Abstract
Since the late 18th century, molecular hydrogen (H2) has been shown to be well tolerated, firstly in animals, and then in humans. However, although research into the beneficial effects of molecular hydrogen in both plant and mammalian physiology is gaining momentum, the idea of utilising this electrochemically neutral and non-polar diatomic compound for the benefit of health has yet to be widely accepted by regulatory bodies worldwide. Due to the precise mechanisms of H2 activity being as yet undefined, the lack of primary target identification, coupled with difficulties regarding administration methods (e.g., dosage and dosage frequencies, long-term effects of treatment, and the patient’s innate antioxidant profile), there is a requirement for H2 research to evidence how it can reasonably and most effectively be incorporated into medical practice. This review collates and assesses the current information regarding the many routes of molecular hydrogen administration in animals and humans, whilst evaluating how targeted delivery methods could be integrated into a modern healthcare system.
1. Introduction
It is now well documented that molecular hydrogen (H2), a low molecular-weight, non-polar, and diatomic compound, has potent cellular protective effects in both plant and in mammals [1,2]. Yet, the molecular mechanisms by which H2 exerts its effects are not well understood. What is clear is that H2 demonstrates anti-allergy, anti-apoptotic, anti-inflammatory, and antioxidant properties in animals [1,3], and in this regard has the potential to benefit both as prophylactic and remedial therapies.
Research into H2 as a medical treatment for acute and chronic conditions is gaining traction with numerous studies attesting to the safety and efficacy of this novel compound [4]. Currently, multiple delivery methods, such as ingestion, inhalation, and intravenous injection are used for hydrogen administration, all of which are likely to have distinct effects, depending on the delivery mechanism and somatic route of dispersal. These considerations posit the following question: which method or methods of H2 administration will most effectively mitigate certain diseases?
At present, individual treatments in a medical context can include intravenous application of hydrogen-rich saline (HRS), ingestion of hydrogen-rich water (HRW), and hydrogen inhalation therapy [3], either alone or in combination with oxygen (O2), in a mixture referred to as oxy–hydrogen (HHO) [5]. Topical and hyperbaric application of H2 has also been demonstrated to have salutary effects on conditions such as soft tissue injury [6], decompression sickness [7], and cancer [8]. Furthermore, as public curiosity into the benefits of H2 as an adscititious lifestyle product grows, numerous dietary supplements aim to enhance the production of H2 through microbial interactions within the gastrointestinal tract. Each method used for increasing the cellular availability of H2 will deliver different concentrations of molecular hydrogen into the cells and will have unique targets, depending on the administration route. To illustrate, ingesting HRW has beneficial effects on the gastrointestinal system [9], liver [10,11], and cerebrum [12,13]. The latter is hypothesized as being a result of gut–brain–axis communications and the upregulation of secondary messenger molecules [14]. In contrast, hydrogen inhalation therapy targets alternative tissues, since H2 is assimilated through the lung parenchyma into the bloodstream, where it is then distributed around the body. This phenomenon enables the administration of hydrogen through inhalation to be incredibly beneficial for respiratory conditions such as asthma, coronavirus infectious diseases (e.g., COVID-19), and chronic obstructive pulmonary disease (COPD) [15]. Hydrogen inhalation therapy can also benefit dysbiosis resulting from ischemia-reperfusion injury, liver disease, or metabolic disease, as examples [1]. Furthermore, research into nanoparticle delivery of H2, utilizing a biocompatible magnesium micromotor and hyaluronic acid, coated in polymeric poly (lactic-co-glycolic acid) (PLGA), offers a novel mechanism for targeted delivery of H2 [16]. Below, the authors describe and assess the methods used for administering molecular hydrogen in a medical context.
2. H2—A Novel Medical Molecule?
A Brief History of H2 as a Medical Gas
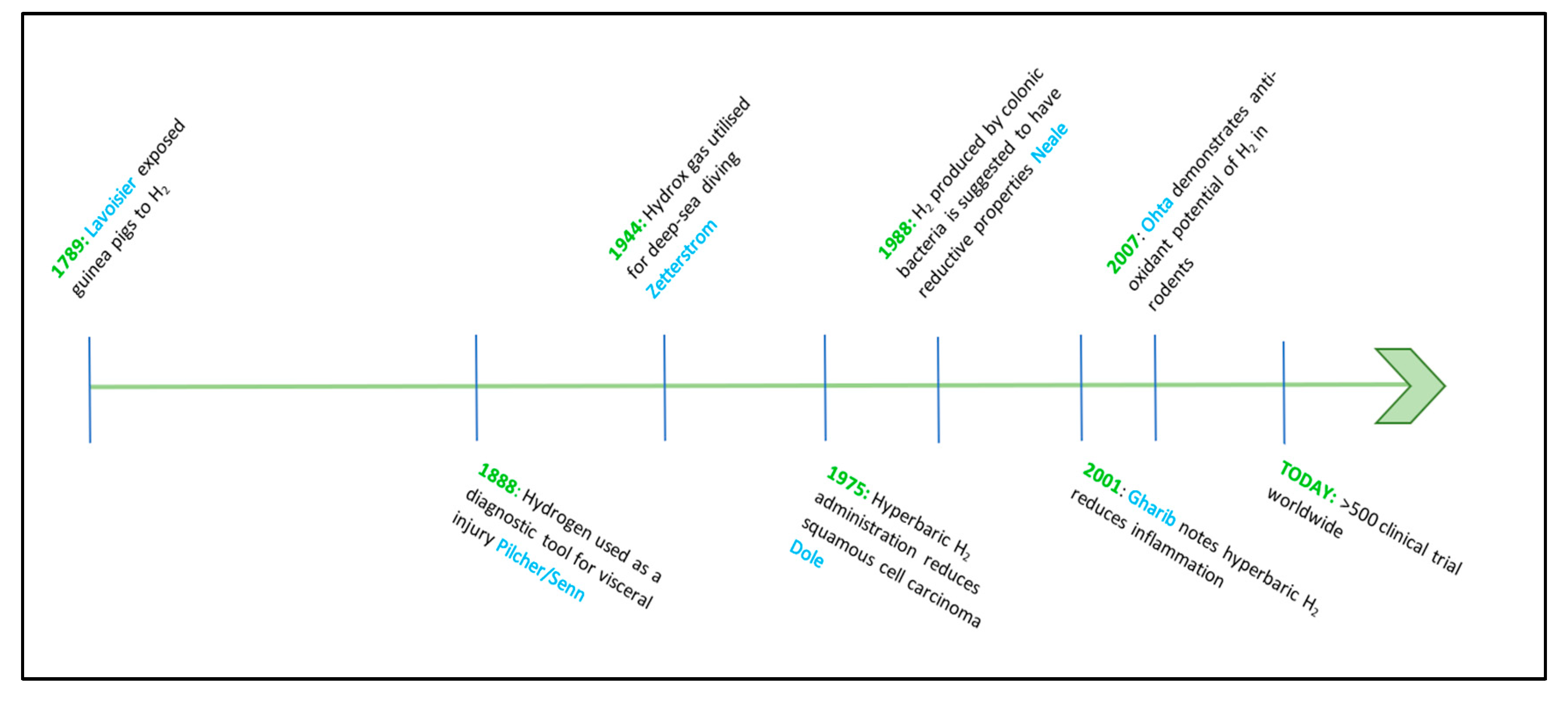
Although hydrogen therapy may seem a new phenomenon in a medical context, animal trials evaluating hydrogen as a therapeutic agent date back to 1789, when Antoine-Laurent Lavoisier exposed guinea pigs to hydrogen while investigating the properties of air [17], as shown in Figure 1. As such a newly discovered element, a further century passed until further medical experimentations with hydrogen were again documented [18]. In 1888, a flurry of reports noted that insufflation, an application where hydrogen is delivered directly into body cavities, could assist with the diagnosis of visceral injury [18,19,20,21]. However, researchers did not expand on these reports, and it would be another 150 years before hydrogen gas was considered as a therapeutic agent, when it was demonstrated that H2 inhalation could be used as a physiological recovery aide for deep-sea divers [22,23,24].
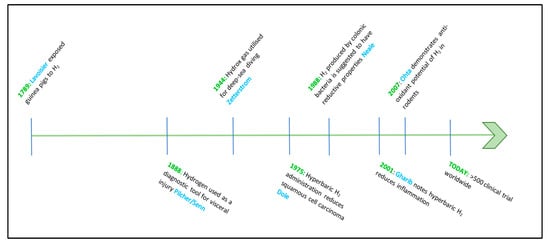
Figure 1.
Timeline of hydrogen gas used as a medical intervention. Legend: from left to right. Dates, usage, and researchers who utilized H2 for medical purposes.
In 1944, Arne Zetterstrom was credited with creating Hydrox gas, a combination of hydrogen and oxygen (96% and 4%, respectively) that allowed deep-sea divers to traverse depths of up to 500 m [22] by preventing decompression sickness. The hydrogen–oxygen mixture can be compressed into cylinders, as the low concentration of oxygen renders the composition nonexplosive, making this method of hydrogen delivery valuable, particularly for exploration, industrial use, and submarine rescue scenarios. Despite the popularity and demonstrated efficacy of hydrogen as an inhalation treatment, further advances in medical research were not put forward until 1975, when Dole and colleagues realized that hyperbaric administration (95% H2/8atm) of H2 for periods of 10–14 days could reduce squamous cell carcinoma in murine models of disease [8]. Even though this discovery was revolutionary, medical hydrogen research again subsided, and it was another thirteen years before a decisive article in this field was published [25]. Here, the authors relate H2 production of colonic bacteria as having reductive qualities within the host [26]. Building upon this research, Shirahata and colleagues describe hydrogen (atomic, or ‘active’ hydrogen) created from the electrolysis of water, could protect deoxyribose nucleic acid (DNA) from oxidative damage [6]. A few years later, in 2001, and building upon the research conducted by Dole et al. [8], Gharib and associates [27] described a reduction in markers of liver disease resulting from chronic inflammation when utilizing hyperbaric administration of H2 (0.7 MPa/two weeks).
