Abstract
Humanity is confronted with one of the most significant challenges in its history. The excessive use of fossil fuel energy sources is causing extreme climate change, which threatens our way of life and poses huge social and technological problems. It is imperative to look for alternate energy sources that can replace environmentally destructive fossil fuels. In this scenario, hydrogen is seen as a potential energy vector capable of enabling the better and synergic exploitation of renewable energy sources. A brief review of the use of hydrogen as a tool for decarbonizing our society is given in this work. Special emphasis is placed on the possibility of storing hydrogen in solid-state form (in hydride species), on the potential fields of application of solid-state hydrogen storage, and on the technological challenges solid-state hydrogen storage faces. A potential approach to reduce the carbon footprint of hydrogen storage materials is presented in the concluding section of this paper.
1. Environmental Concerns
In the last one hundred fifty years, consequently to the second industrial revolution, the life quality of an ever-increasing fraction of the world population (excluding the periods of the 1st and 2nd world wars) has improved sensibly. This improvement has been mostly due to the growing possibility of accessing electricity, transportation, and heat. This, however, has led to an enormous increment in the world’s energy demand. Although not directly, the world’s energy demand is related to the world population. In the last one hundred years, the world population has increased by a factor of 4, whereas the world energy demand has increased by a factor of 24 [1]. Assuming that this trend is maintained also in the incoming decades, a further growth of the world’s energy demand is expected. In fact, considering that the current world population is 7.2 billion inhabitants and is forecasted to grow to about 8.3 billion in 2030 and to 9.6 billion in 2050 [2], the word energy demand is expected to grow by a factor of 4.5. However, this value might be even higher, if the fraction of the population living in the fast-developing countries continues to grow, and if the energy demand in the already developed countries increases even further.
Nowadays, the global energy demand is almost completely supplied by fossil fuel energy sources (e.g., in the year 2016, 80% of the overall energy demand was supplied by fossil fuels, 10% by biofuels, 5% by nuclear power, and only 5% by renewable sources such as hydro, wind, solar, and geothermal) [3,4,5]. It is widely known that in the last century, the extensive use of fossil fuels has led to a continuous increase in the CO2 concentration in the atmosphere. In the pre-industrial revolution period, the calculated average CO2 level in the atmosphere oscillated between circa 180 ppm (during ice ages) and 280 ppm (during interglacial warm periods). In 1958, Charles David Keeling measured the value of CO2 concentration at circa 317 ppm [6]. Since then, this value has increased sharply. In 2017, the CO2 level in the atmosphere settled permanently above 400 ppm, and it is projected to reach 450 ppm by the year 2040 [7]. This, without a shadow of doubt, led to an alteration of the natural atmospheric equilibria, which in turn led to a sensible increase in the average Earth temperature.
The so-called greenhouse effect, which allows the Earth to possess a moderate temperature suitable to host life as we know it, is mainly due to the water vapor present in the atmosphere and to clouds. However, new theoretical atmosphere-ocean climate studies have shown that the planet’s temperature ultimately depends on the atmospheric level of CO2. In fact, CO2 (as with other greenhouse gases present in the atmosphere but in much smaller concentrations such as ozone, N2O, CH4, and chlorofluorocarbons) cannot condense and precipitate from the atmosphere under the actual temperatures, whereas water vapor can and does. The so-called noncondensing greenhouse gases (GHG) account for about 25% of the total Earth’s greenhouse effect, providing a stable temperature structure that determines the current levels of atmospheric water vapor and clouds through feedback processes that control the remaining 75% of the greenhouse effect [8]. Due to the increased amount of noncondensing greenhouse gases in the atmosphere, the average global temperature has risen by approximately 0.7 °C over the last century and is expected to rise by approximately 6 °C this century. However, such an increase in the mean global temperature will lead to environmental and social scenarios that are difficult to foresee and surely not positive. It is clear at this point that, without a prompt and drastic reduction in CO2 emissions, a remarkable and irreversible increment in the Earth’s temperature is expected. This is possible only by abandoning the use of fossil fuels as the main energy supply.
2. Hydrogen Economy
The transition from a carbon-based economy to a carbon-free economy is surely the biggest challenge that humankind will face in the next century. In fact, although energy sources alternative to fossils fuels are known, their full and safe exploitation has yet to be demonstrated. Possible candidates are the so-called “renewable energy sources” (e.g., solar, wind, geothermal, wave, and hydroelectric) and nuclear sources. According to the report published by the International Energy Agency for the year 2016, the amount of electrical energy produced by renewables and nuclear sources in the countries belonging to the Organization for Economic Co-operation and Development (OECD) is equal to 24% and 18%, respectively. However, in the view of a safe and long-lasting energy solution upon which the future development of humankind can be based, the possibility of producing energy through nuclear technology must be discarded because of multiple issues. The already problematic storage of radioactive wastes must be taken into serious consideration when planning a large-scale use of this technology. Moreover, the safety of this technology after the recent Fukushima Daiichi disaster and the continuous issues with the power plants in France and Belgium are seriously debated. Last but not least, the uranium, necessary for powering the nuclear reactors’ reserves, is nowadays scarcely available and not renewable. Due to the lack of harmful products resulting from their exploitation, renewable energy sources appear highly appealing from both environmental and safety points of view. However, their intermittent nature, as well as their uneven distribution on Earth, are drawbacks that necessitate the identification of a suitable energy carrier, as well as the development of (yet undeveloped) technologies that will enable their efficient and synergic utilization. In this regard, the use of hydrogen as the future energy vector within the framework of a fully integrated and sustainable hydrogen-based economy (hydrogen economy) could be the solution to the pressing environmental issue caused by the widespread use of fossil fuels as an energy source. The idea of utilizing hydrogen as an energy vector is not a recent one and dates back to the year 1874, when Jules Verne mentioned the possibility of combining hydrogen and oxygen to obtain “an inexhaustible source of heat and light” in the book The Mysterious Island. However, it was much later that the term “hydrogen economy” was coined (by John Bockris in 1970). The sustainability of such an approach is bonded to the possibility of producing hydrogen via environmentally friendly methods such as electrolysis and (in the future) photocatalytic water splitting. The possibility of producing hydrogen via electrolysis allows for utilizing the excess energy produced by renewable energy sources to produce hydrogen that, in turn, will be utilized to produce electricity, via the use of fuel cells, when electricity cannot be directly supplied by renewable sources. The use of fuel cells for producing electricity from hydrogen is a versatile and scalable approach that can be implemented in many stationary and mobile devices [9]. In the transportation sector, the possibility to replace internal combustion engines with hydrogen-powered fuel cells is extremely interesting both from the efficiency and life quality points of view. In fact, the energetic efficiency of a fuel cell is higher than 60%, whereas, for combustion engines running on gasoline or diesel, it is only 22% or 45%, respectively. The quality of life in densely populated areas will be positively influenced by the replacement of vehicles powered by internal combustion engines with vehicles powered by hydrogen fuel cells. In fact, in a fuel cell, when hydrogen is combined with oxygen, water is the only chemical compound produced, whereas, from the combustion of gasoline and/or diesel in an internal combustion engine, a long series of harmful pollutants and particulates are released into the atmosphere (e.g., CO, CO2, SO2, NOx, N2O, VOCs, HCs, PM10, and PM2.5). Hydrogen possesses a further advantage over conventional fossil fuels. Its gravimetric energy density is considerably high (its lower heating value, i.e., LHV, is equal to 120 MJ/kg and its higher heating value, i.e., HHV, is equal to 140 MJ/kg). For example, the gravimetric energy density of hydrogen is about 3 times higher than that of gasoline. However, as hydrogen is the lightest element in the periodic table, its volumetric density in gaseous (at 20 °C and 1 bar of pressure) or liquid form (at −253 °C and 1 bar of pressure) is very low, i.e., 0.0899 g/L and 70.8 g/L, respectively [10].
