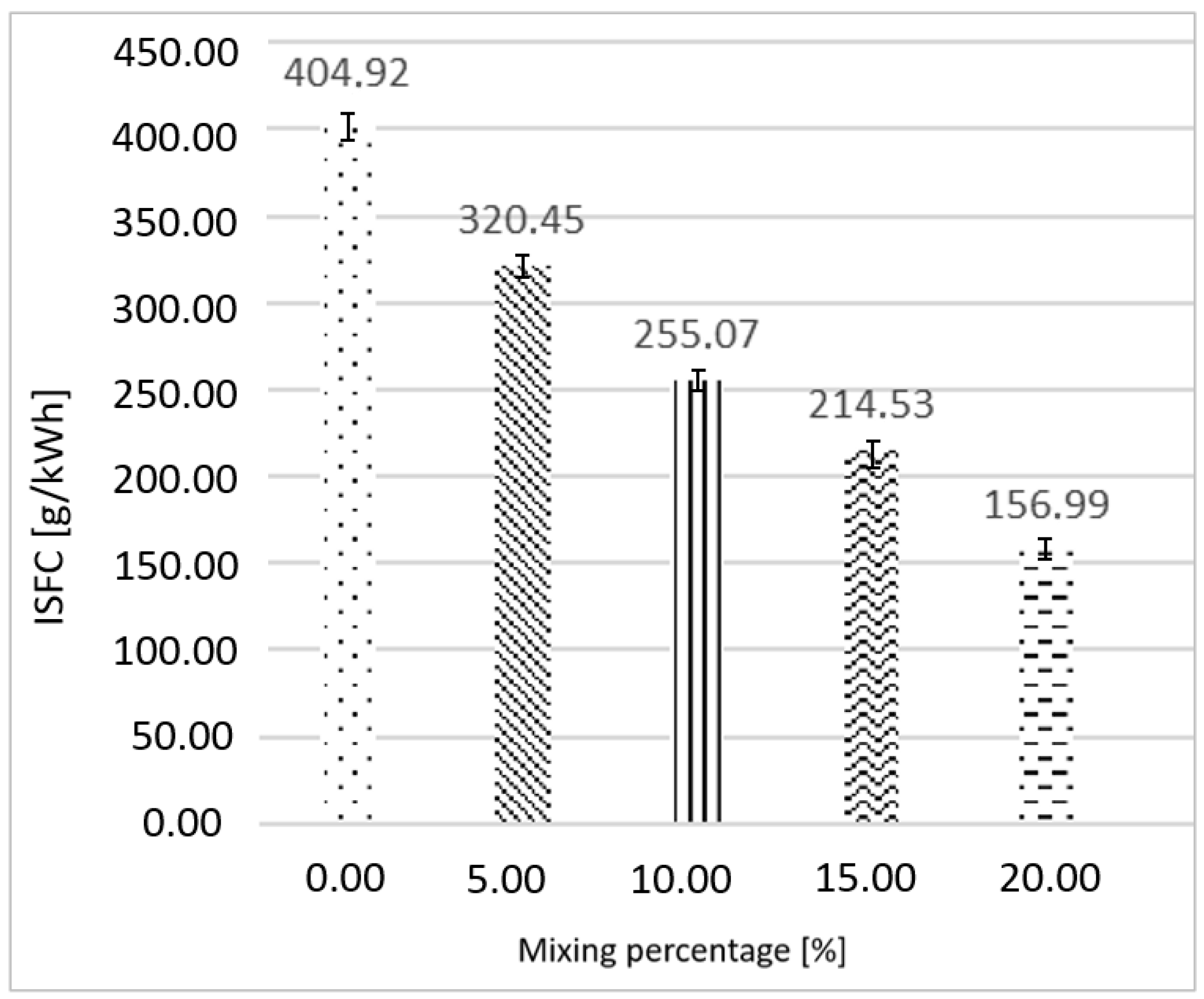
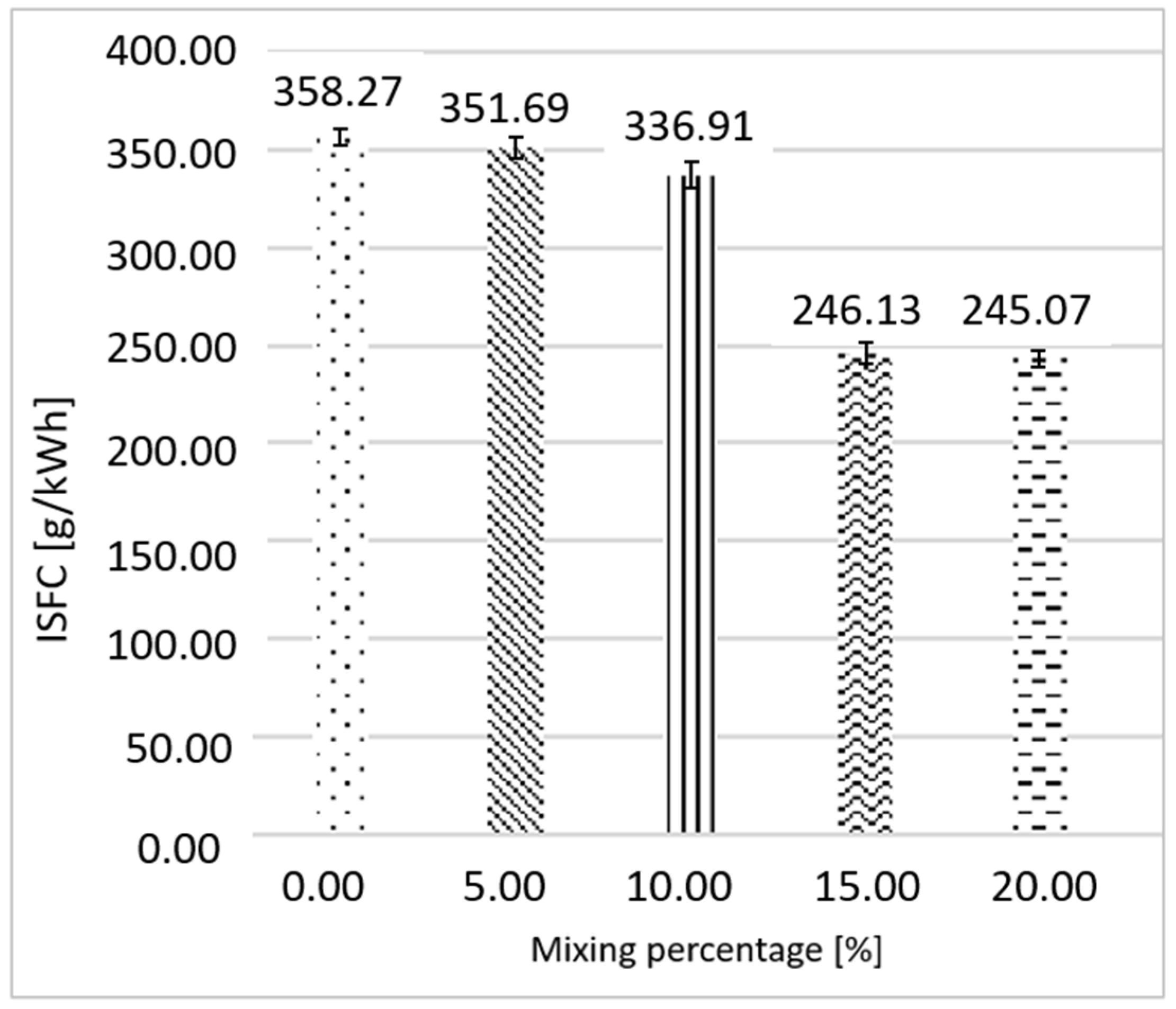
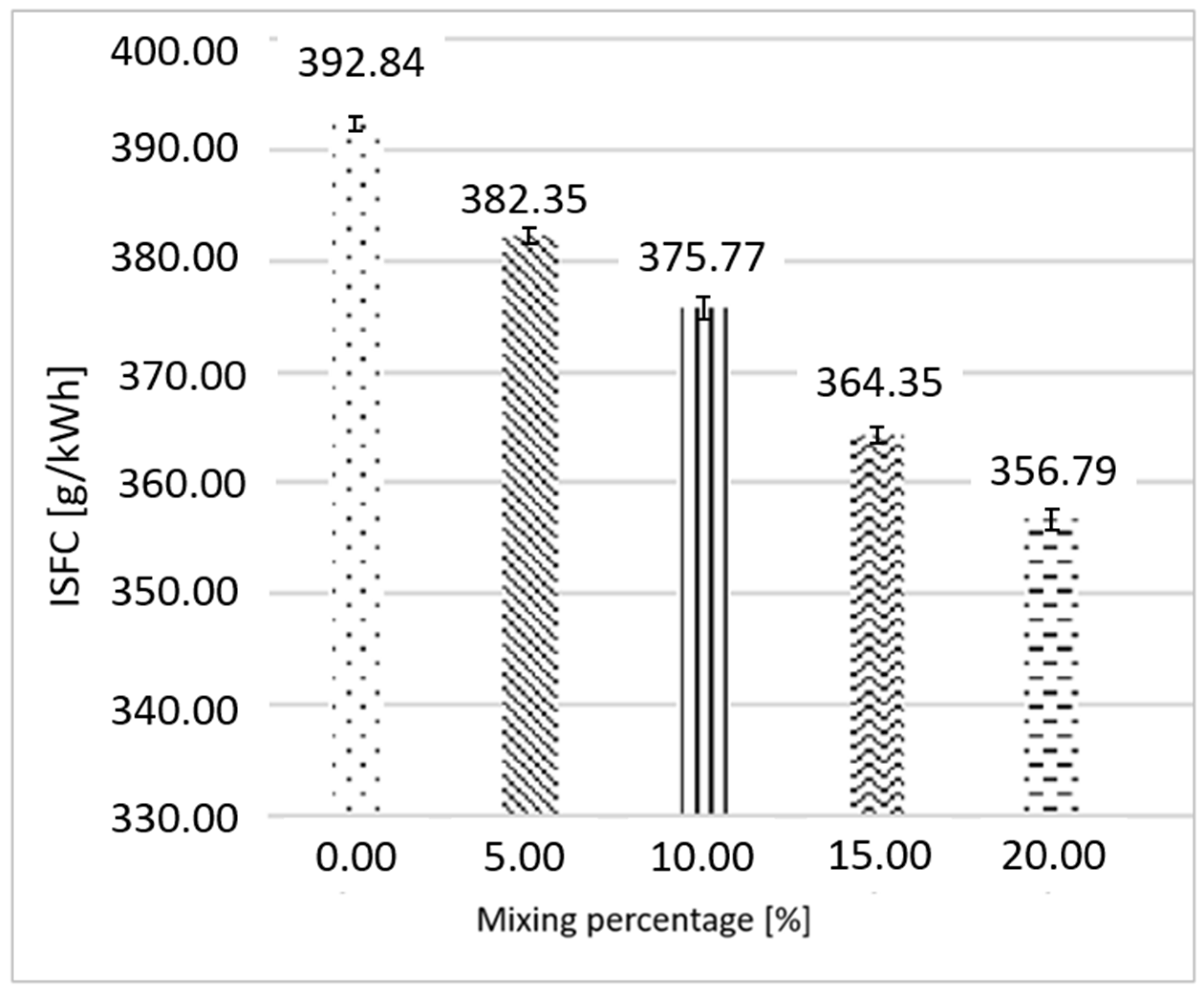
3.1. Ethanol as Primary Fuel
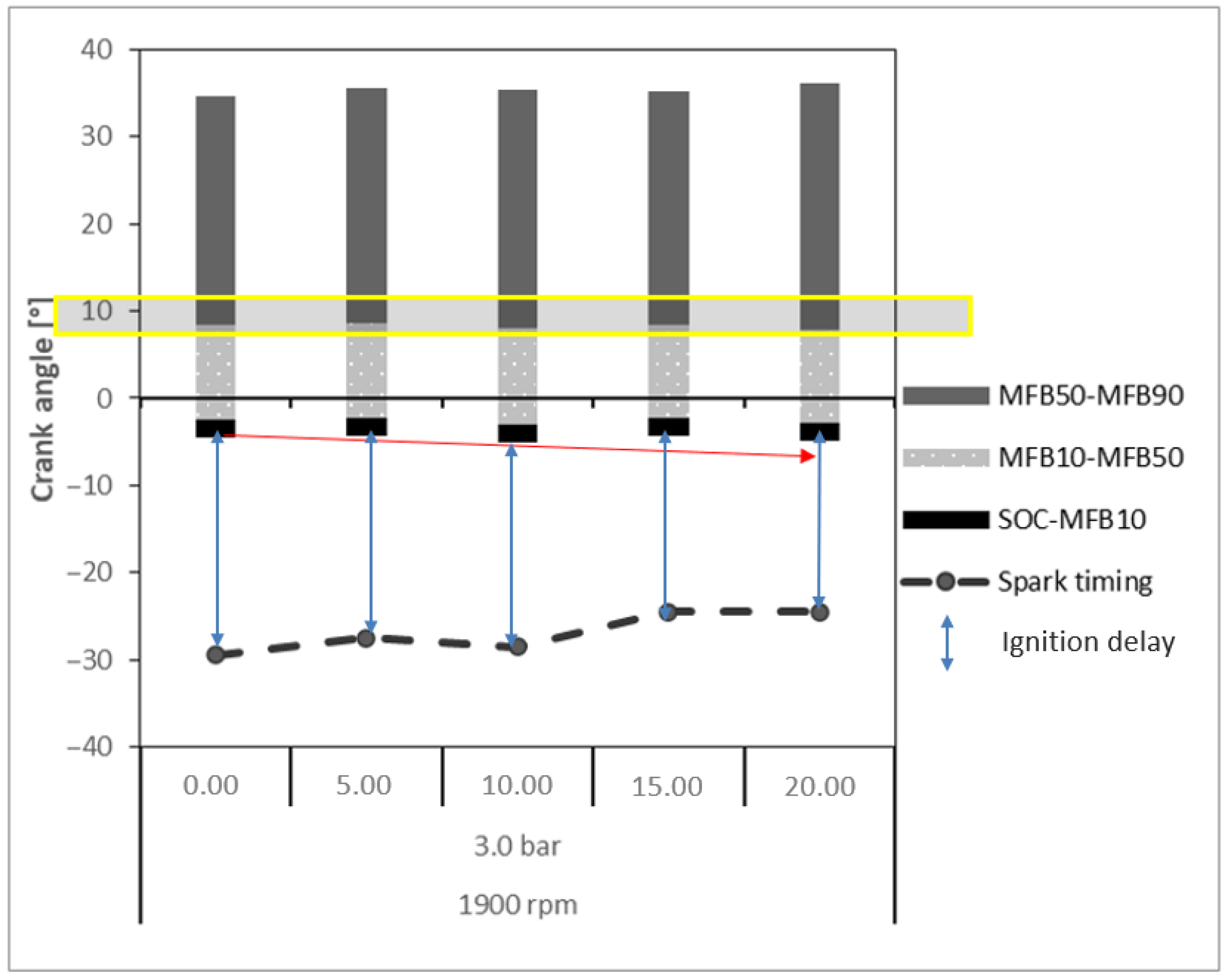
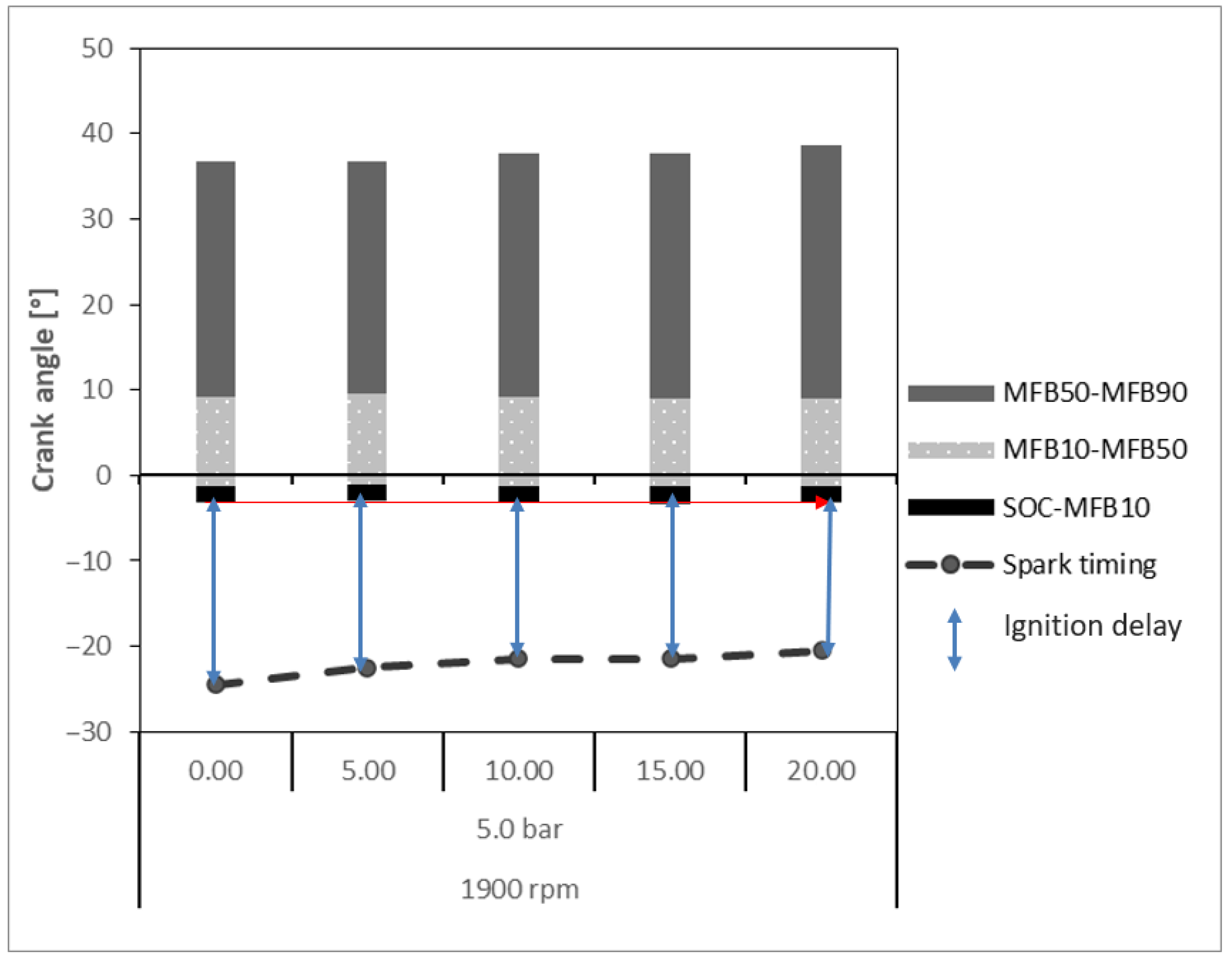
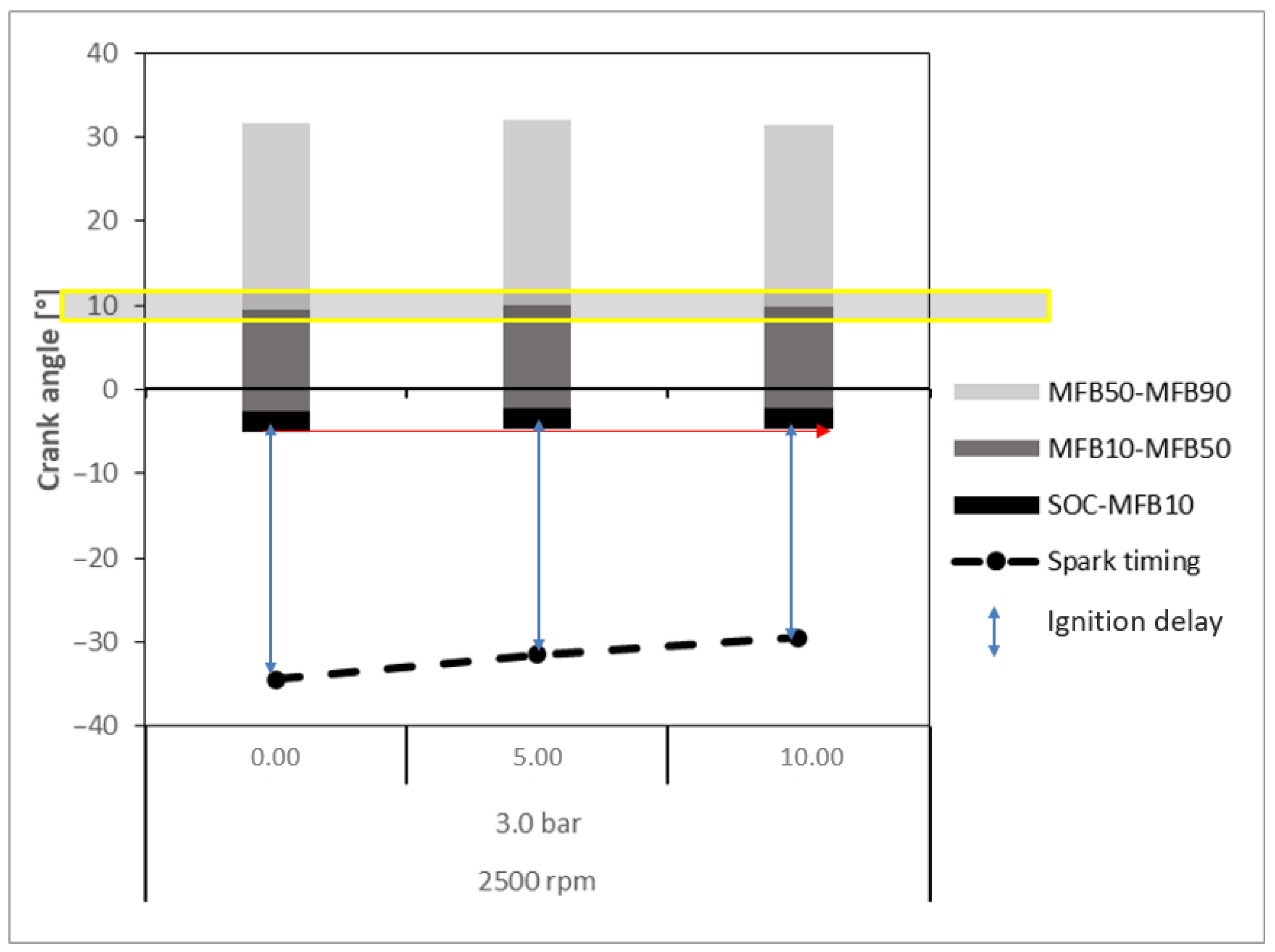
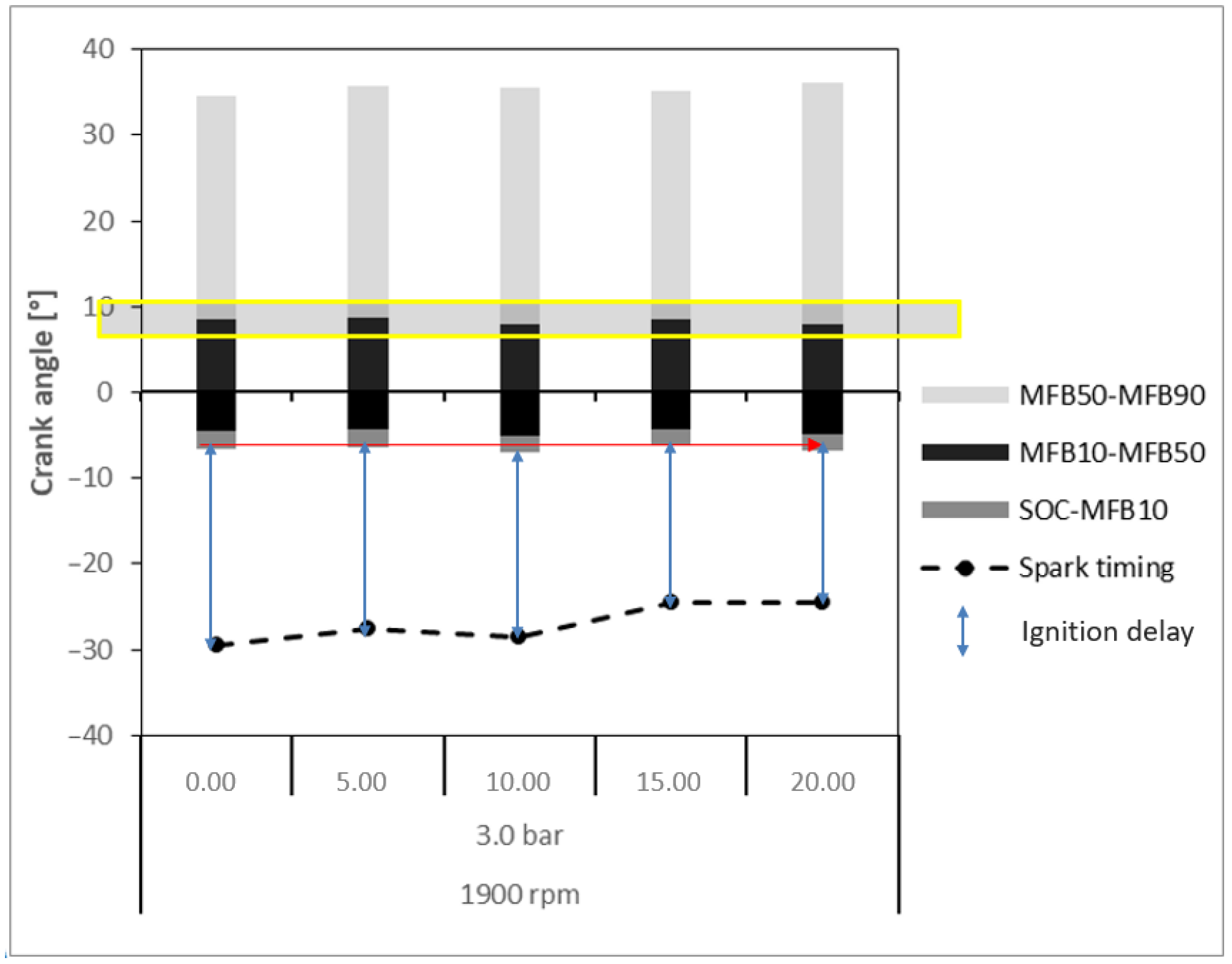
- Ignition Delay: Crankshaft angular range given by the difference between the ignition time (spark) and the SOC. The SOC is the point in the engine cycle at which combustion begins, often coinciding with the rapid increase in cylinder pressure caused by the ignition of the air–fuel mixture. It marks the transition from ignition delay to the combustion phase, where the fuel is actively burning and producing pressure that drives the piston.
- MFB10: Crankshaft angular position for burn at 10% of the mixture of air–fuel, determined by when the ordinate of the curve reaches 10%.
- MFB50: Crankshaft angular position for burn at 50% of the mixture of air–fuel, determined by when the ordinate of the curve reaches 50%.
- MFB90: Crankshaft angular position for burn at 90% of the mixture of air–fuel, determined by when the ordinate of the curve reaches 90%.
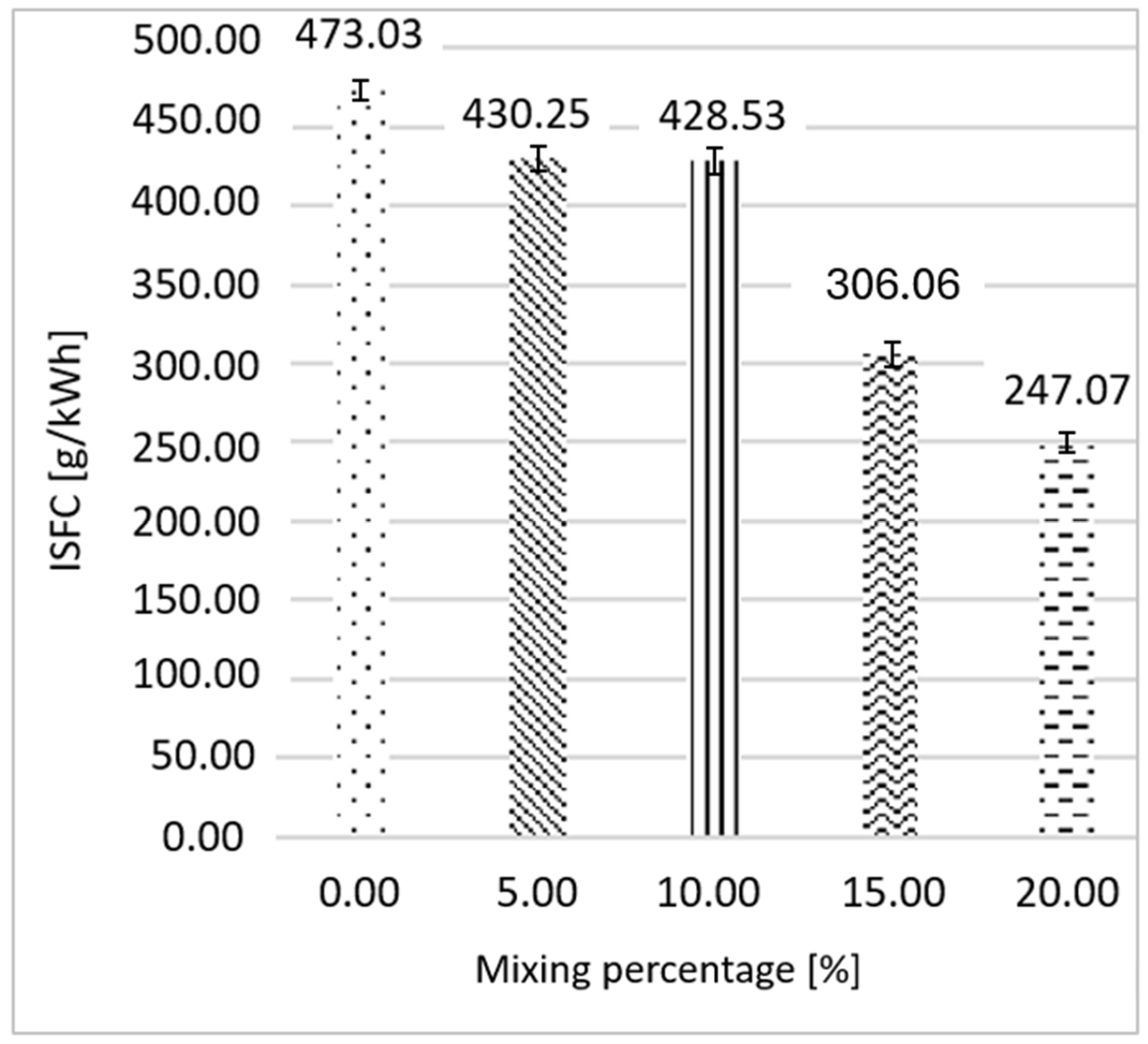