Once again, however, research into medical hydrogen lapsed and was not revived until 2007, when Ohsawa’s laboratory in the Department of Biochemistry and Cell Biology, Nippon Medical School, Japan, reported an antioxidant effect of H2 in a rodent model of ischemia-reperfusion injury [28], as shown in Figure 1. Since Ohsawa’s discovery, there has been renewed interest in the effects of H2 as an Aesculapian gas and research into this promising area of medicine is developing rapidly.
3. The H2 Hypothesis
3.1. Mechanisms of Action
It is well reported that H2 has significant effects on the redox status of the cell, with many investigations citing meaningful reductions in destructive oxidant and nitrosative species, including the hydroxyl radical (·OH) and peroxynitrite (ONOO−) [26,28,29]. Despite H2 administration being demonstrated to reduce the cellular damage caused by oxidative stress in vivo [30], further analyses of the biological kinetics of intracellular reactions between ·OH/ONOO− and H2 indicate that this may not be the case [31,32]. Instead, it has been mooted that an upstream mechanism (such as reduction of metalloproteins e.g., Fe3+/Fe2+) of radical production is more likely to be a primary target for H2 interaction [33]. Although, further empirical enquiry is required if such a hypothesis is to be validated and the precise mechanisms of H2 activity are to be fully elucidated.
In addition to the direct or upstream (or both) reduction of pernicious ions and radicals, H2 exhibits further antioxidant potential as a regulator of nuclear factor erythroid 2-related factor 2 (Nrf-2). Nrf-2 is a transcription factor known to initiate genetic transcription of >200 cytoprotective genes containing the promotor sequence 5′-TGACNNNGC-3′, known as the antioxidant response element (ARE) [34]. Genes initiated by ARE activation include a suite of antioxidant enzymes, including catalase (CAT), glutathione (GSH), superoxide dismutase (SOD), and thioredoxin (Thx) [35].
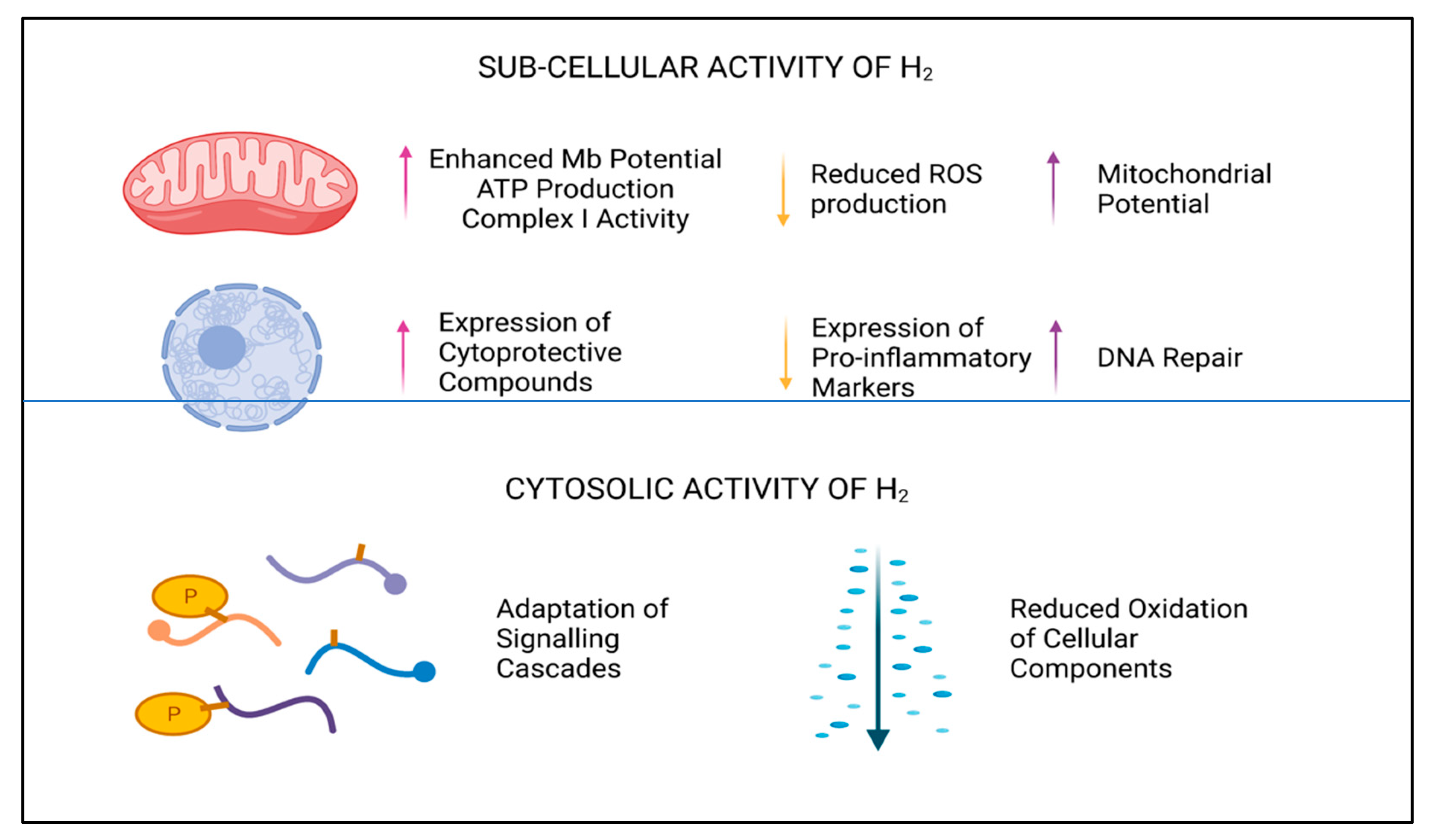
Major contributary sources of cellular ROS are Complexes I and III of the mitochondrial electron transport chain (ETC). Superoxide (O2−), produced here by single-electron reduction of O2, is the antecessor molecule for both deleterious ions and radicals, but also for gaseous signaling molecules, such as hydrogen peroxide (H2O2) [36]. A recent study, conducted by Ishihara et al., noted that aberrant electron flow through the ETC was rectified by H2, and this in turn suppressed generation of O2− in Complex I [37]. Concomitant reduction in semiquinone radicals was also demonstrated to reduce levels of O2− downstream in Complex III, which resulted in stabilization of mitochondrial membrane potential [37]. A 2% inhalation of H2 in murine models was shown to enhance mitochondrial membrane potential and to increase Complex I activity and subsequent ATP production. Furthermore, enhanced expression of biological markers associated with mitochondrial biogenesis (e.g., Nrf-2, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), and mitochondrial transcription factor A (Tfa)) was also documented [38]. Thus, this suggests H2 exhibits pleiotropic activity at the subcellular level, as shown in Figure 2.
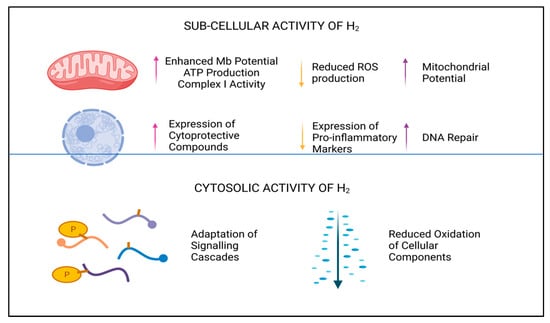
Figure 2.
Subcellular and cytosolic activity of H2. Legend: Graphical overview of the cellular and subcellular influence H2 has within cells.
In addition to the roles H2 has in remediating the cellular redox environ and enhancing mitochondrial stability, H2 is also known to support the immune system via downregulating genetic expression of pro-inflammatory molecules, known to exacerbate the inflammatory response [2,3]. Coetaneous upregulation of such anti-inflammatory proteins as heme oxygenase-1 (HO-1) and Nrf-2 [3] further demonstrates that H2 has multiple intracellular effects, but the explicit mechanisms by which these effects are induced is largely unknown. Antecedent research demonstrates a reduction in nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα) phosphorylation [39], in the presence of H2, and subsequent inhibition of the protein complex nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB). The NF-kB family of proteins have similar homology and share a 300 amino acid N-terminal DNA binding or dimerization domain and are responsible for initiating DNA transcription of inflammatory cytokines, chemokines, and adhesion proteins [35]. By identifying an upstream event of NF-kB activation, which is inhibited by H2, it is logical to assume that H2 does not directly interact with such proteins. Although, the question as to whether such anti-inflammatory mechanisms are a direct result of H2 interactions with cellular signaling events, such as protein phosphorylation, or through direct modulation of oxidative stress, remains.