3. Hydrogen Storage and Applications
Nowadays, the two most common methods commercially used to store hydrogen are in gaseous form at high pressure (350–700 bar at 20 °C) and in liquid form at cryogenic temperature (at −253 °C and 1 bar of pressure) [10]. The pressurization of hydrogen allows for improving the volumetric energy density, which, however, remains remarkably lower than those of the most common fossil fuels. In fact, gaseous hydrogen compressed to 350 bar and 700 bar possesses volumetric energy densities of 3 MJ/L and 5.6 MJ/L, respectively, whereas the volumetric energy density of gasoline is 32 MJ/L (Figure 1).
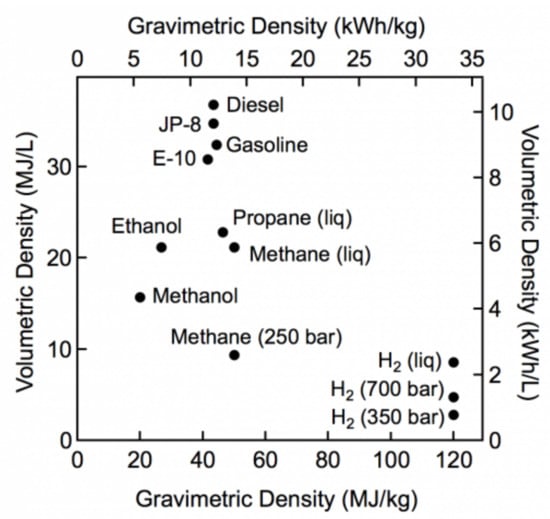
Figure 1.
Comparison of specific energy (energy per mass or gravimetric density) and energy density (energy per volume or volumetric density) for several fuels based on LHV. Courtesy of the U.S. Department of Energy (DOE).
In addition, the compression to 700 bar consumes an amount of energy that is higher than the 13% of the LHV. Although dependent on the scale of the process, the liquefaction of hydrogen at −253 °C is also energy demanding, i.e., 21 to 40% of the LHV is consumed during the condensation process. This storage method is also affected by another drawback. Due to the boil-off phenomenon, a hydrogen loss of about 1% per day is expected. Although this phenomenon can be minimized by improving the thermal insulation of the containing tank, it cannot be completely eliminated. In both the gaseous and liquid forms, hydrogen preserves its molecular structure. A further method to store hydrogen in molecular form is the physisorption on large-surface-area materials at cryogenic temperatures [11]. This storage method takes advantage of the dipolar interactions occurring between the hydrogen molecules and the surface of the absorbent material. Due to the weakness of van der Waals forces (ΔH = ~5 kJ/molH2, not considering the so-called hydrogen bonds), physisorption can occur only at cryogenic temperatures. In the past decades, several materials (e.g., zeolites, carbon-based materials, metal–organic frameworks, and polymers with intrinsic microporosity) have been investigated as potential adsorbents for hydrogen.
Interestingly, due to the high hydrogen packing density, a significant enhancement of the volumetric energy density is obtained when hydrogen is chemically bonded to other elements in solid-state. It is well known that hydrogen can form compounds with most elements in the periodic table, displaying an extremely rich chemistry. In fact, in such compounds, several different chemical bonds and interactions with the different elements can be observed. Among the elements of the periodic table, metals and a few metalloids have a high affinity for hydrogen. This high affinity leads to the breaking of the H-H bonds of the H2 molecules and to the formation of hydride species containing M-H bonds (M = metal/metalloid) [12]. This overall process can be divided into several different steps: (1) molecular hydrogen (H2) approaching the metal surface experiences attractive forces (van der Waals forces [13]) that confine it in a space adjacent to the metal surface (physisorption); (2) H2 dissociates on the metal surface and forms a metal-hydrogen bond (chemisorption); (3) chemisorbed hydrogen diffuses into the bulk lattice sites and forms a solid-solution (α phase Figure 2); (4) once a certain concentration of hydrogen is reached in a certain volume of the crystal lattice, a new phase characterized by a specific atomic metal-hydrogen ratio starts to form (β phase, metal hydride) at the expense of the α phase (Figure 2); (5) the growth of the β phase at the α/β interface proceeds until the formation of the β phase is complete. The process described above can be measured and analyzed by means of pressure–composition isotherms (pcT), as depicted in Figure 2 for a typical intermetallic compound. This process ideally should occur under isobaric conditions. However, in most of the real cases, the equilibrium pressure during the α-to-β transition (and vice versa) is not perfectly constant, but spans in a small range, and the graph displays a slope (e.g., LaNi5 [14,15]). This feature is usually attributed to a small inhomogeneity of composition in the starting material and/or the presence of small traces of contaminants. Furthermore, the equilibrium pressure for the hydrogenation process is often higher than the equilibrium pressure for the dehydrogenation process. This phenomenon is called hysteresis and, although its origin is not fully understood, state-of-the-art knowledge attributes it to the small modification of the stability of involved phases as the consequence of strain and relaxation phenomena that the metal and/or hydride lattices experience during hydrogenation and dehydrogenation [16].
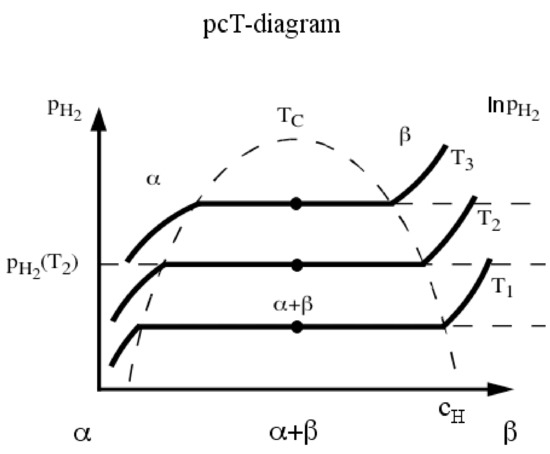
Figure 2.
Schematic pcT-diagram. The α-phase is the solid-solution phase, whereas the β-phase is the hydride phase. Within the (α – β) two-phase region, both the metal-hydrogen solution and the hydride phase coexist [17].
The reaction of the formation of a metal hydride is accompanied by the release of a certain amount of heat (Q) that is normally expressed as kJ per mole of H2 (kJ/molH2). This process can be summarized as follows:
M + (x/2)H2 ↔ MHx + Q
Three different types of strong chemical bonds can be found in hydride species, i.e., covalent (observed in the complex ions of borohydrides, alanates, and amides such as LiBH4, NaBH4, NaAlH4, and LiNH2) [18,19,20], metallic (observed in transition metal-containing hydrides such as LaNi5 and TiFe), and ionic (observed in alkaline and alkaline earth hydrides such as LiH, NaH, KH, CaH2, and MgH2). Although these bonds can be distinguished from the other, in several compounds, it is possible to observe a mixture of these types of bonds (e.g., in LiBH4 and NaAlH4). For these bonds, dissociation enthalpies in the range from 50 to 400 KJ/molH2 are expected.
The dissociation enthalpy or enthalpy of formation (ΔH) is the key parameter that (together with the entropic variation ΔS) determines the temperature (T) and H2 pressure (p) conditions, under which a metal hydride can exist.