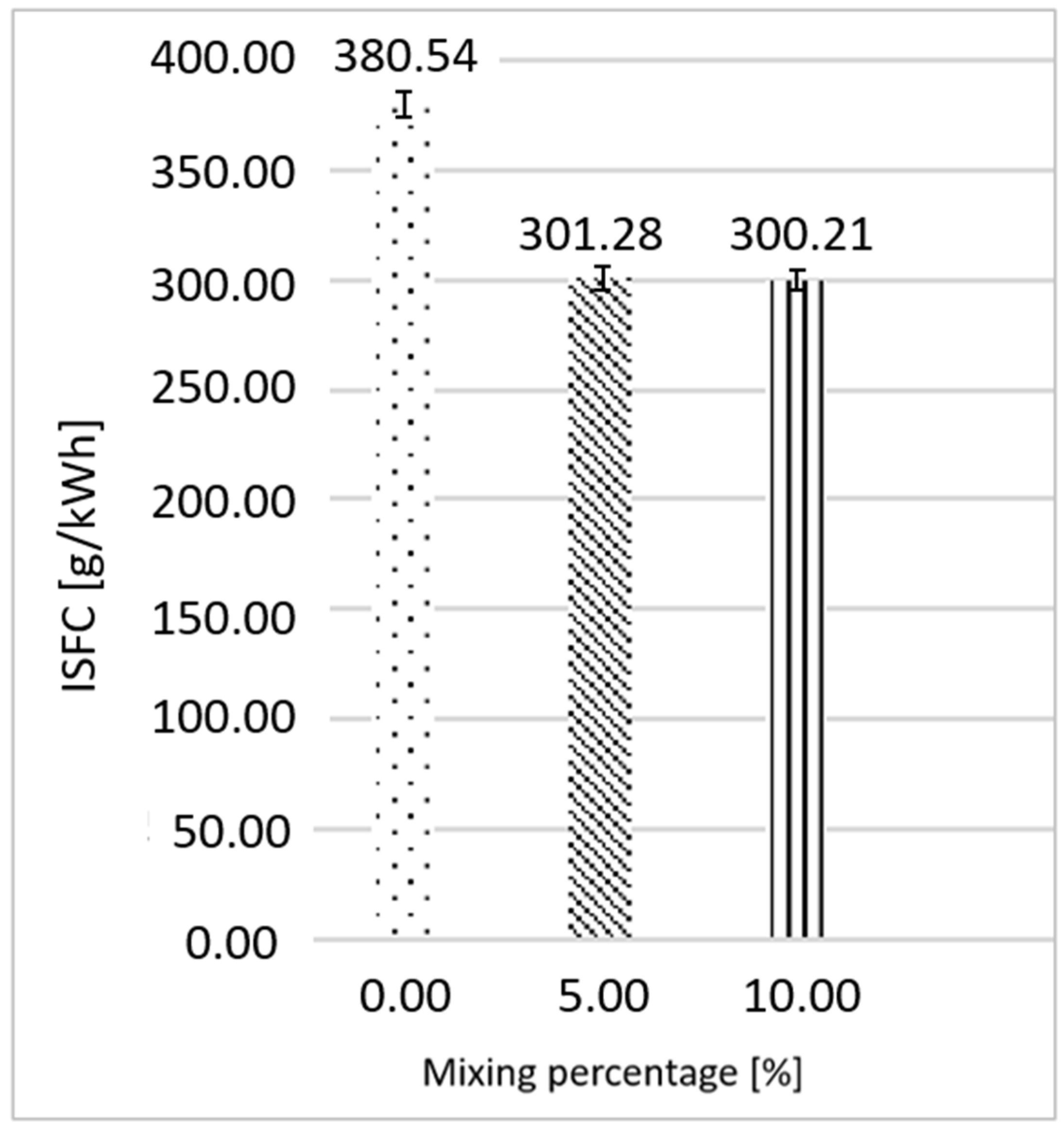
3.2. Gasoline as Primary Fuel
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | Crank Angle |
| CO | Carbon Monoxide |
| CO2 | Carbon Dioxide |
| CH4 | Methane |
| COVIMEP | IMEP Covariance |
| DI | Direct Injection |
| FS | Fumigation System |
| H2 | Hydrogen |
| HC | Hydrocarbon |
| HGM’ | Hydrogen Gaseous Mixture |
| ICE | Internal Combustion Engine |
| IMEP | Indicated Mean Effective Pressure |
| INT | Instituto Nacional de Tecnologia |
| ISFC | Indicated Specific Fuel Consumption |
| LHV | Low Heat Value |
| MFB-10 | 10% Mass burned fraction |
| MFB 10-50 | 10% to 50% Mass burned fraction |
| MFB 50-90 | 50% to 90% Mass burned fraction |
| MFB 50 | 50% Mass burned fraction |
| MP | Particulate Matter |
| NMOGs | Nitrogen oxides (NOx) + Non-methane Organic Gases |
| NOx | Nitrous Oxides |
| MBT | Maximum Brake Torque |
| SCRE | Single Cylinder Research Engine |
| SOC | Start of Combustion |
| WOT | Wide Open Throttle |
References
- UNEP. Conferencia das Nações Unidas Sobre Mudanças Climáticas (UNFCCC COP 28). Available online: https://www.unep.org/pt-br/events/conference/conferencia-das-nacoes-unidas-sobre-mudanca-do-clima-unfccc-cop-28 (accessed on 20 August 2025).
