Abstract
Mediterranean chestnut forests represent ecologically and economically important systems that support biodiversity while providing timber, non-timber forest products, and cultural services. However, traditional management practices are undergoing substantial shifts, with potential implications for forest structure and biodiversity. This study investigates how variation in forest structure and management intensity influences woodpecker communities in chestnut-dominated forests on Mount Paiko, northern Greece. Standardized surveys were conducted at 26 sites stratified by management intensity, and structural forest characteristics such as tree diameter, basal area, and deadwood volume were quantified. Species richness, abundance, and habitat use (feeding and nesting signs) were assessed in relation to these variables. Seven woodpecker species were detected, with distinct differences in species composition across management types. Feeding activity was positively associated with mean tree diameter, while basal area showed a significant negative correlation with woodpecker diversity. Canonical correspondence analysis revealed species-specific preferences along structural gradients, highlighting the association of the Black and Lesser Spotted woodpeckers with larger-diameter trees and deadwood-rich stands. Our results underscore the role of structural heterogeneity in supporting diverse woodpecker assemblages and highlight the need to integrate biodiversity conservation into chestnut forest management, particularly through selective retention of large trees and deadwood elements.
1. Introduction
Mediterranean chestnut forests (Castanea sativa Mill.) constitute an ecologically significant component of temperate and Mediterranean-type ecosystems, providing a wide range of ecosystem services. These forests support biodiversity by offering habitat and food resources for numerous animal species, including cavity-nesting birds, large mammals, and soil fungi communities [,,,]. Structurally diverse chestnut forests, particularly those with a mix of old-growth trees and deadwood elements, also play a crucial role in maintaining high levels of biodiversity, supporting complex ecological interactions across multiple trophic levels [,]. In addition to their ecological contributions, chestnut forests deliver essential regulating services, such as erosion control, water filtration, and climate regulation, particularly in mountainous regions where they mitigate soil degradation and reduce runoff []. The ecological value of these forests in maintaining natural ecosystems has been acknowledged by the European Community Natura 2000 network, which has designated areas with chestnut-dominated forests and old chestnut plantations (9260: Castanea sativa woods) as important for biodiversity conservation [].
Chestnut forests have been actively managed by humans for centuries; thus, their distribution is closely linked to historical land-use practices [,]. Traditionally cultivated for timber and fruit production, chestnut stands remain a valuable economic resource across regions, particularly in southern Europe, where they have been extensively cultivated in orchards and mixed agroforestry systems []. In Greece, chestnut forests are an integral part of rural economies, with the country ranking seventh in global chestnut production as of 2023 []. Depending on management intensity, chestnut stands vary from intensively cultivated plantations, where trees are pruned for nut production, to more natural or abandoned woodlands that retain higher structural complexity [,]. These diverse management regimes influence both forest dynamics and the associated fauna, including bird communities that rely on cavity-bearing trees and deadwood availability [], making chestnut forests particularly relevant for biodiversity conservation and sustainable land-use planning.
Woodpeckers (Picidae) are widely regarded as vital ecological indicators of forest health and sustainability [,], owing to their strong associations with structural forest attributes such as tree diameter, standing deadwood, and basal area [,]. As primary cavity nesters, they create nesting and roosting sites that are subsequently used by secondary cavity-nesting birds and small mammals, contributing to forest ecosystem integrity [,]. Furthermore, woodpeckers play an active role in forest dynamics by controlling insect populations and facilitating fungal dispersal, which accelerates wood decay and nutrient cycling []. However, woodpecker populations are highly sensitive to habitat alterations, with studies demonstrating population declines due to forest fragmentation, logging, and intensive agriculture [,,]. While the response of woodpeckers to habitat fragmentation varies depending on matrix type and tree composition [,], their presence and abundance within a given forest type largely depend on the availability of foraging and nesting resources.
Most studies examining woodpecker responses to forest structure and management have primarily focused on natural and semi-natural mixed forests dominated by Pinus or Quercus spp., rather than chestnut-dominated landscapes [,,,]. Research within Mediterranean chestnut forest ecosystems remains scarce, with studies showing that bird diversity, including woodpecker presence, increased in chestnut forests with older trees, likely due to the availability of cavities and deadwood structures []. Furthermore, chestnut management can enhance habitat diversity and food resources for certain species [,]. However, the extent to which Mediterranean chestnut forest management influences woodpecker community structure, habitat use, and species-specific preferences remains largely unexplored.