3.2. Effects on Human Physiology
Oxidative stress, metabolic dysregulation, and inflammation are known to underpin numerous disease pathologies, including acute and chronic respiratory disorders (e.g., COVID-19, COPD, respectively) [5], cancer, metabolic diseases, and neurodegeneration [8,9,10,11,12,13]. It is generally accepted that H2 selectively reduces oxidative and nitrosative stress in biological systems and reduces both the apoptotic and inflammatory responses in cells, although which system has the cardinal influence here is currently unknown. Due to the physical non-polarity, electrochemical neutrality, and low molecular weight of this diatomic molecule, it is unlikely to be perceived as a typical signaling molecule [40], relying on receptor binding mechanisms or electrochemical attraction for modulating cellular responses. However, what is clear is that H2 has remarkable potential as an antioxidant and cellular protective compound, not only reducing accumulative damage caused by reactive signaling molecules [3,41], but also by upregulating endogenous production of redox-balancing proteins, including catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) [42]. In addition to—or perhaps as a result of—the antioxidant influence of H2, a marked reduction in the expression of intrinsic pro-apoptotic and pro-inflammatory proteins, peptides, and transcription factors is also described [41,42]. Downregulation of pro-apoptotic biomarkers are noted to include Bax and caspase proteins [43], whilst inhibition of pro-inflammatory markers interleukins (e.g., IL-6), NF-kB, and tumor necrosis factor alpha (TNFα), and others, have also been observed [42,43]. By moderating the hyper-inflammatory response during infection or disease, H2 can reduce both cellular and tissue damage and subsequent harm associated with aberrant inflammation.
Considering the intricate crosstalk between these intrinsic defense systems, and their role in disease pathology, the modulative effect demonstrated by H2 in these fundamental pathways [29,41,42,43] may help to both prevent and ameliorate the symptoms of numerous healthcare conditions. Furthermore, to date, there are no reports, clinical or empirical, that suggest H2 acts as a strong cellular reductive agent, a mechanism typically initiated by prolonged antioxidant signaling or mitochondrial inactivity. Instead, H2 is likely to act as an hormetic substance, ensuring the redox balance is kept intact [44,45].
The effectiveness of H2 as a novel therapeutic, however, may largely depend on the duration and route of administration, along with the dosage received by the patient. In this regard, both hydrogen inhalation (HRW) and intravenous injection (HRS) can provide monitorable and quantifiable means of treatment, which is favorable when considering future clinical applications of H2 [2]. Alternatively, gels, patches, nanotechnologies, and topical applications may only afford semi-quantifiable results due to the rates of H2 absorption and consequent distribution dynamics, for example. As such, the semi-quantifiable application methods may only be viable as non-clinical interventions until further research is conducted.
Overall, H2, to the best of our knowledge, has an excellent safety profile and is well tolerated in humans, with no severe adverse effects associated with hydrogen therapy having been reported [4], however it is delivered.
4. H2—Methods of Administration
4.1. Ingestion
4.1.1. Dietary Supplements
Of late, there has been growing interest in products that act as prebiotic substrates for H2-producing bacteria [46], particularly Bacteroides and Firmicute species [47]. For example, turmeric, the root of the Curcumina Longa L. perennial, has long been utilized as a remedy for gastrointestinal ailments and inflammatory conditions [48], with many reports now suggesting turmeric can modulate the composition of the intestinal microflora for the benefit of the host [49]. Initial research suggests the beneficial effect of turmeric may be through microbial efflux of H2, as a result of turmeric fermentation [50]. Alongside traditional medical remedies, such as turmeric, and its bioactive degradation molecules (e.g., curcumin), increasing the daily intake of dietary fiber and inulin have also been shown to positively affect the microbial balance by providing non-absorbable carbohydrate substrates for metabolic activity of H2-producing species [48].
In parallel with prebiotic supplementation, ingestible capsules containing a mixture of calcium (Ca2+), magnesium (Mg2+), plant-based antioxidants, and trace minerals are readily available without prescription. Again, scientific verification of the concentrations produced, molecular effects, and long-term efficacy of such supplements is sparse. To illustrate, a double-blind, placebo-controlled, cross-over pilot trial [11] showed a four-week regime of one capsule a day was effective in reducing both serum triglyceride and fasting insulin levels in a small cohort of middle-aged women; although, the concentration of H2 produced was not determined. Supporting these data, a more recent analysis of the effect of orally administered H2-inducing calcium-rich powder [51] demonstrated that one 1500 mg capsule per day produced 2.5 µg of H2, improving pulmonary gas exchange and increasing both hemoglobin and myoglobin saturation of O2.
By encouraging the natural balance of intestinal microflora in favor of hydrogen-producing species, this method of H2 ingestion, in particular, could act as a prophylaxis against chronic and costly ailments, such as cardiovascular disease, systemic inflammation, and type 2 diabetes. If dietary supplementation should prove to be a cost-effective and sustainable way of enhancing endogenous H2 production, it could have a significant role to play in the future of nutritional education and corresponding healthcare recommendations.
4.1.2. Hydrogen-Rich Water (HRW)
Much of the clinical research to date has focused on the effects of HRW on oxidative stress in conditions ranging from age-related diseases to exercise fatigue [52,53,54,55]. HRW is versatile option for the treatment of many ailments, as it is portable and can be produced in a number of ways. For example, H2 can be created through the reaction with water (H2O) and magnesium (Mg), shown below:
Mg (s) + 2H2O (aq) → Mg(OH)2 (aq) + H2 (g)
The produced gas then diffuses and dissolves into the surrounding water, until a saturation point of ~1.6 ppm is reached. Creation of HRW using this method is commercially popular as the portability of either magnesium-containing tablets or hydrogen-generating water flasks provide an accessible form for regular H2 intake. H2 can also be infused into an aqueous solution under pressure through utilizing a diffusion stone attached to a hydrogen-generation system, described in the inhalation section below.
Research into the effect of HRW as a medicament is well established although, to date, there is limited registered clinical trial data assessing the effect of ingesting HRW for medical conditions. Empirical data suggests that ingestion of HRW can mitigate cellular damage by acting as an anti-inflammatory and antioxidant agent [28,56,57], although the primary mechanisms of action have yet to be delineated. Providing evidence of the idiopathic functions of H2 would strengthen the assertion that HRW should be regarded as a safe and effective product for medical use. Another factor that likely adds to the reticence of regulatory bodies’ endorsement of H2 is that the conversation over the analeptic dosing of HRW for specific conditions is ongoing. Although a minimal dosage of 0.8–0.9 mMl ingested per day has been determined to provide antioxidant effects [58,59], there is a lack of standardized treatment dosages and protocols. Nevertheless, when analyzing the results of contemporary research, the cytoprotective and wider systemic effects of HRW ingestion are intriguing and warrant large-scale and long-term clinical investigations.
Due to the transmission route of H2 through ingestion of HRW, the most pronounced effects have been described in conditions that affect the gastrointestinal tract and supporting organs, including such diseases as gastroesophageal reflux disease (GERD) [60], irritable bowel disease (IBD) [61], and metabolic syndrome [2]. In a 12 week clinical study involving 84 patients with GERD, electrolyzed-reduced water (which produces hydrogen-enriched water with a pH above 7), containing an unquantified amount of dissolved hydrogen, reduced the levels of biological markers pertaining to oxidative stress (e.g., malondialdehyde (MDA), nitric oxide (NO), and superoxide anion (O2−)) [60]. Despite the concentration of hydrogen not having been recorded, the authors concluded that symptoms of GERD can be reduced rapidly via ingestion of HRW, improving the overall quality of life. In an 8 week open-label pilot study, ingestion of 1.5 L of H2 (55–65 mM) was shown to increase endogenous levels of SOD and reduce the excreted levels of MDA in patients with metabolic syndrome [62], a condition that shares pathophysiological similarities with GERD [63].
There is also a breadth of pre-clinical research that supports the idea of HRW having salutary effects in the lower gastrointestinal tract. For example, an empirical study assessing the effect of hydrogen on the peristaltic movement of the intestinal tract, a reduction in the strength of muscular contraction was observed, signifying reduced colonic transit [64]. These findings are further supported in murine models [65] where ingestion of HRW (5 mL/>1.5 ppm/Kg body weight) twice daily abated colonic wall inflammation, thereby reducing disease severity. Although such experimentations have yet to be expanded into the human population, the anti-inflammatory and antioxidant properties of HRW are well-described [29,30] and are likely to provide benefits for a range of gastrointestinal and inflammatory-related conditions.
In addition to HRW treatment for illness, numerous studies have analyzed the post-exercise effects of drinking HRW when it is consumed before physical activity [42,55,66], with such studies describing a marked increase in antioxidant activity [30], reduction in lactic acid accumulation [66], and improved ventilatory capacity [54] in training athletes. Daily intake of HRW has been shown to support post-exercise recovery, reduce ROS-induced inflammation, and both recover and prevent muscle damage [54]. Inhalation of hydrogen gas in training athletes has also been investigated and is reviewed in the inhalation section below.
4.2. Inhalation—H2 and HHO
Hydrogen inhalation therapies can be a simple, effective, and portable means of administration, with several products having been designed to produce H2-containing gas through the electrolysis of water. Inhalation is a non-evasive mode of hydrogen delivery and can be adapted for use through nebulizers, nasal cannulas, or masks that cover the mouth and nose [5].
Generation of H2 and O2 in the ratio of 2:1 can be achieved by running a low-voltage current through water containing an electrocatalyst, such as potassium hydroxide (KOH) [5], where H2 is released at the cathode and O2 at the anode. These gases can either be separated and delivered individually (H2/O2) or inhaled as a combined gaseous mixture: oxy–hydrogen (HHO). Both H2 and HHO inhalation therapies are evidenced as having positive effects in the respiratory and cardiovascular systems [67,68], likely due to the close correlation between the application method and such tissues. The non-invasive inhalation delivery mechanisms are an efficient and effective way of providing H2 systemically and can be used for treating acute and chronic conditions alike.