Thus, the enthalpy variation associated with the dissociation or formation of a hydride also determines its theoretical operating conditions (possible variations of these parameters, due to kinetic constrains, must be considered separately). The relation between T, p, ΔH, and ΔS is described by the van’t Hoff equation [21]:
where R is the universal gas constant. For many metal hydrides, the value of ΔS is approximated to the standard entropy value of hydrogen S300K = 130.77 J/(K∙molH2). A graphical representation of the effect of ΔH on the stability of three hypothetical metal hydrides is provided in Figure 3. The regions of temperature and pressure on the left side of each p and T equilibrium curve are those conditions where the hydride phases are stable. On the contrary, the regions on the right side of the curves indicate those conditions under which the hydride phases are not stable and therefore decompose. The p and T equilibrium curves also indicate that, for a given pressure value, the increment in ΔH leads to a shift in the equilibrium temperature toward higher values.
lnp = −ΔH/RT + ΔS/R
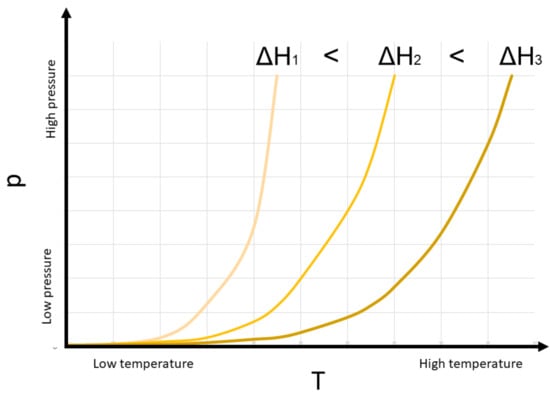
Figure 3.
Schematic p–T diagram presenting the equilibrium line for three hypothetic metal hydrides possessing increasing dissociation enthalpy, i.e., ΔH1 < ΔH2 < ΔH3.
Among the above-mentioned hydrides, MgH2 has been one of the most studied materials for hydrogen storage purposes due to its high gravimetric and volumetric hydrogen storage capacity (i.e., ρm = 7.6 wt% and ρv = 110 g H/L) and to its high natural abundance (e.g., Mg is present in minerals such as magnesite, dolomite, and in salt and brine deposits) [22]. However, there are practical constraints to its employment in large-scale applications. In fact, unfavorable thermodynamics and sluggish kinetics lead to possible operative conditions that are far from desired. Pure MgH2 possesses an hydrogen equilibrium pressure of 1 bar at 283 °C (calculated considering values of enthalpy and entropy equal to 74.6 ± 0.42 kJ/molH2 and 133.4 ± 0.7 J/(mol K), respectively). The dehydrogenation and re-hydrogenation kinetics of MgH2 were found to be highly sensitive to the material preparation route and to the presence of additives [23,24,25,26,27]. In fact, the nanostructuring via ball milling, infiltration into a nanometrically structured carbon-based scaffold, as well as the addition of transition metal (TM)-based additives, oxides, and halides, significantly improve the reaction kinetics [28,29,30,31].
The issue of the high thermodynamic stability of several metal hydrides, including MgH2, was already investigated at the end of the 1950s and during the 1960s.
In that period, Libowitz and coworkers noticed that for a fixed temperature, ZrNiH3 shows a hydrogen equilibrium pressure much higher than the one measured for ZrH2. Thus, the use of an alloy of Ni and Zr in the stoichiometric ratio of 1:1 leads, upon hydrogenation, to the formation of a hydride species (i.e., ZrNiH3) that possesses a reaction enthalpy lower than that of ZrH2 [32]. Similarly, Reilly and Wiswall [33,34] reported the possibility of tuning the reaction enthalpy of MgH2 by mixing Mg with other compounds in a proper stoichiometric ratio. In particular, they showed that the dehydrogenation enthalpies of 3MgH2 + MgCu2 and of Mg2NiH4 to form 2Mg2Cu + 3H2 and Mg2Ni + 2H2 are lower than those of pure MgH2. Unfortunately, the gained reduction in the reaction enthalpy is at the expense of the system’s hydrogen capacity.
Recently, owing to their high hydrogen storage capacities, “complex hydrides” have attracted considerable attention as potential hydrogen storage materials for mobile and stationary applications [18,19,20,35,36]. The term “complex hydrides” originates from the presence of an anionic non-metal–hydrogen complex (e.g., [NH2]−, [BH4]−, and [AlH4]−) or metal–hydrogen complex (e.g., [CoH5]4−, [FeH6]4−, [NiH4]4−, [ZnH4]2−, and [MnH6]5−) (often) bonded to a cationic alkali or transition metal. Based on this definition, complex metal hydrides can be divided into two main groups, i.e., non-transition metal complex hydrides (such as LiNH2, LiBH4, and LiAlH4) and transition metal complex hydrides (such as Mg2NiH4, Mg2CoH5, Mg2FeH6, Mg3MnH7, and K2ZnH4). Complex hydrides have been known for a long time (the first report on pure metal amides was published in 1809); however, they were initially not considered as potential hydrogen storage materials but rather as a laboratory curiosity or as reducing agents in chemical syntheses. The interest in hydrogen as an energy carrier began around the 1960s, and it has grown significantly since the 1990s. However, also in this period of intensive work, non-transition metal complex hydrides were considered as unappealing hydrogen storage materials due to their apparent irreversibility, and more generally due to the difficulty of producing them on a large scale. This scenario changed drastically in the year 1996 when Bogdanovic and Schwickardi as the first ones demonstrated the possibility to reversibly store hydrogen at moderate temperature and pressure conditions in NaAlH4 doped with Ti-based additives [37]. Since then, many efforts have been made to investigate and optimize the kinetic properties of complex hydrides.
Despite the noticeable scientific achievements that followed the work of Bogdanovic and Schwickardi, it has been necessary to wait until the beginning of the 2000s to discover a new concept that allows the obtaining of hydrogen storage systems based on complex hydrides that are fully reversible and possess low reaction enthalpies.
In fact, the approach of Libowitz et al., and Reilly and Wiswall has been improved by Chen et al. [38], Vajo et al. [39], and Barkhordarian et al. [40]. These researchers destabilized light-weight hydrides using a second hydride or multiple hydrides. Such hydride mixtures are also called Reactive Hydride Composites (RHCs). This concept offers the advantage of obtaining a reversible hydrogen storage system with a still high theoretical hydrogen storage capacity (i.e., the weighted average of the capacities of the hydrides composing the system) that possesses a reaction enthalpy lower than those of the single hydrides composing the system. Upon dehydrogenation, the hydrides might react directly among each other (mutual or concerted reaction) or with decomposition intermediates or their respective dehydrogenation products. A schematic representation of the RHC approach for a two-component system (AHx and BHy) that, upon dehydrogenation, forms a single compound (AB) plus hydrogen is given in Figure 4. This approach has been successfully applied to several families of transition complex hydrides and non-transition complex hydrides.
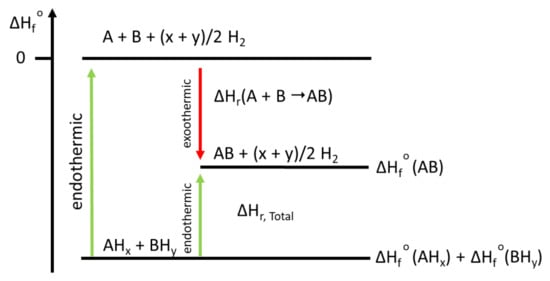
Figure 4.
Schematic enthalpy diagram depicting the RHC approach applied to a two-component system (AHx and BHy), which, upon dehydrogenation, forms a single compound (AB) plus hydrogen. ΔHf° = standard enthalpy of formation.