- International Energy Agency. Latin America Energy Outlook: Brazil Overview. Energy Outlook: Brazil. 2023. Available online: https://iea.blob.core.windows.net/assets/76db9baf-1d00-40a1-939c-2c9f21127c11/BrazilenergyprofileEN.pdf (accessed on 21 August 2025).
- IEA. Global Hydrogen Review 2024; IEA: Paris, France, 2024; Available online: https://iea.blob.core.windows.net/assets/89c1e382-dc59-46ca-aa47-9f7d41531ab5/GlobalHydrogenReview2024.pdf (accessed on 21 August 2025).
- CDA. Copper Drives Electric Vehicles; EUA: McLean, VA, USA; Available online: https://www.copper.org/publications/pub_list/pdf/A6191-ElectricVehicles-Factsheet.pdf (accessed on 23 August 2025).
- CDA. How Copper Drives Electric Vehicles; EUA: McLean, VA, USA; Available online: https://www.copper.org/publications/pub_list/pdf/A6192_ElectricVehicles-Infographic.pdf (accessed on 23 August 2025).
- Siciliano, B.; Da Silva, C.M.; Loureiro, L.N.; Vicentini, P.C.; Arbilla, G. Hydrocarbon emissions in flex fuel vehicles using ethanol: Preliminary results using a method implemented in Brazil. Fuel 2021, 287, 119506. [Google Scholar] [CrossRef]
- Mendiburu, A.Z.; Lauermann, C.H.; Hayashi, T.C.; Mariños, D.J.; Da Costa, R.B.R.; Coronado, C.J.R.; Roberts, J.J.; De Carvalho, J.A., Jr. Ethanol as a renewable biofuel: Combustion characteristics and application in engines. Energy 2022, 257, 124688. [Google Scholar] [CrossRef]
- Akhtar, M.U.S.; Asfand, F.; Mishamandani, A.S.; Mishra, R.; Khan, M.I. Hydrogen as a sustainable combustion fuel: Performance, challenges, and pathways for transition to low-carbon propulsion systems. Renew. Sustain. Energy Rev. 2025, 223, 116004. [Google Scholar] [CrossRef]
- Di Nardo, A.; Portarapillo, M.; Russo, D.; Di Benedetto, A. Hydrogen production via steam reforming of different fuels: Thermodynamic comparison. Int. J. Hydrogen Energy 2024, 55, 1143–1160. [Google Scholar] [CrossRef]
- Zbigniew, S. A Comprehensive Overview of Hydrogen-Fueled Internal Combustion Engines: Achievements and Future Challenges. Energies 2021, 14, 6504. [Google Scholar] [CrossRef]
- BRASIL Ministério do Meio Ambiente (MMA). Conselho Nacional do Meio Ambiente (CONAMA). Resolução MMA/CONAMA nº 490, de 23 de novembro de 2018. Estabelece a Fase PROCONVE P8 de exigências do Programa de Controle da Poluição do Ar por Veículos Automotores—PROCONVE para o Controle das Emissões de Gases Poluentes e de Ruído para Veículos Automotores Pesados novos de uso Rodoviário e dá Outras Providências. Diário Oficial da União, Brasília, DF, 2018. Available online: https://conama.mma.gov.br/?option=com_sisconama&task=arquivo.download&id=767 (accessed on 29 August 2025).
- Machado, G.; De Melo, T.; Da Silva, K. GDI flex fuel engine: Influence of different fuels on the performance. Int. J. Engine Res. 2020, 22, 3407–3414. [Google Scholar] [CrossRef]
- Kroyan, Y.; Wojcieszyk, M.; Kaario, O.; Larmi, M. Modelling the end-use performance of alternative fuel properties in flex-fuel vehicles. Energy Convers. Manag. 2022, 269, 116080. [Google Scholar] [CrossRef]
- Masaki, N.; Kohei, M.; Satoshi, S.; Kotaro, T.; Mitsuru, K. Effects of hydrogen addition on engine performance in a spark ignition engine with a high compression ratio under lean burn conditions. Int. J. Hydrogen Energy 2019, 44, 15565–15574. [Google Scholar] [CrossRef]
- Teng, S.; Changwei, J.; Shuofeng, W.; Xiaoyu, C.; Lei, S. Improving the combustion performance of a gasoline rotary engine by hydrogen enrichment at various conditions. Int. J. Hydrogen Energy 2018, 43, 1902–1908. [Google Scholar] [CrossRef]
- Greenwood, J.B.; Erickson, P.A.; Hwang, J.; Jordan, E.A. Experimental results of hydrogen enrichment of ethanol in an ultra-lean internal combustion engine. Int. J. Hydrogen Energy 2014, 39, 12980–12990. [Google Scholar] [CrossRef]
- Catapano, F.; Di Iorio, S.; Magno, A.; Sementa, P.; Vaglieco, B.M. A comprehensive analysis of the effect of ethanol, methane and methane-hydrogen blend on the combustion process in a PFI (port fuel injection) engine. Energy 2015, 88, 101–110. [Google Scholar] [CrossRef]
- Ren, Z.; Lin, C.; Haiqiao, W.; Jiaying, P.; Jinguang, L.; Penghui, Y.; Rui, C. Optical study on the effects of the hydrogen injection timing on lean combustion characteristics using a natural gas/hydrogen dual-fuel injected spark-ignition engine. Int. J. Hydrogen Energy 2021, 46, 20777–20789. [Google Scholar] [CrossRef]
- Hanyuyang, Z.; Gesheng, L.; Yanxiang, L.; Zunhua, Z.; Wenwen, W.; Mengni, Z. Numerical study on combustion and emission characteristics of a spark-ignition ammonia engine added with hydrogen-rich gas from exhaust-fuel reforming. Fuel 2023, 332, 125939. [Google Scholar] [CrossRef]