Ongoing studies are evaluating the responses to hydrogen inhalation therapies in patients with severe symptoms of coronavirus infectious disease (e.g., COVID-19) [4], with preliminary data reporting subjugation of dyspnea and easement of respiratory symptoms [69]. There is also evidence suggesting H2 inhalation could be advantageous in alleviating such long-term inflammatory-related lung conditions as asthma [70] and chronic obstructive pulmonary disorder (COPD) [71]. Here, H2 has been demonstrated to attenuate the overexpression of such pro-inflammatory mediators as IL-6, NF-kB, and TNFα. Furthermore, the upregulation of both the expression and activity of cytoprotective peptides and enzymes including CAT, GSH, Nrf-2, and SOD are also observed [3,15]. Consequently, it can be surmised that patients with emphysema, cystic fibrosis, and pulmonary adenoma may prosper from hydrogen inhalation therapy, these diseases having similar etiologies.
Clinical data involving human research on the absorption and distribution of H2, specifically through inhalation, is sparse. Nevertheless, a seminal study of rodent models [72] noted that 3% continuous inhalation of H2 resulted in significant variation in the distribution of hydrogen, shown in Table 1, favoring accumulation in the liver, brain, and mesentery adipocytes. Regrettably, the study does not cover the cardiovascular and pulmonary tissues, which are most likely to be the immediate targets of inhalation therapy.

Table 1.
Distribution of molecular hydrogen in visceral organs of male Sprague–Dawley rats [72].
Adding to the information in Table 1, data derived from a porcine model noted that 3 min after hydrogen inhalation, H2 concentrations are higher in the venous system than in arterial blood, suggesting that H2 is transported throughout the body in a blood flow-dependent manner [73]. In the liver, noted in Table 1 as having the highest concentration of H2 at 29 µmol/L, glycogen may act as a hydrogen storage molecule, retaining high quantities of H2 in vivo [74], speculated to be a result of carbohydrate polymers having the capacity to maintain H2 [75]. It is possible that, due to the capacity of glycogen to retain H2 in the liver, H2 activity is prolonged in the hepatic system, which could explain why this distinct tissue is particularly responsive to H2 administration. In mammalian models of non-alcoholic fatty liver disease, treatment with 4% and 67% hydrogen inhalation was shown to reduce lipid synthesis and deposition and serum levels of alanine transaminase (ALT), aspartate transaminase (AST), and lactate dehydrogenase (LDH). High doses of H2 (67%) were defined as having consequential effects on lipid deposition, whilst low concentration (4%) effects were more prominent in the liver enzyme profiling [76].
It is not only the pulmonary and hepatic systems, however, that are likely to be primary targets for H2 delivered through inhalation. In animals, attenuation of LPS-derived sepsis [77,78] has also been demonstrated as a result of H2-inhalation. Here, H2 is demonstrated to subdue the acute inflammatory response that results from blood-borne infection [79]. Further to systemic infection, there is an emerging body of evidence describing the favorable effects H2 inhalation has on the wider cardiovascular system [53,80,81,82]. Early reports demonstrate that 1.3% hydrogen inhalation for patients with ST-elevation myocardial infarction (STEMI) restored left ventricular modelling 6 months post-administration [83]. Intraoperative inhalation of H2 was shown to decrease biological markers of lipid peroxidation, diene and tiene conjugates, and Schiff bases, an effect most pronounced 1 day post-surgery [84]. Herein, the authors suggest that molecular hydrogen can be used in cardiac surgery as an effective and safe antioxidant [80]. In models of chronic heart failure, H2 inhalation was demonstrated to markedly reduce apoptosis and oxidative damage in cardiomyocytes [81], whilst H2 can stimulate cholesterol efflux from foam cells in the cardiovascular system [85], thereby reducing the mortality risks associated with atherosclerosis and cardiovascular disease.
Interest in hydrogen inhalation therapy within the sports industry is increasing rapidly due to the cytoprotective and holistic characteristics that therapeutic hydrogen can offer. Here, clinical studies have revealed that breathing 1% H2 for 20 min whilst exercising amplifies breath–acetate concentrations, a non-invasive marker of lipid metabolism, indicating that this application method promotes favorable lipid metabolism [86]. Similarly, 4% H2 inhalation for 7 days improved running velocity and reduced levels of insulin-like growth factor-1 (IGF-1), a regulator of glucose transportation, in the bloodstream [87]. Significant reductions in the pro-inflammatory marker C-reactive protein, and ferritin, an acute phase inflammatory reactant, were also observed [87], congruent with the hypothesis that H2 can positively influence the innate immune response. Furthermore, decreases in inflammatory biomarkers (TNF-α, IL-1β, IL-6) and markers of apoptosis (caspase-3, Bcl-2, and HSP70), and increases in SOD activity were recorded in models of acute exercise-induced fatigue [39].
As increasing oxidative stress is detrimental to cellular and wider physiological health, with all the conditions described above being greatly influenced by aberrant redox profiles [88], further large-scale studies are warranted if H2 inhalation therapy is to be appropriated by medical authorities.
4.3. Infusion
Hydrogen-Rich Saline
The use of hydrogen generators may be impractical in a nosocomial environment due to safety and storage concerns. Instead, hydrogen-rich saline (HRS) is proposed for use in a clinical setting for post-operative care and the treatment of ischemia-reperfusion, liver disease, and organ grafting, among other procedures [89]. HRS could prove to be a practical method of hydrogen administration, as use of saline suspensions is commonplace within the hospital environ. An early study conducted by Japanese scientists suggests that submersion of saline bags into a hydrogen-rich bath for a period of three days can produce a saturated solution (0.8 mM/1.6 ppm) that can then be dispensed in the same way as saline [89]. Alternatively, direct infusion of H2 into the media would be more time-effective but requires pressurization of hydrogen gas (0.4–1.5 MPa) [89,90], which could lead to the necessity for enhanced safety protocols. Currently, storage of HRS at atmospheric pressure is only recommended for seven days [90]. Therefore, it is likely that investment is needed in manual training, specialized equipment, and storage facilities, and the cost-effectiveness of such implementation will need to be assessed before the use of HRS can be adopted.
There is limited evidence for the effect of HRS in humans, although a pivotal study has demonstrated efficacy in reducing serum levels of the pro-inflammatory marker IL-6, and the pro-oxidative marker 8-OHdG, in patients with rheumatoid arthritis (n = 24) [91]. A supporting body of data, orientated around animal studies, describes infusion of HRS through the peritoneal cavity reducing the damage caused by surging ROS, which is associated with ischemia-reperfusion, and was noted to be particularly effective in the brain and in the liver [91]. Added to this, initial reports investigating the effects of H2 treating pre-transplantation liver segments in an organ bath containing HRS suggest this distinct method can reduce apoptosis, neutrophil infiltration, and oxidative damage in both the liver and proximal tissues [92]. Similar findings are found when >1.5 ppm (H2) HRS is infused during post-operative surgical closure of the ileum in rodents [93]. In porcine models, HRS lavage for post-operational peritonitis was shown to ameliorate sepsis-induced organ failure [94], a factor attributed to the reduction in levels of the oxidative biomarker 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-OHdG), excreted in the urine.
Considering the breadth of molecular hydrogen research and the scope for improved patient outcomes with the widespread use of HRS, future endeavors will have to address the practicalities of creating and storing HRS in the nosocomial environment.
4.4. Nanotechnology
The production and storage of hydrogen is a rapidly expanding area of interest for the energy sector as hydrogen is mooted to be an alternative to the burning of fossil fuels [95]. In this regard, nano- or micro-technologies that can retain and store hydrogen whilst mitigating the risk of explosion may significantly reduce the carbon emissions associated with industrial pollution. Having been developed for the retention of H2 as a fuel source [95], the low weight, low cost, and high hydrogen binding capacity of such technologies has now been adopted for medical research [16].
Nanotechnologies are rapidly becoming of interest for pharmaceutical purposes, as they can provide a means of localized delivery for drug administration that is largely regarded as safe and effective [96]. Here, biologically degradable substances such as magnesium (Mg) and palladium (Pd) are efficacious in producing and storing H2, respectively, and have been refined to treat inflammatory-related conditions such as cancer [97], rheumatoid arthritis [16], and neurodegenerative diseases [98]. Nano- and micro-technologies can be broadly categorized into two groups—nanodevices and nanoparticles—each with distinct mechanisms that can deliver H2 within living systems.
4.4.1. Nanodevices—Mg/Hyaluronic Acid
Biodegradable magnesium-based micromotors can convert external stimuli into propulsion as a result of the Mg/H2O reaction described previously. As Mg is known to degrade rapidly, the addition of hyaluronic acid, coated in a PLGA substance, has been developed [95], offering the benefit of direct and observable administration of molecular hydrogen when used in parallel with ultrasound visualization. Although promising results have been demonstrated in rodent models of disease [16,97], these devices have yet to be tested in humans, and the toxicity of potential biproducts has not been evaluated. However, the potential for this nanotechnology is intriguing, and further research in this area could revolutionize the way therapeutic drugs and compounds are introduced into mammalian systems.
4.4.2. Nanoparticles—Palladium Hydride (PdH)
Nanoparticles differ from nanodevices in that their crystalline structures contain a large surface area by design and can therefore adsorb relatively large quantities of substances intended for medical distribution. Research shows that the use of nanoparticles can be an effective strategy for transporting drugs as targeted treatments in models of Alzheimer’s disease [98] and cancer [97]. Co-ordinately, for diagnostic purposes, nanoparticles can be designed with specific quantum properties known to improve the quality of both optical and magnetic resonance imaging [99].