In the year 2007, Nakamori et al. reported a theoretical study that underlined a linear dependency between the thermal stability of borohydrides and their formation enthalpy (ΔH). Following this work, Nakamori et al. [41] published the experimental proof of the dependency of the thermal stability of several borohydrides on the Pauling electronegativity of the metal cation. These findings opened the path to the tailoring of the thermodynamic properties of complex hydrides via either cation substitution or anion modification. In this regard, several works describing the successful thermodynamic destabilization of alanates via cation substitution, to obtain bialkali alanates, were published. Unfortunately, the application of the cation substitution approach to other complex hydrides, such as borohydrides, did not lead to significant modifications of their thermodynamic properties. The modification of the thermodynamic stability of complex hydrides via anion modification (e.g., via the intramolecular partial replacement of F− for H−) is commonly referred to as the “functional anion concept” [42]. To achieve this result, complex hydrides are often mixed with metal halides. The driving force of this approach relies on the strong Pauling electronegativity of halogen atoms, which potentially weakens the force of hydrogen bonding in the complex anion, thus facilitating the hydrogen release. Although significant thermodynamic destabilizations were observed for several complex hydrides (e.g., Na3AlH6 and LiBH4), the decomposition products are usually mixtures of stable halogens, and the purity of the released hydrogen is poor.
It is clear, at this point, that metal hydride-based systems display a large range of hydrogen storage properties (e.g., hydrogen storage capacity) and operating conditions (e.g., temperature and hydrogen pressures). This diversity makes it possible to imagine their employment in several different technological fields.
Considering the European energy scenario, the final energy consumption assessed by sector for the year 2017 indicates that the highest fraction of consumed energy is due to the transport sector (31%), followed by the households (27%), industry (25%), and services (15%) sectors [43]. Therefore, it is clear that the transport sector together with the household and industry sectors are the main potential fields of application for hydrogen storage technologies. Based on the requirements dictated by the Department of Energy (DoE) for solid-state hydrogen storage in mobile and stationary applications [44,45], the possibility to store hydrogen through physisorption is unlikely. On the contrary, the storage of hydrogen in metal hydrides, complex hydrides, and RHCs, owing to their high achievable hydrogen storage capacities coupled with tunable thermodynamic and kinetic properties, appears to be a suitable solution for both mobile and stationary applications.
Regarding the transportation sector, the possibility to successfully employ hydride-based materials as hydrogen storage media depends on, in addition to possessing high hydrogen storage capacity (both volumetric and gravimetric), the possibility to achieve operating conditions suitable for their use in combination with proton-exchange-membrane (PEM) fuel cells, i.e., dehydrogenation temperatures lower than 120 °C at hydrogen pressures between 1 and 10 bar [46]. For this reason, in recent decades, thorough research in identifying new hydride species capable of satisfying at once all the automotive requirements was carried out [18,19,35,47,48,49,50]. However, despite the considerable progress made, among the complex metal hydrides, NaAlH4 (Ti-doped) is the only material that has been tested in custom-built tanks under automotive demand cycles. In this regard, the most important contributions available in the literature are those from the United Technology Center (UT), Sandia National Laboratories/General Motors (SNL/GM), and Gesellschaft für Kernenergieverwertung in Schiffbau und Schifffahrt (GKSS), now Hereon (Figure 5) [51,52,53,54]. The design of the tanks in these three cases followed different aims. The UT tank was designed to maximize tank storage capacity regardless of tank cost, while the SNL/GM tank was designed to strike a balance between storage capacity and cost, and the GKSS tank was designed to achieve rapid recharging times. The UT tank contained about 19 kg of Ti-doped NaAlH4 loaded in an aluminum foam (to increase the heat conductivity into the powder bed) and had an overall hydrogen content of 450 g H2. The GKSS tank contained about 8 kg of Ti-doped NaAlH4 mixed with graphite (divided into seven 1.4571 stainless-steel tubes) and had an overall hydrogen content of 450 g H2. The use of graphite has been necessary to increase the heat conductivity into the powder bed. These two tanks were constructed in a single module, whereas the storage system constructed by the SNL/GM consortium consisted of four interconnected modules, each containing 21.5 kg of Ti-doped NaAlH4 mixed with graphite (divided into twelve 316L stainless-steel tubes). The overall hydrogen content of the SNL/GM was about 3 kg. Despite the fact that these tanks could meet automotive demand cycles, the systems’ low hydrogen gravimetric capacity (around 1 wt%) precluded their utilization in commercial applications. For this to happen, the development of a better storage material that will allow solid-state tanks to reach and/or exceed the gravimetric storage capacity of modern compressed gas tanks is necessary.

Figure 5.
Prototype NaAlH4 storage tank developed in GKSS laboratories [53].
In addition to the use of NaAlH4, the use of metal hydrides from the families AB2 and AB5 [55] for the development of automotive hydrogen storage tanks has been investigated in recent decades [56,57,58,59]. These hydrides are characterized by high volumetric hydrogen storage capacities but low gravimetric hydrogen storage capacities. Aimed at obtaining a metal hydride-based tank with good gravimetric hydrogen storage capacity, a hybrid high-pressure/solid-state approach was recently proposed for automotive tanks, but at the cost of the system volumetric capacity (in comparison to low-pressure metal hydride-based tanks). Using this method, metal hydrides are placed in a standard high-pressure tank. Such a concept has been pursued by a number of companies, including Toyota Motor Company, which developed a 7.3 kg hydrogen tank based on the alloy Ti1.1CrMn [60] that could be refilled to 80% of its capacity in only 5 min [57]. Unfortunately, using such alloys inside the high-pressure tank results in a significant increase in vehicle weight (compared to high-pressure tank vehicles), which can only be partially offset by using higher-cost, lighter-weight materials for the vehicle’s other components. Furthermore, the high cost of the raw materials required for the synthesis of the best-performing alloys (e.g., Ti, Cr, V, and Mn), and the high costs for manufacturing the high-pressure vessels are barriers to the widespread use of this technology.
An additional potential field of application for hydride-based systems, closely related to the transportation sector, is that of hydrogen compression units. This technology was developed in the 1970s, and it represents a good alternative to compressing hydrogen through methods such as mechanical, electrochemical, and ionic liquid compression [61]. The advantages of using hydride-based compression units are their simplicity of design, absence of moving parts, safety, and reliability. A further advantage of this technology is that it can be operated using waste heat in an industrial application context. For understanding which kinds of metal hydride-based systems are suitable to be operated using waste heat, it should be considered that the majority of waste heat lies in the temperature range of 100–200 °C. However, industrial waste heat with a temperature lower than 100 °C is also available from the food and drink industrial sector (i.e., mostly from drying and preheating processes) [62]. Thus, hydride-based systems possessing reaction enthalpies up to about 60 kJ/molH2 could be potentially used as materials for hydrogen compressing units.
The fields of application proposed thus far do not contemplate the possibility of employing hydride-based systems possessing reaction enthalpies higher than 60 kJ/molH2 and/or operating temperatures higher than 200 °C. However, in the framework of a hydrogen economy based on renewable energies, these materials might find application in heat storage systems for solar and related applications [63,64]. Concentrating solar thermal power (CSP) plants successfully store thermal energy using, in most cases, a mixture of molten NaNO3/KNO3 salts, which possesses a latent heat of fusion of about 10 kJ/mol [65]. However, the use of such material presents some criticalities, such as the very large quantity of molten salt necessary to store energy for the night cycle, the high corrosivity, and a maximum possible operating temperature of about 565 °C [66]. These issues could be solved by replacing the NaNO3/KNO3 salt mixture with metal hydrides. In fact, due to the high energy density of some hydrides (e.g., formation enthalpies of MgH2, CaH2, and LiH are −74.6 kJ/molH2 [67], −181.5 kJ/molH2 [68], and −133.0 kJ/molH2 [69], respectively), a significant reduction in the material mass and volume (up to 10 times) needed for the heat storage unit is possible. Moreover, using metal hydrides, operating temperatures up to almost 1000 °C can be achieved (Figure 6) [70].