Given the historical anthropogenic management of Mediterranean chestnut forests and the ongoing shifts in land-use intensity, understanding the relationships between forest structure and woodpecker communities is crucial for biodiversity conservation. This study aims to investigate how variations in forest structure, driven by differing management intensities, influence the abundance, composition, and habitat use of woodpecker species in Mediterranean chestnut forests. These results will highlight how different management practices and structural features of chestnut forests jointly shape woodpecker communities, providing insights for sustainable forest management.
2. Materials and Methods
The research was conducted in Mediterranean chestnut forests located in the eastern slopes of Mount Paiko in Kilkis, Greece, an area characterized by a range of forest types, including pure and mixed stands of chestnut (Castanea sativa), oaks (Quercus cerris, Quercus petraea), and European beech (Fagus sylvatica), extending from 340 to 1420 m in elevation. The area is part of the NATURA 2000 network (GR1240003), mainly because of the rich vegetation mosaic creating an important diversity of habitats. The chestnut forests are historically anthropogenic in terms of their origin and structure [] and exhibit a wide range of management regimes, from intensively cultivated orchards to abandoned or naturally regenerating stands interspersed with extensive oak woodlands.
Data collection took place during the breeding season (1–30 April 2023) to capture peak woodpecker activity. A total of twenty-one sites were selected in areas dominated by chestnut to capture the range of forest management intensities present in the region. Sites were further classified into three levels of management intensity: cultivated (n = 10), partly cultivated (n = 7), and unmanaged (n = 4), based on evidence of active nut harvesting, pruning, and thinning operations observed during fieldwork, as well as consultation with local forest records. Additionally, five oak-dominated sites were included to provide ecological context and illustrate differences in woodpecker assemblages between forest types. Within each site, random points were generated using ArcGIS Pro 3.4 tool “Create Random Points”, keeping a minimum distance of approximately 300 m among them to avoid double counting of individuals [,]. Upon arrival at each point, observers allowed three to five minutes of acclimatization to reduce disturbance before conducting standardized woodpecker surveys []. Surveys combined visual detections and acoustic cues, supplemented by playback calls to increase detection probability []. A standardized playback protocol was used, consisting of calls and drumming sequences for eight target species (Dryobates minor, Dendrocopos syriacus, Leiopicus medius, Dendrocopos major, Picus viridis, Picus canus, Dendrocopos leucotos, and Dryocopus martius). The playback was ordered from smallest to largest species to minimize interference from dominant species. Each species’ vocalization (call followed by drumming) was played for approximately 60 s, followed by two to three minutes of silent observation before proceeding to the next. Observers noted the presence and number of individuals for each woodpecker species, along with any evidence of nest trees or feeding marks. All woodpecker surveys were completed during morning hours (6:30–12:00) to capture the peak of bird activity. Time spent at each survey point ranged from thirty to forty-five minutes, depending on the number of species responses to the audio playback ensuring standardization of effort across sites.
For the chestnut-dominated sites, more detailed forest structure measurements were obtained, focusing on the features most relevant to cavity-nesting birds and their foraging behavior [,,]. Within circular plots of twenty meters radius centered on the survey points (i.e., 1256 m2 area; []), all living trees with a diameter at breast height (DBH) above five centimeters were measured, and their DBH values were recorded to derive the mean and standard deviation of living tree diameters. The number, height, and DBH of standing dead trees, as well as the cut height and diameter of stumps were also documented if present. Downed logs were measured for length and diameter, and their decomposition stage was categorized using a standard five-class scale []. These data allowed the calculation of deadwood volume, stump volume, and basal area as a measure of stand density []. Observers noted whether trees exhibited grafting, pruning, or signs of historical orchard practices, thereby helping to classify sites into management intensity categories: cultivated (actively managed for chestnut production), partly cultivated (abandoned or less intensive management), or unmanaged. During this same visit, all trees within the plot were carefully inspected for signs of woodpecker activity, including cavities used as nests and the presence of characteristic feeding marks.