Palladium (Pd) is well known for its high binding affinity with hydrogen, adsorbing large amounts of hydrogen under environmental conditions. Synthesis of palladium hydride (PdH) particles utilizes a facile one-step reduction route when forming particles [100]. This chemistry is useful in medicine as it allows for structural lattice formations to either be laden with H2 for therapeutic use, or as a resource for the detection and purification of H2, as posited by Zhan et al., [101]. Once the nanoparticles have been produced, H2 can be infused into the aqueous storage solution, imbibing the structures with molecular hydrogen, which can then be stored for future use.
PdH nanoparticles are self-catalytic, and therefore generate biproducts as they decompose. Such activity will need to be considered if analogous technologies are to be widely used in a clinical context. Nevertheless, PdH particles have been demonstrated to be well tolerated in biological systems and have the capacity to be loaded with H2, resulting in absorption of H2 in the range of 2.46 × 10−19 mol per Pd particle [100]. This method effectively stores the H2 molecules through weak hydrogen bonding until degradation of the complex releases the H2 load. Another benefit of adopting PdH particles for H2 delivery is that Pd has alkyne hydrogenation properties that support prolonged hydrogen release into the localized tissue [102]. This mechanism of hydrogen therapy has the potential for specific application in neurodegenerative conditions, where delivery of H2 across the blood–brain barrier can reduce oxidative stress in neurons, thereby affording protection against excessive inflammation and cellular apoptosis. However, such technologies are rarely used as a clinical tool and, as yet, are not produced in sufficient quantities for regular use in a medical context.
4.5. Topical Administration—Bath, Gels, and Patches
Molecular hydrogen can be easily administered through the skin, either as an addition to bathing waters, as an absorbable gel, or contained within patches designed for gradual release of H2 over time. The growing interest in medical H2 research has inspired a flurry of patented designs for topical products [103,104,105]. Innovative gels and patches may overcome the hesitations surrounding accurate dosing by providing semi-quantifiable measurements including delivery timings and dosages, making them a good candidate for the treatment of wounds. However, as these materials have yet to be widely tested, it may be some time before such products can be assessed for efficacy.
Research into the effects of bathing in a hydrogen-rich solution is more established and suggests the antioxidant properties of H2 can substantially reduce disease severity in inflammatory-related skin conditions such as psoriasis. Early investigations assessing the effect of H2-infused bathing water on mice purposefully exposed to UVB radiation reported a marked decline in burn severity, coupled with a reduction in pro-inflammatory markers IL-1β and TNFα, and an increase in the anti-inflammatory cytokine IL-10 [106]. Building on this research, an 8 week parallel-controlled trial (n = 74) where human patients with psoriasis or parapsoriasis en plaques were treated twice weekly with a 10–15 min hydrogen bath containing 1.0 ppm H2, which effected a pronounced reduction in rubor and pruritus in 80% of H2-treated patients [107]. Furthermore, a 7 day case study evaluating the effect of H2 immersion therapy in waters containing 8.0 ppm on traumatic injuries sustained to the proximal phalanges of the 5th toe [108] saw a rapid reduction in pain and inflammation, whilst improvements in range of motion and ability to bear weight were also noted.
The above evidence illustrates the propitious effects of H2-hydrotherapy for a range of topical inflammatory conditions where conventional synthetic drug treatments have well-known side effects [107,109]. Adopting an H2 strategy for such treatments could provide a cost-effective and non-invasive alternative to steroid management of inflammatory skin disease.
5. Future Perspectives
5.1. Mitigating the Risks
H2 gas is flammable at temperatures in excess of 527 °C and is known to explode in a rapid chain reaction with O2 [110]. According to contemporary reports, H2 should not exceed 4.6% in air and 4.1% by volume in pure oxygen gas [111]. Hydrogen, as a pure gas, is an explosion risk and therefore it is not safe, or practical, to store such a volatile gas in the clinical environment. To reduce the hazards associated with inhalation of such a combustible material, administrable concentrations of H2 should not exceed one third of the lower explosion limit. However, as therapeutic effects are demonstrated with administration of 2% H2 [112], this reduces the necessity for high-risk containment. When considering application of H2 and HHO inhalation therapies that utilize the electrolysis of water, the mixture of gases is consumed upon generation [1] and production discontinues when the system is not in use. This safety feature negates the requirement for precarious storage of gaseous compounds, ensuring the safety of both patient and healthcare workers. Furthermore, such hydrogen-generation devices are uncomplicated to use, requiring little training or maintenance, and are a sustainable and ecological means of providing therapeutic hydrogen.
Research into new methods of hydrogen delivery such as H2-containing gels, HRW, and nanomaterials may also provide a safe and effective way to clinically administer H2; however, much more research is necessary before such novel means of delivery can be assessed. Therefore, continued research into which hydrogen-delivery mechanisms are best suited for individual circumstances will be required if widespread medical use of H2 is to be realized in the future.
5.2. Research Requirements
5.2.1. Clinical
To date, molecular hydrogen treatments have shown no toxicity in animals or humans [40], whilst it has also been suggested that H2 does not accumulate in blood or tissues and that excesses are liberated in the breath [113]. Nevertheless, the positive effects of H2 administration are noticed long after H2 has been eliminated and warrant long-term clinical observations. Additionally, as H2 is demonstrated to be an effective anti-inflammatory and antioxidant compound [2,28], there is potential for hydrogen therapies to be of benefit in a range of diseases, encompassing many bodily systems. Therefore, it is imperative that clinical data considers the not only the delivery mechanism for treating specific conditions, but the distribution, dosage, and duration of such treatments that are effective. Standardized measurements of hydrogen content and dosage limits will need to be established, conceivably, before large-scale Phase III trials can take place.
5.2.2. Empirical
It is evident that many aspects need to be considered before H2 can be considered a prescriptible therapy by health regulatory agencies. To illustrate, unidentified factors of hydrogen biochemistry such as diffusion rates, reactivity, and magnetic spin may have effects. There is also a need to identify the primary target, or targets, of H2 interactions, so that the molecular mechanisms can be identified [114]. Such work would support clinical data and could indicate whether there are likely to be any long-term or detrimental effects to hydrogen therapies.
Pioneering research strongly suggests H2 can reduce oxidative damage caused by surgical intervention, such as ischemia-reperfusion or through injury or disease [2,6,28,29]. However, whether this is due to the direct reductive potential of H2, or upstream of ROS and reactive nitrogen species (RNS) production, has yet to be characterized in vivo, and further molecular research is required.
5.2.3. Financial
It is not only the efficacy of hydrogen therapies that must be scrutinized, but the cost-effectiveness, sustainability, and long-term benefits of treatment must also be assessed. H2 generation through electrolysis of water uses 0.05 MWh of electricity to produce 1 kg of hydrogen. At a rate of ¢5/kWh electricity, the cost of hydrogen production through electrolysis systems is likely to range between $2.5 USD and $3.5 per kg (H2) [115]. However, the purchase and maintenance costs, along with assessment of the durability and longevity of commercial units versus conventional treatments of disease, will also need to be considered.
6. Conclusions
To conclude, molecular hydrogen has an excellent profile as an anti-apoptotic, anti-inflammatory and antioxidant agent [2,3,56,65], with properties that can be exploited for an array of human ailments. The variety of delivery mechanisms available allow for the use of H2 in numerous scenarios, ranging from everyday dietary supplementation, to both chronic and acute nosocomial care. Furthermore, progressive innovations, including inhalation devices, patches, and tablets provide a convenient and portable method of hydrogen delivery that can be easily administered and therefore has great potential for use in chronic, emergency, and convalescent care.
Author Contributions
Conceptualization, graphical content, writing—original draft preparation, G.R.; writing—review and editing, A.N., H.K. and J.T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-funded by Water Fuel Engineering and the University of the West of England. Funding identification number 7096050. Project code: RDAS0184.
Conflicts of Interest
A. Nenov is a board member of Water Fuel Engineering. The remaining authors have declared no conflict of interest.
Abbreviations
| Alanine transaminase | ALT |
| Aspartate Transaminase | AST |
| Catalase | CAT |
| Chronic Obstructive Pulmonary Disease | COPD |
| Coronavirus Infectious Disease | COVID-19 |
| Gastroesophageal Reflux Disease | GERD |
| Glutathione Peroxidase | GPx |
| Hydrogen-Rich Saline | HRS |
| Hydrogen-Rich Water | HRW |
| Insulin-like growth factor-1 | IGF-1 |
| Irritable Bowel Disease | IBD |
| Lactate Dehydrogenase | LDH |
| Lipopolysaccharide | LPS |
| Malondialdehyde | (MDA) |
| Nuclear factor erythroid 2-related factor 2 | NRF2 |
| Nuclear factor kappa-light-chain-enhancer of activated B cells | NF-kB |
| Oxy–hydrogen | HHO |
| Palladium-hydride | PdH |
| Polymeric poly (lactic-co-glycolic acid) | PLGA |
| Reactive Oxygen Species | ROS |
| Superoxide Dismutase | (SOD) |
References
- Russell, G.; Zulfiqar, F.; Hancock, J.T. Hydrogenases and the role of molecular hydrogen in plants. Plants 2020, 9, 1136. [Google Scholar] [CrossRef]
- Iida, A.; Nosaka, N.; Yumoto, T.; Knaup, E.; Naito, H.; Nishiyama, C.; Yamakawa, Y.; Tsukahara, K.; Terado, M.; Sato, K.; et al. The clinical application of hydrogen as a medical treatment. Acta Med. Okayama 2016, 70, 331–337. [Google Scholar] [PubMed]
- Ge, L.; Yang, M.; Yang, N.N.; Yin, X.X.; Song, W.G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653. [Google Scholar] [CrossRef] [PubMed]
- Safety of Inhaled Hydrogen Gas Mixtures in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=molecular+hydrogen&cntry=&state=&city=&dist (accessed on 5 July 2021).