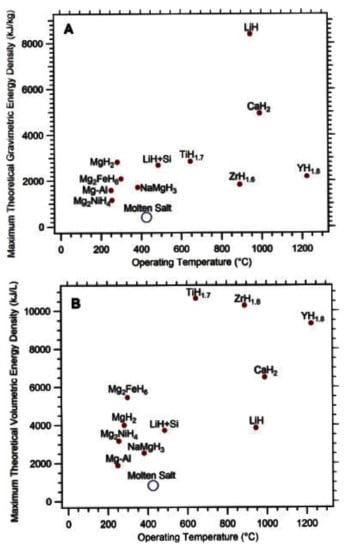
Figure 6.
Maximum theoretical (A) gravimetric and (B) volumetric energy storage densities of select metal hydrides compared to existing molten salt technology [66].
Hydride-based systems might also be utilized to replace diesel generators as a reliable on-demand power source in so-called “off-the-grid” applications and as a material for inter-seasonal energy storage [71]. It is well known that many facilities around the world are permanently not connected to the electric energy grid or have to continuously operate even in the event of a sudden interruption of the electrical energy supply. Despite the issues related to an adequate design and heat management, the viability of using hydride-based devices for enabling off-grid installations for power generation and energy storage was recently proved in several demonstration projects [72,73,74,75].
In the near future, owing to the possibility of using waste heat, high-energy efficiency, and environmental friendliness (i.e., use of environmentally harmless fluids), metal hydrides might find application in cooling systems. Such systems are commonly referred to as MHCSs (i.e., metal hydride cooling systems) [76]. For such a purpose, two metal hydrides possessing different thermodynamic stabilities are utilized [77,78]. Considering a given hydrogen pressure value, the metal hydride operating at a higher temperature is referred to as high-temperature hydride (A), whereas the hydride operating at a lower temperature is referred to as low-temperature hydride (B). The simplest MHCS operates using two reactors, one containing hydride A and the other containing hydride B. Hydride A is operated between the heat source temperature (Ths) and the ambient temperature (Tamb), whereas hydride B is operated between Tamb and the cooling temperature (Tc). The overall operational process can be divided into two half cycles, namely the cooling cycle (CC) and the regeneration cycle (RC).
At the beginning of the CC operation, hydride B is fully charged, while hydride A is in the dehydrogenated state. Once the two reactors are connected, with hydride A having an equilibrium pressure higher than that of hydride B, the hydrogen flows from the reactor containing hydride B to the reactor containing hydride A. As the dehydrogenation process is endothermic, until the maximum obtainable desorbed fraction is achieved, the reactor containing hydride B provides the required cooling effect (Qc). On the contrary, as the hydrogenation reaction is an exothermic process, the heat generated in the reactor containing hydride A will have to be dispersed in the ambient environment (Qamb 1).
During the RC, the heat provided from the available sources (Qhs) is supplied to the reactor containing hydride A, causing its dehydrogenation once its equilibrium pressure rises above that of hydride B. In this case also, the heat generated by the hydrogenation of hydride B (Qamb 2) will have to be released into the ambient environment. A schematic representation of the functioning principle of a MHCS is presented in Figure 7.
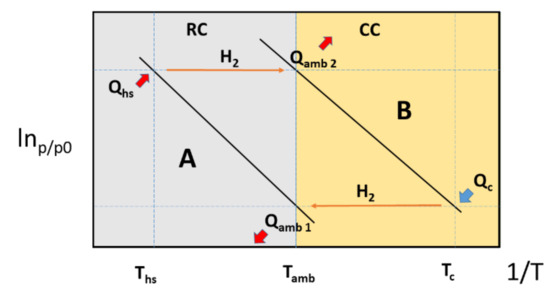
Figure 7.
Schematic illustration of a MCHS operating principle using a van’t Hoff plot.
Interestingly, the potential field of application of hydride-based systems as cooling systems can be extended further beyond the Earth boundaries into outer space, as demonstrated by the works of Strumpf and Bowman [79,80]. In their works, they described the possibility to provide cooling or refrigeration for a variety of space applications that require constant operating temperatures (e.g., optics cooling, detectors cooling, personal astronaut cooling during extravehicular activity, food refrigeration and freezing, extraordinary medical needs, and habitation cabin cooling) using hydride-based systems (Figure 8). These cooling devices are widely called cryocoolers and operate exploiting the Joule–Thomson (J–T) effect. In this context, the advantages provided by the use of such hydride-based technology are mostly related to the compactness of the cooling device, as well as the complete lack of vibrations that can limit the performance of highly sensitive devices to record undistorted signals.
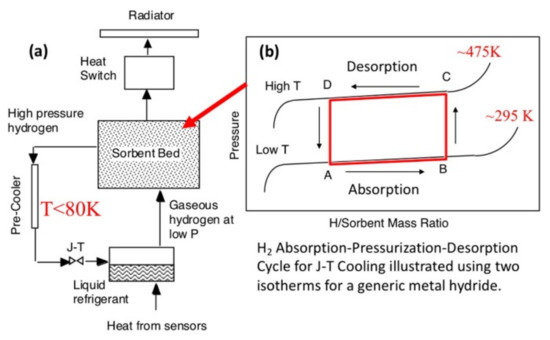
Figure 8.
(a) Schematic diagram of a single-stage sorption cryocooler with a metal hydride sorbent bed producing liquid hydrogen and (b) the idealized closed-loop cycle A–B–C–D that is superimposed on the isotherms for a representative metal hydride [79].
From the discussion above, it is possible to infer that to achieve the final goal of a fully decarbonized hydrogen economy, the development of a large variety of solid-state hydrogen storage materials, each of them possessing peculiar properties suited to the targeted application, is necessary. Moreover, to sustain this epochal transition, the developed solutions must also be cost-effective, scalable, and environmentally sustainable.
The demand for metals for hydrogen storage applications is currently negligible, but it will certainly increase in the following decades, posing huge environmental and social concerns that must be handled immediately on a global scale. Deforestation, water pollution, and soil contamination are the most visible evidence of the environmental impact that metal mining has. Furthermore, mining is an energy-intensive operation that necessitates the use of significant amounts of fuel and electricity, resulting in the release into the atmosphere of large amounts of GHG emissions. Additionally, the treatment of the mined minerals, such as crushing, greening, thermal or electrochemical reduction, and so on, leads to the further release of GHG. Thus, a rethinking of the procedures used to obtain metal hydride-based hydrogen storage systems is required if we wish to consider the usage of hydrogen and its storage in solid-state as practical strategies for climate change mitigation.
A possible solution can certainly come from the use of circular economy strategies and concepts such as recycling and by-product synergy. Although, for most of the metals used in industrial applications, well-established functional recycling processes are in place, a considerable part of the metals ends up in land fields. These scraps, once sorted, could be used to make hydrogen storage systems with a minimal carbon footprint when compared to systems made from high-purity metals and/or metal compounds. Another obstacle that prevents the widespread adoption of metal hydride-based hydrogen storage systems, namely their high-cost, will be addressed by such an approach.
Until recently, studies on the possibility of producing metal-based hydrogen storage systems from recycled metal scraps were virtually nonexistent in the literature. This lack of publications can be traced to the interest of the scientific community in deeply understanding material physicochemical properties and correlating them to specific material compositions, whereas the utilization of recycled material will inevitably introduce into the system several different contaminants. A pioneering work on the use of metal scraps for the production of hydrogen storage materials has been the one from Bergemann et al. [81]. In this work, based on the ball milling technique [82], NaAlH4 was synthesized using aluminum obtained from a waste incineration plant and commercial NaH.