- Ayad, S.M.M.E.; Belchior, C.R.P.; Da Silva, G.L.R.; Lucena, R.S.; Carreira, E.S.; De Miranda, P.E.V. Analysis of performance parameters of an ethanol fueled spark ignition engine operating with hydrogen enrichment. Int. J. Hydrogen Energy 2019, 45, 5588–5606. [Google Scholar] [CrossRef]
- Prasad, B.S.N.; Jayashish, K.P.; Kumar, G.N. Effect of hydrogen enrichment on performance, combustion, and emission of a methanol fueled SI engine. Int. J. Hydrogen Energy 2021, 46, 25294–25307. [Google Scholar] [CrossRef]
- Guven, G.; Bahri, S.; Mehmet, F.H. Influences of hydrogen and various gas fuel addition to different liquid fuels on the performance characteristics of a spark ignition engine. Int. J. Hydrogen Energy 2022, 47, 12421–12431. [Google Scholar] [CrossRef]
- Ayad, S.M.M.E.; Belchior, C.R.P.; Sodré, J.R. Hydrogen addition to ethanol-fuelled engine in lean operation to improve fuel conversion efficiency and emissions. Int. J. Hydrogen Energy 2024, 49, 744–752. [Google Scholar] [CrossRef]
- Amaral, L.V.; Malaquias, A.C.T.; Fraga, M.A.; Torres, R.B.; Sebastião Rde Cde, O.; Pujatti, F.J.P. Combustion and specific fuel consumption evaluation of a single-cylinder engine fueled with ethanol, gasoline, and a hydrogen-rich mixture. Case Stud. Therm. Eng. 2024, 57, 104316. [Google Scholar] [CrossRef]
- Araujo, N.R.S.; Carvalho, F.S.; Amaral, L.V.; Braga, J.P.; Pujatti, F.J.P.; Sebastião Rde Cde, O. Kinetic study of the combustion process in internal combustion engines: A new methodological approach employing an artificial neural network. Fuel 2025, 382, 133739. [Google Scholar] [CrossRef]
- Malaquias, A.C.T.; Araújo GHde, P.; Amaral, L.V.; Filho Mde, C.T.; Chavda, N.B.; Pujatti, F.J.P.; Baeta, J.G.C. Experimental characterization of ethanol controlled auto-ignition in a single-cylinder research engine. Case Stud. Therm. Eng. 2025, 70, 106128. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals, 2nd ed.; McGraw-Hill Education: Columbus, OH, USA, 2018. [Google Scholar]
- Ayala, F.A.; Gerty, M.D.; Heywood, J.B. Effects of Combustion Phasing, Relative Air-Fuel Ratio, Compression Ratio, and Load on SI Engine Efficiency; SAE Technical Paper 2006-01-0229; SAE International: Warrendale, PA, USA, 2006. [Google Scholar] [CrossRef]
- Turns, S.R. An Introduction to Combustion: Concepts and Applications, 3rd ed.; McGraw-Hill Education: Columbus, OH, USA, 2012. [Google Scholar]


| Pollutants | Emissions Limits (mg/km) |
|---|---|
| Carbon monoxide (CO) | 1000 |
| Particulate matter (MP) | 6 |
| Aldehydes | 15 |
| Nitrogen oxides (NOx) + Non-methane organic gases (NMOGs) | 80 |
| Item | Specification |
|---|---|
| Bore × Stroke | 82.0 × 86.0 mm |
| Connecting rod length | 144.0 mm |
| Displaced volume | 454.16 cm3 |
| Compression ratio | 11.5:1 |
| Number of valves | 4 |
| Intake valve diameter | 34 mm |
| Exhaust valve diameter | 28 mm |
| Property | Uncertainty |
|---|---|
| Displaced volume [cm3] | 1.28 × 10−6 |
| Indicated mean effective pressure [bar] | 0.08 |
| Indicated power [kW] | 0.03 |
| Indicated torque [Nm] | 0.12 |
| Indicated fuel specific consumption [g/kWh] | 3.08 |
| Fuel conversion efficiency | 0.01 |
| Chemical Entity | Composition [%v/v] |
|---|---|
| H2 | 51.6 |
| CO | 6.0 |
| CH4 | 24.3 |
| CO2 | 18.10 |
| Engine Speed [rpm] | IMEP [bar] |
|---|---|
| 1900 | 3 and 5 |
| 2500 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, L.V.; Teixeira Malaquias, A.C.; Araújo, G.H.d.P.; Torres Filho, M.d.C.; Fraga, M.A.; Belchior Torres, R.; Sebastião, R.d.C.d.O.; Pujatti, F.J.P. Hydrogen-Rich Gaseous Mixture for Enhanced Combustion in a Flex-Fuel Engine: An Experimental Analysis. Hydrogen 2025, 6, 99. https://doi.org/10.3390/hydrogen6040099
Amaral LV, Teixeira Malaquias AC, Araújo GHdP, Torres Filho MdC, Fraga MA, Belchior Torres R, Sebastião RdCdO, Pujatti FJP. Hydrogen-Rich Gaseous Mixture for Enhanced Combustion in a Flex-Fuel Engine: An Experimental Analysis. Hydrogen. 2025; 6(4):99. https://doi.org/10.3390/hydrogen6040099
Chicago/Turabian StyleAmaral, Lucimar Venancio, Augusto César Teixeira Malaquias, Gabriel Heleno de Paula Araújo, Marcos de Carvalho Torres Filho, Marco André Fraga, Ricardo Belchior Torres, Rita de Cássia de Oliveira Sebastião, and Fabricio José Pacheco Pujatti. 2025. "Hydrogen-Rich Gaseous Mixture for Enhanced Combustion in a Flex-Fuel Engine: An Experimental Analysis" Hydrogen 6, no. 4: 99. https://doi.org/10.3390/hydrogen6040099
APA StyleAmaral, L. V., Teixeira Malaquias, A. C., Araújo, G. H. d. P., Torres Filho, M. d. C., Fraga, M. A., Belchior Torres, R., Sebastião, R. d. C. d. O., & Pujatti, F. J. P. (2025). Hydrogen-Rich Gaseous Mixture for Enhanced Combustion in a Flex-Fuel Engine: An Experimental Analysis. Hydrogen, 6(4), 99. https://doi.org/10.3390/hydrogen6040099