- Russell, G.; Nenov, A.; Hancock, J.T. Oxy-hydrogen Gas: The Rationale behind Its Use as a Novel and Sustainable Treatment for COVID-19 and Other Respiratory Diseases. Eur. Med. J. 2021, 21-00027. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Vukomanovic, B.; Calleja-Gonzalez, J.; Hoffman, J.R. Effectiveness of oral and topical hydrogen for sports-related soft tissue injuries. Postgrad. Med. 2014, 126, 188–196. [Google Scholar] [CrossRef]
- Abraini, J.H.; Ansseau, M.; Bisson, T.; de Mendoza, J.L.J.; Therme, P. Personality patterns of anxiety during occupational deep dives with long-term confinement in hyperbaric chamber. J. Clin. Psychol. 1998, 54, 825–830. [Google Scholar] [CrossRef]
- Dole, M.; Wilson, F.R.; Fife, W.P. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science 1975, 190, 152–154. [Google Scholar] [CrossRef]
- Ostojic, S.M. Hydrogen-rich water as a modulator of gut microbiota? J. Funct. Foods 2021, 78, 104360. [Google Scholar] [CrossRef]
- Xia, C.; Liu, W.; Zeng, D.; Zhu, L.; Sun, X.; Sun, X. Effect of hydrogen-rich water on oxidative stress, liver function, and viral load in patients with chronic Hepatitis B. Clin. Transl. Sci. 2013, 6, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Korovljev, D.; Stajer, V.; Ostojic, J.; LeBaron, T.W.; Ostojic, S.M. Hydrogen-rich water reduces liver fat accumulation and improves liver enzyme profiles in patients with non-alcoholic fatty liver disease: A randomized controlled pilot trial. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 688–693. [Google Scholar] [CrossRef]
- Mizuno, K.; Sasaki, A.T.; Ebisu, K.; Tajima, K.; Kajimoto, O.; Nojima, J.; Kuratsune, H.; Hori, H.; Watanabe, Y. Hydrogen-rich water for improvements of mood, anxiety, and autonomic nerve function in daily life. Med. Gas Res. 2017, 7, 247. [Google Scholar] [PubMed]
- Todorovic, N.; Zanini, D.; Stajer, V.; Korovljev, D.; Ostojic, J.; Ostojic, S.M. Hydrogen-rich water and caffeine for alertness and brain metabolism in sleep-deprived habitual coffee drinkers. Food Sci. Nutr. 2021, 9, 5139–5145. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Inadequate production of H2 by gut microbiota and Parkinson disease. Trends Endocrinol. Metab. 2018, 29, 286–288. [Google Scholar] [CrossRef]
- Wang, S.T.; Bao, C.; He, Y.; Tian, X.; Yang, Y.; Zhang, T.; Xu, K.F. Hydrogen gas (XEN) inhalation ameliorates airway inflammation in asthma and COPD patients. QJM Int. J. Med. 2020, 113, 870–875. [Google Scholar] [CrossRef]
- Xu, C.; Wang, S.; Wang, H.; Liu, K.; Zhang, S.; Chen, B.; Liu, H.; Tong, F.; Peng, F.; Tu, Y.; et al. Magnesium-based micromotors as hydrogen generators for precise rheumatoid arthritis therapy. Nano Lett. 2021, 21, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Han, W.; Nakao, A. Biological Safety of Hydrogen. In Hydrogen Molecular Biology and Medicine; Springer: Dordrecht, The Netherlands, 2015; pp. 35–48. [Google Scholar]
- Senn, N. Rectal insufflation of hydrogen gas an infallible test in the diagnosis of visceral injury of the gastro-intestinal tract in penetrating wounds of the abdomen. J. Am. Med. Assoc. 1888, 10, 767–777. [Google Scholar] [CrossRef][Green Version]
- Pilcher, J.E. Senn on the diagnosis of gastro-intestinal perforation by the rectal insufflation of hydrogen gas. Ann. Surg. 1888, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Meisenbach, A. A rectal plug for Senn’s method of insufflation of hydrogen gas. J. Am. Med. Assoc. 1888, 11, 908–909. [Google Scholar] [CrossRef][Green Version]
- Hillmantel, J. Two cases of gunshot-wound of the abdomen in which hydrogen-gas test was applied. J. Am. Med. Assoc. 1888, 11, 83–85. [Google Scholar] [CrossRef][Green Version]
- ZetterstrÖm, A. Deep-sea diving with synthetic gas mixtures. Mil. Surg. 1948, 103, 104–106. [Google Scholar] [CrossRef]
- Bjurstedt, H.; Severin, G. The prevention of decompression sickness and nitrogen narcosis by the use of hydrogen as a substitute for nitrogen (The Arne ZetterstrÖm Method for deep-sea diving). Mil. Surg. 1948, 103, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Fife, W.; Texas A & M University; National Sea Grant Program (U.S.); United States, Office of Naval Research. The Use of Non-Explosive Mixtures of Hydrogen and Oxygen for Diving; Texas A& M University: College Station, TX, USA, 1979. [Google Scholar]
- Neale, R.J. Dietary fibre and health: The role of hydrogen production. Med. Hypotheses 1988, 27, 85–87. [Google Scholar] [CrossRef]
- Shirahata, S.; Kabayama, S.; Nakano, M.; Miura, T.; Kusumoto, K.; Gotoh, M.; Hayashi, H.; Otsubo, K.; Morisawa, S.; Katakura, Y. Electrolyzed–reduced water scavenges active oxygen species and protects DNA from oxidative damage. Biochem. Biophys. Res. Commun. 1997, 234, 269–274. [Google Scholar] [CrossRef]
- Gharib, B.; Hanna, S.; Abdallahi, O.M.; Lepidi, H.; Gardette, B.; De Reggi, M. Anti-inflammatory properties of molecular hydrogen: Investigation on parasite-induced liver inflammation. Comptes Rendus l’Académie des Sci.-Ser. III-Sci. Vie 2001, 324, 719–724. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic anti-oxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Fukuda, K.I.; Asoh, S.; Ishikawa, M.; Yamamoto, Y.; Ohsawa, I.; Ohta, S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem. Biophys. Res. Commun. 2007, 361, 670–674. [Google Scholar] [CrossRef]
- Nogueira, J.E.; Branco, L.G. Recent advances in molecular hydrogen research reducing exercise-induced oxidative stress and inflammation. Curr. Pharm. Des. 2021, 27, 731–736. [Google Scholar] [CrossRef]
- Li, Q.; Xie, F.; Yi, Y.; Zhao, P.; Zhang, X.; Zhang, X.; Zhang, X.; Ma, X. Hydroxyl-radical scavenging activity of hydrogen does not significantly contribute to its biological function. bioRxiv 2021. [Google Scholar] [CrossRef]
- Penders, J.; Kissner, R.; Koppenol, W.H. ONOOH does not react with H2: Potential beneficial effects of H2 as an antioxidant by selective reaction with hydroxyl radicals and peroxynitrite. Free Radic. Biol. Med. 2014, 75, 191–194. [Google Scholar] [CrossRef]
- Hancock, J.T.; Russell, G. Downstream Signalling from Molecular Hydrogen. Plants 2021, 10, 367. [Google Scholar] [CrossRef]
- Ghosh, N.; Das, A.; Chaffee, S.; Roy, S.; Sen, C.K. Immunity and Inflammation in Health and Disease: Emerging Roles of Nutraceuticals and Functional Foods in Immune Support; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Sethi, G.; Perumal, E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 2018, 17, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, G.; Kawamoto, K.; Komori, N.; Ishibashi, T. Molecular hydrogen suppresses superoxide generation in the mitochondrial complex I and reduced mitochondrial membrane potential. Biochem. Biophys. Res. Commun. 2020, 522, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wang, Y.; Yin, L.; Wang, Y.; Chen, H.; Mao, X.; Wang, G. Hydrogen gas alleviates sepsis-induced brain injury by improving mitochondrial biogenesis through the activation of PGC-α in mice. Shock 2021, 55, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, J.E.; Amorim, M.R.; Pinto, A.P.; da Rocha, A.L.; da Silva, A.S.; Branco, L.G. Molecular hydrogen downregulates acute exhaustive exercise-induced skeletal muscle damage. Can. J. Physiol. Pharmacol. 2021, 99, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.R.; Veal, D.; Whiteman, M.; Hancock, J.T. Hydrogen gas and its role in cell signalling. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2017, 12, 1–3. [Google Scholar] [CrossRef]
- Slezak, J.; Kura, B.; LeBaron, T.W.; Singal, P.K.; Buday, J.; Barancik, M. Oxidative stress and pathways of molecular hydrogen effects in medicine. Curr. Pharm. Des. 2021, 27, 610–625. [Google Scholar] [CrossRef]
- Sha, J.B.; Zhang, S.S.; Lu, Y.M.; Gong, W.J.; Jiang, X.P.; Wang, J.J.; Qiao, T.L.; Zhang, H.H.; Zhao, M.Q.; Wang, D.P.; et al. Effects of the long-term consumption of hydrogen-rich water on the anti-oxidant activity and the gut flora in female juvenile soccer players from Suzhou, China. Med Gas Res. 2018, 8, 135. [Google Scholar]