The possibility of producing Mg-based hydrogen storage materials using recycled magnesium alloy scraps was recently investigated by Pistidda et al. [24], Hardian et al. [83], and Cao et al. [84]. In these works, the materials used as magnesium sources were scraps of AZ91 alloy and Mg-10 wt% Gd alloy. The results showed that the MgH2 formed during the hydrogenation of the milled scraps can be used to reversibly store considerable amounts of hydrogen (i.e., AZ91 up to 6 wt%) despite being stored under atmospheric conditions for a long time. Even after several dehydrogenation/hydrogenation cycles, the material showed quick reaction kinetics and complete reversibility. Surprisingly, the presence of transition metal elements in the starting alloy appears to act as dehydrogenation/hydrogenation catalysts either alone or after generating oxide species (during exposure to the environment). Using the MgH2 obtained from scrap AZ91 alloy, Cao et al. [84] synthesized first Mg(NH2)2 and then used it in the RHC system Mg(NH2)2-2LiH RHC. The identical system was produced using Mg(NH2)2 obtained from highly pure MgH2 as a comparison. The hydrogenation/dehydrogenation kinetics studies demonstrate that the waste material composite can reach a hydrogen capacity of 4.6 wt% in 100 min after five cycles, whereas the composite containing pure Mg(NH2)2 can reach a capacity of 4.4 wt% in 100 min after five cycles. Furthermore, the Mg(NH2)2-2LiH composites generated from waste alloy have a hydrogen desorption peak temperature of 220 °C, which is 15 °C lower than the material synthesized from pure Mg.
Although preliminary, these findings show that high-quality hydrogen storage materials can be made from metal scraps, as well as the enormous potential that using circular economy strategies could have in reducing both the carbon footprint and the cost of metal hydride-based hydrogen storage materials.
4. Conclusions
In the following decades, humankind will face enormous challenges related to climate change and energy supply. A swift transition to a more sustainable energy system is necessary, and hydrogen is the key element of this transition. In this work, it was displayed that metal hydride-based systems provide a suitable solution to store large quantities of hydrogen for mobile and stationary applications, owing to their large variety of physicochemical properties. For these reasons, in the near future, metal hydride-based materials are expected to play an important role in realizing a fully decarbonized hydrogen-based economy. However, a rethinking of the way metal hydrides are produced is necessary for avoiding issues connected to their environmental sustainability and high costs. A possible solution is the application of circular economy strategies involving the use of industrial metal scraps to be used as metal sources for the synthesis of high-quality metal hydride systems.
Funding
This research was funded by DFG (Deutsche Forschungsgemeinschaft), grant number PU 131/16-1.
Acknowledgments
The author of this manuscript would like to thank the Helmholtz association for their financial support. The author of this manuscript would like to thank Agnieszka Rzeszutek-Pistidda and Lucia Anna Pistidda for their kind support.
Conflicts of Interest
The author declares no conflict of interest.
References
- Züttel, A. Introduction. In Hydrogen as a Future Energy Carrier; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 1–6. [Google Scholar]
- United States Census Bureau. The 2012 Statistical Abstract of the United States, the National Data Book, International Statistics, Total Word Population; United States Census Bureau: Suitland-Silver Hill, MD, USA, 2012.
- Klebanoff, L. Hydrogen Storage Technology: Materials and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Monastersky, R. Climate crunch: A burden beyond bearing. Nature 2009, 458, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.; Archer, D. Climate change: Too much of a bad thing. Nature 2009, 458, 1117–1118. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.C. Charles david keeling and the story of atmospheric CO2 measurements. Anal. Chem. 2010, 82, 7865–7870. [Google Scholar] [CrossRef] [PubMed]
- Website NCC. Available online: https://climate.nasa.gov/climate_resources/7/graphic-carbon-dioxide-hits-new-high/ (accessed on 1 November 2021).
- Lacis, A. Atmospheric CO2: The Greenhouse Thermostat. 2011. Available online: https://judithcurry.com/2011/10/09/atmospheric-co2-the-greenhouse-thermostat/ (accessed on 1 November 2021).
- Martín, A.J.; Hornés, A.; Martínez-Arias, A.; Daza, L. Chapter 15—Recent advances in fuel cells for transport and stationary applications. In Renewable Hydrogen Technologies; Gandía, L.M., Arzamendi, G., Diéguez, P.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 361–380. [Google Scholar]
- Klell, M. Storage of hydrogen in the pure form. In Handbook of Hydrogen Storage; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 1–37. [Google Scholar]
- Panella, B.; Hirscher, M. Physisorption in porous materials. In Handbook of Hydrogen Storage; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 39–62. [Google Scholar]
- Huot, J. Metal hydrides. In Handbook of Hydrogen Storage; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 81–116. [Google Scholar]
- Chen, P.; Akiba, E.; Orimo, S.; Züttel, A.; Schlapbach, L. Hydrogen Storage by Reversible Metal Hydride Formation. In Hydrogen Science and Engineering: Materials, Processes, Systems and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 763–790. [Google Scholar]
- Kuijpers, F.A.; van Mal, H.H. Sorption hysteresis in the LaNi5-H and SmCo5-H systems. J. Less-Common Met. 1971, 23, 395–398. [Google Scholar] [CrossRef]
- Blasse, G. Some considerations and experiments on concentration quenching of characteristic broad-band fluorescence. Philips Res. Repts. 1969, 23, 344. [Google Scholar]
- Sandrock, G.D. The metallurgy and production of rechargeable hydrides. In Hydrides for Energy Storage; Andresen, A.F., Maeland, A.J., Eds.; Pergamon: Bergama, Turkey, 1978; pp. 353–393. [Google Scholar]
- Dornheim, M. Thermodynamics of Metal Hydrides: Tailoring Reaction Enthalpies of Hydrogen Storage Materials. In Thermodynamics; IntechOpen: London, UK, 2011; p. 33. [Google Scholar]
- Puszkiel, J.; Garroni, S.; Milanese, C.; Gennari, F.; Klassen, T.; Dornheim, M.; Pistidda, C. Tetrahydroborates: Development and potential as hydrogen storage medium. Inorganics 2017, 5, 74. [Google Scholar] [CrossRef]
- Milanese, C.; Garroni, S.; Gennari, F.; Marini, A.; Klassen, T.; Dornheim, M.; Pistidda, C. Solid state hydrogen storage in alanates and alanate-based compounds: A review. Metals 2018, 8, 567. [Google Scholar] [CrossRef]
- Milanese, C.; Jensen, T.R.; Hauback, B.C.; Pistidda, C.; Dornheim, M.; Yang, H.; Lombardo, L.; Zuettel, A.; Filinchuk, Y.; Ngene, P.; et al. Complex hydrides for energy storage. Int. J. Hydrogen Energy 2019, 44, 7860–7874. [Google Scholar] [CrossRef]
- The Nobel Prize in Chemistry 1901, Jacobus H. van ′t Hoff Biographical. 1901. Available online: https://www.nobelprize.org/prizes/chemistry/1901/hoff/biographical/ (accessed on 1 November 2021).
- Shang, Y.; Pistidda, C.; Gizer, G.; Klassen, T.; Dornheim, M. Mg-based materials for hydrogen storage. J. Magnes. Alloy. 2021, in press. [Google Scholar] [CrossRef]
- Huot, J.; Liang, G.; Boily, S.; Van Neste, A.; Schulz, R. Structural study and hydrogen sorption kinetics of ball-milled magnesium hydride. J. Alloys Compd. 1999, 293, 495–500. [Google Scholar] [CrossRef]
- Pistidda, C.; Bergemann, N.; Wurr, J.; Rzeszutek, A.; Møller, K.T.; Hansen, B.R.S.; Garroni, S.; Horstmann, C.; Milanese, C.; Girella, A.; et al. Hydrogen storage systems from waste Mg alloys. J. Power Sources 2014, 270, 554–563. [Google Scholar] [CrossRef]
- Stander, C.M. Kinetic of formation of magnesium hydride from magnesium and hydrogen. Z. Fur Phys. Chem.-Frankf. 1977, 104, 229–238. [Google Scholar] [CrossRef]
- Stander, C.M. Kinetic of decomposition of magnesium hydride. J. Inorg. Nucl. Chem. 1977, 39, 221–223. [Google Scholar] [CrossRef]
- Vigeholm, B.; Kjoller, J.; Larsen, B.; Pedersen, A.S. Formation and decomposition of magnesium hydride. J. Less-Common Met. 1983, 89, 135–144. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Fast hydrogen sorption kinetics of nanocrystalline Mg using Nb2O5 as catalyst. Scr. Mater. 2003, 49, 213–217. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Catalytic mechanism of transition-metal compounds on Mg hydrogen sorption reaction. J. Phys. Chem. B 2006, 110, 11020–11024. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Kinetic investigation of the effect of milling time on the hydrogen sorption reaction of magnesium catalyzed with different Nb2O5 contents. J. Alloys Compd. 2006, 407, 249–255. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R.U. Effect of Nb2O5 content on hydrogen reaction kinetics of Mg. J. Alloys Compd. 2004, 364, 242–246. [Google Scholar] [CrossRef]
- Libowitz, G.G.; Hayes, H.F.; Gibb, T.R.P. The system zirconium-nickel and hydrogen. J. Phys. Chem. 1958, 62, 76–79. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Reaction of hydrogen with alloys of magnesium and copper. Inorg. Chem. 1967, 6, 2220–2223. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. The reaction of hydrogen with alloys of magnesium and nikel and formation of Mg2NiH4. Inorg. Chem. 1968, 7, 2254–2256. [Google Scholar] [CrossRef]
- Ley, M.B.; Jepsen, L.H.; Lee, Y.S.; Cho, Y.W.; von Colbe, J.M.B.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage—New perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Orimo, S.I.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef]
- Bogdanovic, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials1. J. Alloy. Compd. 1997, 253–254, 1–9. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.; Luo, J.; Lin, J.; Tan, K.L. Interaction between lithium amide and lithium hydride. J. Phys. Chem. B 2003, 107, 10967–10970. [Google Scholar] [CrossRef]
- Vajo, J.J.; Mertens, F.; Ahn, C.C.; Bowman, R.C.; Fultz, B. Altering hydrogen storage properties by hydride destabilization through alloy formation: Lih and MgH2 destabilized with Si. J. Phys. Chem. B 2004, 108, 13977–13983. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Dornheim, M.; Bormann, R. Unexpected kinetic effect of MgB2 in reactive hydride composites containing complex borohydrides. J. Alloys Compd. 2007, 440, L18–L21. [Google Scholar] [CrossRef]
- Nakamori, Y.; Miwa, K.; Ninomiya, A.; Li, H.; Ohba, N.; Towata, S.I.; Züttel, A.; Orimo, S.I. Correlation between thermodynamical stabilities of metal borohydrides and cation electronegativites: First-principles calculations and experiments. Phys. Rev. B Condens. Matter Mater. Phys. 2006, 74, 045126. [Google Scholar] [CrossRef]
- Yin, L.-C.; Wang, P.; Kang, X.-D.; Sun, C.-H.; Cheng, H.-M. Functional anion concept: Effect of fluorine anion on hydrogen storage of sodium alanate. Phys. Chem. Chem. Phys. 2007, 9, 1499–1502. [Google Scholar] [CrossRef]
- European Union (EU) and European Economic Area. Final Energy Consumption by Sector and Fuel in Europe. 2020. Available online: https://www.eea.europa.eu/data-and-maps/indicators/final-energy-consumption-by-sector-10/assessment (accessed on 1 November 2021).
- (DOE) USDoE. DOE Technical Targets for Hydrogen Delivery. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-hydrogen-delivery (accessed on 1 November 2021).
- (DOE) USDoE. DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles (accessed on 1 November 2021).
- Sandia National Laboratories Project Report, FY2005 Annual Progress Report for the DOE Hydrogen Program. 2005. Available online: http://www.hydrogen.energy.gov/annual_progress05_storage.html (accessed on 1 November 2021).
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H.W. Hydrogen—A sustainable energy carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Černý, R.; Ravnsbæk, D.B.; Filinchuk, Y.; Dornheim, M.; Besenbacher, F.; Jensen, T.R. Metal borohydrides and derivatives-synthesis, structure and properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef] [PubMed]
- Rude, L.H.; Nielsen, T.K.; Ravnsbæk, D.B.; Bösenberg, U.; Ley, M.B.; Richter, B.; Arnbjerg, L.M.; Dornheim, M.; Filinchuk, Y.; Besenbacher, F.; et al. Tailoring properties of borohydrides for hydrogen storage: A review. Phys. Status Solidi A Appl. Mater. Sci. 2011, 208, 1754–1773. [Google Scholar] [CrossRef]
- Schouwink, P.; Ley, M.B.; Tissot, A.; Hagemann, H.; Jensen, T.R.; Smrčok, L.; Černý, R. Structure and properties of complex hydride perovskite materials. Nat. Commun. 2014, 5, 5706. [Google Scholar] [CrossRef] [PubMed]
- Mosher, D.A.; Tang, X.; Brown, R.J.; Arsenault, S.; Saitta, S.; Laube, B.L.; Dold, R.H.; Anton, D.L. High Density Hydrogen Storage System Demonstration Using NaAlH4 Based Complex Compound Hydrides; United Technologies Research Center: East Hartford, CT, USA, 2007. [Google Scholar]
- Johnson, T.A.; Jorgensen, S.W.; Dedrick, D.E. Performance of a full-scale hydrogen-storage tank based on complex hydrides. Faraday Discuss. 2011, 151, 327–352. [Google Scholar] [CrossRef] [PubMed]
- Na Ranong, C.; Höhne, M.; Franzen, J.; Hapke, J.; Fieg, G.; Dornheim, M.; Eigen, N.; Bellosta von Colbe, J.M.; Metz, O. Concept, design and manufacture of a prototype hydrogen storage tank based on sodium alanate. Chem. Eng. Technol. 2009, 32, 1154–1163. [Google Scholar] [CrossRef]
- Bellosta Von Colbe, J.M.; Metz, O.; Lozano, G.A.; Pranzas, P.K.; Schmitz, H.W.; Beckmann, F.; Schreyer, A.; Klassen, T.; Dornheim, M. Behavior of scaled-up sodium alanate hydrogen storage tanks during sorption. Int. J. Hydrogen Energy 2012, 37, 2807–2811. [Google Scholar] [CrossRef]