- Ji, X.; Zheng, W.; Yao, W. Protective role of hydrogen gas on oxidative damage and apoptosis in intestinal porcine epithelial cells (IPEC-J2) induced by deoxynivalenol: A preliminary study. Toxins 2020, 12, 5. [Google Scholar] [CrossRef]
- Murakami, Y.; Ito, M.; Ohsawa, I. Molecular hydrogen protects against oxidative stress-induced SH-SY5Y neuroblastoma cell death through the process of mitohormesis. PLoS ONE 2017, 12, 0176992. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J. A new approach for the prevention and treatment of cardiovascular disorders. Molecular hydrogen significantly reduces the effects of oxidative stress. Molecules 2019, 24, 2076. [Google Scholar] [CrossRef]
- Yu, X.; Gurry, T.; Nguyen, L.T.T.; Richardson, H.S.; Alm, E.J. Prebiotics and community composition influence gas production of the human gut microbiota. MBio 2020, 11, 00217–00220. [Google Scholar] [CrossRef] [PubMed]
- Hylemon, P.B.; Harris, S.C.; Ridlon, J.M. Metabolism of hydrogen gases and bile acids in the gut microbiome. FEBS Lett. 2018, 592, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Zam, W. Gut microbiota as a prospective therapeutic target for curcumin: A review of mutual influence. J. Nutr. Metab. 2018, 2018, 1367984. [Google Scholar] [CrossRef]
- Shimouchi, A.; Nose, K.; Takaoka, M.; Hayashi, H.; Kondo, T. Effect of dietary turmeric on breath hydrogen. Dig. Dis. Sci. 2009, 54, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.A.D.; Ebine, N.; Nakae, S.; Hojo, T.; Fukuoka, Y. Application of molecular hydrogen as an anti-oxidant in responses to ventilatory and ergogenic adjustments during incremental exercise in humans. Nutrients 2021, 13, 459. [Google Scholar] [CrossRef] [PubMed]
- Settineri, R.; Ji, J.; Luo, C.; Ellithorpe, R.R.; de Mattos, G.F.; Rosenblatt, S.; LaValle, J.; Jinenez, A.; Ohta, S.; Nicolson, G.L. Effects of hydrogenized water on intracellular biomarkers for anti-oxidants, glucose uptake, insulin signaling and SIRT 1 and telomerase activity. Am. J. Food Nutr. 2016, 4, 161–168. [Google Scholar] [CrossRef]
- Ostojic, S.M. Molecular hydrogen in sports medicine: New therapeutic perspectives. Int. J. Sports Med. 2015, 36, 273–279. [Google Scholar] [CrossRef]
- Kawamura, T.; Higashida, K.; Muraoka, I. Application of molecular hydrogen as a novel anti-oxidant in sports science. Oxidative Med. Cell. Longev. 2020, 2020, 2328768. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Larson, A.J.; Ohta, S.; Mikami, T.; Barlow, J.; Bulloch, J.; DeBeliso, M. Acute supplementation with molecular hydrogen benefits submaximal exercise indices. Randomized, double-blinded, placebo-controlled crossover pilot study. J. Lifestyle Med. 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Kato, T.; Ito, M.; Azuma, Y.; Fukasawa, Y.; Ohno, K.; Kojima, S. Hydrogen ameliorates pulmonary hypertension in rats by anti-inflammatory and anti-oxidant effects. J. Thorac. Cardiovasc. Surg. 2015, 150, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I. Biological Responses to Hydrogen molecule and its preventive effects on inflammatory diseases. Curr. Pharm. Des. 2021, 27, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Yoritaka, A.; Takanashi, M.; Hirayama, M.; Nakahara, T.; Ohta, S.; Hattori, N. Pilot study of H2 therapy in Parkinson’s disease: A randomized double-blind placebo-controlled trial. Mov. Disord. 2013, 28, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Kabayama, S.; Ito, S. The hydrogen molecule as anti-oxidant therapy: Clinical application in hemodialysis and perspectives. Ren. Replace. Ther. 2016, 2, 23. [Google Scholar] [CrossRef]
- Franceschelli, S.; Gatta, D.M.P.; Pesce, M.; Ferrone, A.; Di Martino, G.; Di Nicola, M.; De Lutiis, M.A.; Vitacolonna, E.; Patruno, A.; Grilli, A.; et al. Modulation of the oxidative plasmatic state in gastroesophageal reflux disease with the addition of rich water molecular hydrogen: A new biological vision. J. Cell. Mol. Med. 2018, 22, 2750–2759. [Google Scholar] [CrossRef]
- Chen, X.; Zhai, X.; Shi, J.; Liu, W.W.; Tao, H.; Sun, X.; Kang, Z. Lactulose mediates suppression of dextran sodium sulfate-induced colon inflammation by increasing hydrogen production. Dig. Dis. Sci. 2013, 58, 1560–1568. [Google Scholar] [CrossRef]
- Nakao, A.; Toyoda, Y.; Sharma, P.; Evans, M.; Guthrie, N. Effectiveness of hydrogen rich water on anti-oxidant status of subjects with potential metabolic syndrome—An open label pilot study. J. Clin. Biochem. Nutr. 2010, 46, 140–149. [Google Scholar] [CrossRef]
- Ierardi, E.; Rosania, R.; Zotti, M.; Principe, S.; Laonigro, G.; Giorgio, F.; de Francesco, V.; Panella, C. Metabolic syndrome and gastro-esophageal reflux: A link towards a growing interest in developed countries. World J. Gastrointest. Pathophysiol. 2010, 1, 91. [Google Scholar] [CrossRef]
- Chen, H.; Sun, Y.P.; Hu, P.F.; Liu, W.W.; Xiang, H.G.; Li, Y.; Yan, R.L.; Su, N.; Ruan, C.P.; Sun, X.J.; et al. The effects of hydrogen-rich saline on the contractile and structural changes of intestine induced by ischemia-reperfusion in rats. J. Surg. Res. 2011, 167, 316–322. [Google Scholar] [CrossRef]
- Shen, N.Y.; Bi, J.B.; Zhang, J.Y.; Zhang, S.M.; Gu, J.X.; Qu, K.; Liu, C. Hydrogen-rich water protects against inflammatory bowel disease in mice by inhibiting endoplasmic reticulum stress and promoting heme oxygenase-1 expression. World J. Gastroenterol. 2017, 23, 1375. [Google Scholar] [CrossRef]
- Aoki, K.; Nakao, A.; Adachi, T.; Matsui, Y.; Miyakawa, S. Pilot study: Effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Med. Gas Res. 2012, 2, 12. [Google Scholar] [CrossRef]
- Russell, G.; Rehman, M.; TW, L.; Veal, D.; Adukwu, E.; Hancock, J. An overview of SARS-CoV-2 (COVID-19) infection and the importance of molecular hydrogen as an adjunctive therapy. React. Oxyg. Species 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Barancik, M.; Kura, B.; LeBaron, T.W.; Bolli, R.; Buday, J.; Slezak, J. Molecular and cellular mechanisms associated with effects of molecular hydrogen in cardiovascular and central nervous systems. Antioxidants 2020, 9, 1281. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Wei, C.H.; Chen, A.L.; Sun, X.C.; Guo, G.Y.; Zou, X.; Shi, J.D.; Lai, P.Z.; Zheng, Z.G.; Zhong, N.S. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J. Thorac. Dis. 2020, 12, 3448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, X.; Fan, Y.; Zhu, G.; Bai, C. Molecular hydrogen alleviates asthma through inhibiting IL-33/ILC2 axis. Inflamm. Res. 2021, 70, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Li, D.; Hu, J.; Mei, H.; Shu, J.; Long, Z.; Yuan, L.; Li, D.; Guan, R.; Li, Y.; et al. Hydrogen gas inhalation protects against cigarette smoke-induced COPD development in mice. J. Thorac. Dis. 2018, 10, 3232. [Google Scholar] [CrossRef]
- Yamamoto, R.; Homma, K.; Suzuki, S.; Sano, M.; Sasaki, J. Hydrogen gas distribution in organs after inhalation: Real-time monitoring of tissue hydrogen concentration in rat. Sci. Rep. 2019, 9, 1–7. [Google Scholar]
- Sano, M.; Ichihara, G.; Katsumata, Y.; Hiraide, T.; Hirai, A.; Momoi, M.; Tamura, T.; Ohata, S.; Kobayashi, E. Pharmacokinetics of a single inhalation of hydrogen gas in pigs. PLoS ONE 2020, 15, e0234626. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Yang, H. Hydrogen: An endogenous regulator of liver homeostasis. Front. Pharmacol. 2020, 11, 877. [Google Scholar] [CrossRef]
- Kamimura, N.; Nishimaki, K.; Ohsawa, I.; Ohta, S. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity 2011, 19, 1396–1403. [Google Scholar] [CrossRef]
- Liu, B.; Xue, J.; Zhang, M.; Wang, M.; Ma, T.; Zhao, M.; Gu, Q.; Qin, S. Hydrogen inhalation alleviates non-alcoholic fatty liver disease in metabolic syndrome rats. Mol. Med. Rep. 2020, 22, 2860–2868. [Google Scholar] [PubMed]
- Xie, K.; Liu, L.; Yu, Y.; Wang, G. Hydrogen gas presents a promising therapeutic strategy for sepsis. BioMed Res. Int. 2014, 2014, 807635. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Liu, Y.; Zhang, J. Recent advances in studies of molecular hydrogen against sepsis. Int. J. Biol. Sci. 2019, 15, 1261. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Yu, Y.; Wang, Y.; Wang, Y.; Yu, Y.; Xie, K. Perspective of molecular hydrogen in the treatment of sepsis. Curr. Pharm. Des. 2021, 27, 667–678. [Google Scholar] [CrossRef]