- Bowman, R.C.; Fultz, B. Metallic hydrides I: Hydrogen storage and other gas-phase applications. MRS Bull. 2002, 27, 688–693. [Google Scholar] [CrossRef]
- Førde, T.; Næss, E.; Yartys, V.A. Modelling and experimental results of heat transfer in a metal hydride store during hydrogen charge and discharge. Int. J. Hydrogen Energy 2009, 34, 5121–5130. [Google Scholar] [CrossRef]
- Mori, D.; Hirose, K. Recent challenges of hydrogen storage technologies for fuel cell vehicles. Int. J. Hydrogen Energy 2009, 34, 4569–4574. [Google Scholar] [CrossRef]
- Shinpei, M.; Mituo, M.; Komiya, K.; Daigoro, M.; Makoto, T.; Shintaro, W.; Keiji, T.; Hidehito, K.; Seiichiro, M.; Norihiko, H.; et al. High-Pressure Hydrogen-Absorbing Alloy Tank for Fuel Cell Vehicles; SAE International: Warrendale, PA, USA, 2010. [Google Scholar]
- Mori, D.; Haraikawa, N.; Shinozawa, T.; Matsunaga, T.; Toh, K.; Fujita, K. Development of high-pressure metal hydride tank for fuel cell vehicles with Ti-Cr-V-Mo BCC alloy. Nihon Kikai Gakkai Ronbunshu B Hen/Trans. Jpn. Soc. Mech. Eng. Part B 2007, 73, 1236–1242. [Google Scholar] [CrossRef][Green Version]
- Corgnale, C.; Sulic, M. High pressure thermal hydrogen compression employing Ti1.1CrMn metal hydride material. J. Phys. Energy 2019, 2, 014003. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Yartys, V.A.; Pollet, B.G.; Bowman, R.C. Metal hydride hydrogen compressors: A review. Int. J. Hydrogen Energy 2014, 39, 5818–5851. [Google Scholar] [CrossRef]
- Papapetrou, M.; Kosmadakis, G.; Cipollina, A.; La Commare, U.; Micale, G. Industrial waste heat: Estimation of the technically available resource in the EU per industrial sector, temperature level and country. Appl. Therm. Eng. 2018, 138, 207–216. [Google Scholar] [CrossRef]
- Rönnebro, E.C.E.; Whyatt, G.; Powell, M.; Westman, M.; Zheng, F.; Fang, Z.Z. Metal hydrides for high-temperature power generation. Energies 2015, 8, 8406–8430. [Google Scholar] [CrossRef]
- Felderhoff, M.; Bogdanović, B. High temperature metal hydrides as heat storage materials for solar and related applications. Int. J. Mol. Sci. 2009, 10, 335–344. [Google Scholar] [CrossRef]
- Kamimoto, M.; Tanaka, T.; Tani, T.; Horigome, T. Investigation of nitrate salts for solar latent heat storage. Sol. Energy 1980, 24, 581–587. [Google Scholar] [CrossRef]
- Paskevicius, M.; Sheppard, D.A.; Williamson, K.; Buckley, C.E. Metal hydride thermal heat storage prototype for concentrating solar thermal power. Energy 2015, 88, 469–477. [Google Scholar] [CrossRef]
- Dornheim, M.; Eigen, N.; Barkhordarian, G.; Klassen, T.; Bormann, R. Tailoring hydrogen storage materials towards application. Adv. Eng. Mater. 2006, 8, 377–385. [Google Scholar] [CrossRef]
- Varunaa, R.; Ravindran, P. Structural phase stability in fluorinated calcium hydride. AIP Conf. Proc. 2017, 1832, 030005. [Google Scholar]
- Javadian, P.; Sheppard, D.A.; Jensen, T.R.; Buckley, C.E. Destabilization of lithium hydride and the thermodynamic assessment of the Li–Al–H system for solar thermal energy storage. RSC Adv. 2016, 6, 94927–94933. [Google Scholar] [CrossRef]
- Lai, Q.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.; Mao, J.; Huang, Z.; Liu, H.K.; et al. Hydrogen storage materials for mobile and stationary applications: Current state of the art. ChemSusChem 2015, 8, 2789–2825. [Google Scholar] [CrossRef]
- Bellosta von Colbe, J.; Ares, J.-R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Gray, E.M.; Webb, C.J.; Andrews, J.; Shabani, B.; Tsai, P.J.; Chan, S.L.I. Hydrogen storage for off-grid power supply. Int. J. Hydrogen Energy 2011, 36, 654–663. [Google Scholar] [CrossRef]
- Bielmann, M.; Vogt, U.F.; Zimmermann, M.; Züttel, A. Seasonal energy storage system based on hydrogen for self sufficient living. J. Power Sources 2011, 196, 4054–4060. [Google Scholar] [CrossRef]
- Delhomme, B.; Lanzini, A.; Ortigoza-Villalba, G.A.; Nachev, S.; De Rango, P.; Santarelli, M.; Marty, P.; Leone, P. Coupling and thermal integration of a solid oxide fuel cell with a magnesium hydride tank. Int. J. Hydrogen Energy 2013, 38, 4740–4747. [Google Scholar] [CrossRef]
- Bhogilla, S.S.; Ito, H.; Segawa, T.; Kato, A.; Nakano, A. Experimental study on laboratory scale Totalized Hydrogen Energy Utilization System using wind power data. Int. J. Hydrogen Energy 2017, 42, 13827–13838. [Google Scholar] [CrossRef]
- Weckerle, C.; Dörr, M.; Linder, M.; Bürger, I. A compact thermally driven cooling system based on metal hydrides. Energies 2020, 13, 2482. [Google Scholar] [CrossRef]
- Izhvanov, L.A.; Solovey, A.I.; Frolov, V.P.; Shanin, Y.I. Metal hydride heat pump—New type of heat converter. Int. J. Hydrogen Energy 1996, 21, 1033–1038. [Google Scholar] [CrossRef]
- Muthukumar, P.; Groll, M. Metal hydride based heating and cooling systems: A review. Int. J. Hydrogen Energy 2010, 35, 3817–3831. [Google Scholar] [CrossRef]
- Bowman, R.C., Jr. Metal hydride compressors with gas-gap heat switches: Concept, development, testing, and space flight operation for the planck sorption cryocoolers. Inorganics 2019, 7, 139. [Google Scholar] [CrossRef]
- Strumpf, H.J. Metal hydrides cooling for space applications. In Proceedings of the IV Minsk International Seminar “Heat Pipes, Heat Pumps, Refrigerators”, Minsk, Belarus, 4–7 September 2000. [Google Scholar]
- Bergemann, N.; Pistidda, C.; Milanese, C.; Girella, A.; Hansen, B.R.S.; Wurr, J.; Bellosta Von Colbe, J.M.; Jepsen, J.; Jensen, T.R.; Marini, A.; et al. NaAlH4 production from waste aluminum by reactive ball milling. Int. J. Hydrogen Energy 2014, 39, 9877–9882. [Google Scholar] [CrossRef]
- Bellosta von Colbe, J.M.; Felderhoff, M.; Bogdanovic, B.; Schuth, F.; Weidenthaler, C. One-step direct synthesis of a Ti-doped sodium alanate hydrogen storage material. Chem. Commun. 2005, 37, 4732–4734. [Google Scholar] [CrossRef]
- Hardian, R.; Pistidda, C.; Chaudhary, A.L.; Capurso, G.; Gizer, G.; Cao, H.; Milanese, C.; Girella, A.; Santoru, A.; Yigit, D.; et al. Waste Mg-Al based alloys for hydrogen storage. Int. J. Hydrogen Energy 2018, 43, 16738–16748. [Google Scholar] [CrossRef]
- Cao, H.; Pistidda, C.; Castro Riglos, M.V.; Chaudhary, A.L.; Capurso, G.; Tseng, J.C.; Puszkiel, J.; Wharmby, M.T.; Gemming, T.; Chen, P.; et al. Conversion of magnesium waste into a complex magnesium hydride system: Mg(NH2)2-LiH. Sustain. Energy Fuels 2020, 4, 1915–1923. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).