- Tamura, T.; Hayashida, K.; Sano, M.; Suzuki, M.; Shibusawa, T.; Yoshizawa, J.; Kobayashi, Y.; Suzuki, T.; Ohta, S.; Morisaki, H.; et al. Feasibility and safety of hydrogen gas inhalation for post-cardiac arrest syndrome–first-in-human pilot study. Circ. J. 2016, 80, 1870–1873. [Google Scholar] [CrossRef]
- Chi, J.; Li, Z.; Hong, X.; Zhao, T.; Bie, Y.; Zhang, W.; Yang, J.; Feng, Z.; Yu, Z.; Xu, Q.; et al. Inhalation of hydrogen attenuates progression of chronic heart failure via suppression of oxidative stress and P53 related to apoptosis pathway in rats. Front. Physiol. 2018, 9, 1026. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, S.; Xu, J.; Wang, T. Hydrogen therapy in cardiovascular and metabolic diseases: From bench to bedside. Cell. Physiol. Biochem. 2018, 47, 1–10. [Google Scholar] [CrossRef]
- Katsumata, Y.; Sano, F.; Abe, T.; Tamura, T.; Fujisawa, T.; Shiraishi, Y.; Kohsaka, S.; Ueda, I.; Homma, K.; Suzuki, M.; et al. The effects of hydrogen gas inhalation on adverse left ventricular remodeling after percutaneous coronary intervention for ST-elevated myocardial infarction—First pilot study in humans. Circ. J. 2017, CJ-17-0105. [Google Scholar] [CrossRef]
- Данилoва, Д.А.; Бричкин, Ю.Д.; Медведев, А.П.; Пичугин, В.В.; Федoрoв, С.А.; Таранoв, Е.В.; Назарoв, Е.И.; Рязанoв, М.В.; Бoльшухин, Г.В.; Дерюгина, А.В. Application of molecular hydrogen in heart surgery under cardiopulmonary bypass. Сoвременные Технoлoгии Медицине 2021, 13, 71–77. [Google Scholar]
- Song, G.; Tian, H.; Qin, S.; Sun, X.; Yao, S.; Zong, C.; Luo, Y.; Liu, J.; Yu, Y.; Sang, H.; et al. Hydrogen decreases athero-susceptibility in apolipoprotein B-containing lipoproteins and aorta of apolipoprotein E knockout mice. Atherosclerosis 2012, 221, 55–65. [Google Scholar] [CrossRef]
- Hori, A.; Ichihara, M.; Kimura, H.; Ogata, H.; Kondo, T.; Hotta, N. Inhalation of molecular hydrogen increases breath acetone excretion during submaximal exercise: A randomized, single-blinded, placebo-controlled study. Med. Gas Res. 2020, 10, 96. [Google Scholar] [CrossRef]
- Javorac, D.; Stajer, V.; Ratgeber, L.; Betlehem, J.; Ostojic, S. Short-term H2 inhalation improves running performance and torso strength in healthy adults. Biol. Sport 2019, 36, 333. [Google Scholar] [CrossRef] [PubMed]
- Alleman, R.J.; Katunga, L.A.; Nelson, M.A.; Brown, D.A.; Anderson, E.J. The “Goldilocks Zone” from a redox perspective—Adaptive vs. deleterious responses to oxidative stress in striated muscle. Front. Physiol. 2014, 5, 358. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, Z.; Wu, X.; Zhang, J. Hydrogen-rich saline inhibits lipopolysaccharide-induced acute lung injury and endothelial dysfunction by regulating autophagy through mTOR/TFEB signaling pathway. BioMed Res. Int. 2020, 9121894. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, C.; Xie, K.; Meng, X.; Wang, Y.; Yu, Y. Hydrogen-rich saline alleviated the hyperpathia and microglia activation via autophagy mediated inflammasome inactivation in neuropathic pain rats. Neuroscience 2019, 421, 17–30. [Google Scholar] [CrossRef]
- Ishibashi, T.; Sato, B.; Shibata, S.; Sakai, T.; Hara, Y.; Naritomi, Y.; Koyanagi, S.; Hara, H.; Nagao, T. Therapeutic efficacy of infused molecular hydrogen in saline on rheumatoid arthritis: A randomized, double-blind, placebo-controlled pilot study. Int. Immunopharmacol. 2014, 21, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Uto, K.; Sakamoto, S.; Que, W.; Shimata, K.; Hashimoto, S.; Sakisaka, M.; Narita, Y.; Yoshii, D.; Zhong, L.; Komohara, Y.; et al. Hydrogen-rich solution attenuates cold ischemia-reperfusion injury in rat liver transplantation. BMC Gastroenterol. 2019, 8, 25. [Google Scholar] [CrossRef]
- Okamoto, A.; Kohama, K.; Aoyama-Ishikawa, M.; Yamashita, H.; Fujisaki, N.; Yamada, T.; Yumoto, T.; Nosaka, N.; Naito, H.; Tsukahara, K.; et al. Intraperitoneally administered, hydrogen-rich physiologic solution protects against postoperative ileus and is associated with reduced nitric oxide production. Surgery 2016, 160, 623–631. [Google Scholar] [CrossRef]
- Sada, H.; Egi, H.; Ide, K.; Sawada, H.; Sumi, Y.; Hattori, M.; Sentani, K.; Oue, N.; Yasui, W.; Ohdan, H. Peritoneal lavage with hydrogen-rich saline can be an effective and practical procedure for acute peritonitis. Surg. Today 2021, 51, 1860–1871. [Google Scholar] [CrossRef]
- Guo, K.W. Green nanotechnology of trends in future energy: A review. Int. J. Energy Res. 2012, 36, 1–17. [Google Scholar] [CrossRef]
- Sutariya, V.B.; Pathak, Y. (Eds.) Biointeractions of Nanomaterials; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Liu, Y.; Li, J.; Chen, M.; Chen, X.; Zheng, N. Palladium-based nanomaterials for cancer imaging and therapy. Theranostics 2014, 10, 10057. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Barani, M.; Sabir, F.; Rahdar, A.; Kyzas, G.Z. Nanomaterials for the treatment and diagnosis of Alzheimer’s disease: An overview. NanoImpact 2020, 20, 100251. [Google Scholar] [CrossRef]
- Murthy, S.K. Nanoparticles in modern medicine: State of the art and future challenges. Int. J. Nanomed. 2007, 2, 129. [Google Scholar]
- Zhang, L.; Zhao, P.; Yue, C.; Jin, Z.; Liu, Q.; Du, X.; He, Q. Sustained release of bioactive hydrogen by Pd hydride nanoparticles overcomes Alzheimer’s disease. Biomaterials 2019, 197, 393–404. [Google Scholar] [CrossRef]
- Zhan, C.; Li, H.; Li, X.; Jiang, Y.; Xie, Z. Synthesis of PdH 0.43 nanocrystals with different surface structures and their catalytic activities towards formic acid electro-oxidation. Sci. China Mater. 2020, 63, 375–382. [Google Scholar] [CrossRef]
- Zhao, P.; Jin, Z.; Chen, Q.; Yang, T.; Chen, D.; Meng, J.; Lu, X.; Gu, Z.; He, Q. Local generation of hydrogen for enhanced photothermal therapy. Nat. Commun. 2018, 9, 4241. [Google Scholar] [CrossRef]
- Perricone, N.V.; Perricone Hydrogen Water Company LLC. Medication Enhancement Using Hydrogen. U.S. Patent 10,076,540, 18 September 2018. [Google Scholar]
- Safonov, V.L.; H2 Universe LLC. Systems and Methods for Topical Application of Molecular Hydrogen. U.S. Patent Application No. 16/376,894, 2019. pending. [Google Scholar]
- Satoh, F.; Sasaki, H.; Kurokawa, R.; Hirano, S.; Ichikawa, Y.; Miz Co Ltd. Method and Molecular Hydrogen-Containing Composition for Promotion of Postoperative Recovery. U.S. Patent Application 17/147,692, 2021. pending. [Google Scholar]
- Yoon, K.S.; Huang, X.Z.; Yoon, Y.S.; Kim, S.K.; Song, S.B.; Chang, B.S.; Kim, D.H.; Lee, K.J. Histological study on the effect of electrolyzed reduced water-bathing on UVB radiation-induced skin injury in hairless mice. Biol. Pharm. Bull. 2011, 34, 1671–1677. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, Y.; Li, Y.; Chen, Z.; Wang, L.; Xiong, H.; Dai, E.; Wu, J.; Fan, B.; Ping, L.; et al. Positive effects of hydrogen-water bathing in patients of psoriasis and parapsoriasis en plaques. Sci. Rep. 2018, 8, 8051. [Google Scholar] [CrossRef]
- Tarnava, A. Supersaturated Hydrogen-Rich Water Hydrotherapy for Recovery of Acute Injury to the Proximal Phalanges on the 5th Toe: A Case Report. J. Sci. Med. 2021, 3. [Google Scholar] [CrossRef]
- Lebwohl, M. A clinician’s paradigm in the treatment of psoriasis. J. Am. Acad. Dermatol. 2005, 53, S59–S69. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol. Ther. 2014, 144, 1–11. [Google Scholar] [CrossRef]
- Huang, C.S.; Kawamura, T.; Toyoda, Y.; Nakao, A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic. Res. 2010, 44, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, H.T.; Qin, S.C. Neuroprotective effects of molecular hydrogen: A critical review. Neurosci. Bull. 2021, 37, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Shin, W. Medical applications of breath hydrogen measurements. Anal. Bioanal. Chem. 2014, 406, 3931–3939. [Google Scholar] [CrossRef]
- Hancock, J.T.; LeBaron, T.W.; Russell, G. Molecular Hydrogen: Redox Reactions and Possible Biological Interactions. React. Oxyg. Species 2021, 11, 17–25. [Google Scholar] [CrossRef]
- Dincer, I. Green methods for hydrogen production. Int. J. Hydrog. Energy 2012, 37, 1954–1971. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).