We firstly provide descriptive statistics to summarize relative woodpecker abundance by tree species composition and by management category. The relationship between habitat use indicators (nest trees, feeding marks) and management intensity was then examined through Chi-squared tests to determine whether sites subjected to higher levels of cultivation supported fewer nesting and feeding opportunities. Furthermore, to compare DBH among sites with and without habitat use indicators, we calculated Cohen’s d. This effect-size measure quantifies the standardized difference in mean DBH between two groups (e.g., presence vs. absence of feeding marks). A higher Cohen’s d suggests a more pronounced difference in DBH between groups. Generalized linear models (GLMs) were run to assess whether any forest structural variable could predict total woodpecker abundance. In addition, the association of DBH variability and basal area with woodpecker diversity (Shannon index) was evaluated using Spearman’s rank correlation. To understand whether forest structure drives broader patterns in species composition, we ran a canonical correspondence analysis (CCA). This multivariate approach uses the abundance of each woodpecker species as the response variable, while forest structural characteristics including DBH, deadwood volume, and basal area, served as explanatory variables. Variance inflation factors were calculated to assess multicollinearity among structural predictors. All analyses were implemented in R.4.2.0 [] using packages “vegan” v.2.6. [], “effsize” v. 0.8.1 [], and “mgcv” 1.9 [].
3. Results
Woodpeckers were detected at 20 of the 21 surveyed points within chestnut-dominated stands, while all 5 oak-dominated sites also yielded at least 1 woodpecker detection. Overall, 7 species were recorded, with a total of 57 individuals in chestnut sites and 11 in oak sites. In chestnut stands, the most abundant species were the Middle spotted woodpecker (Leiopicus medius, 20 individuals) and the Green woodpecker (Picus viridis, 13 individuals). Less abundant in chestnut stands were the Lesser spotted woodpecker (Dryobates minor, five individuals) and the Syrian woodpecker (Dendrocopos syriacus, three individuals). In oak-dominated sites, the Lesser spotted woodpecker proved most frequent (four individuals), followed by the Syrian and Middle spotted woodpeckers (two individuals each). The Black woodpecker and the Great spotted woodpecker each appeared only once in oak stands, while the Green woodpecker did not respond at any of the surveyed oak points. In one site, located on a steep slope with older oak individuals, a White-backed woodpecker (Dendrocopos leucotos) responded to the playback, representing the only detection of this rare species in the survey.
Actively managed chestnut orchards generally hosted a larger proportion of Great spotted woodpeckers, whereas less intensively managed or abandoned stands showed an increased relative abundance of Black woodpeckers (Figure 1a). Unmanaged stands exhibited greater relative abundance of Lesser spotted woodpeckers, whereas the Middle spotted woodpecker was the most abundant species in less intensively managed or abandoned stands. In regard to tree species composition, in mixed chestnut stands with beech and oak along with ash, the Middle spotted and Green woodpeckers were the most abundant, whereas mixed chestnut stands with oaks and beech showed a different relative abundance profile, with lower species diversity and the Lesser spotted woodpecker being the most frequent species (Figure 1b).
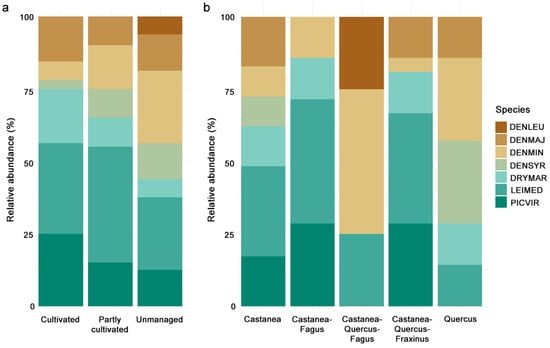
Figure 1.
Stacked bar plot of the relative abundance of each woodpecker species for (a) different levels of management intensity and (b) different tree composition of stands. Bars represent proportions of total individuals recorded per site type.
Observations of nest trees were less frequent in actively managed orchards, albeit marginally significant (χ2 = 5.7, p-value = 0.059), whereas the frequency of feeding marks did not show any pronounced differences among management intensity categories (Figure 2). Mean DBH was found to have a large effect on the presence of feeding marks (Cohen’s d = −0.92), implying that feeding is associated with larger average DBH. The effect size of DBH on stands with observed nests was negligible (Cohen’s d = 0.1).
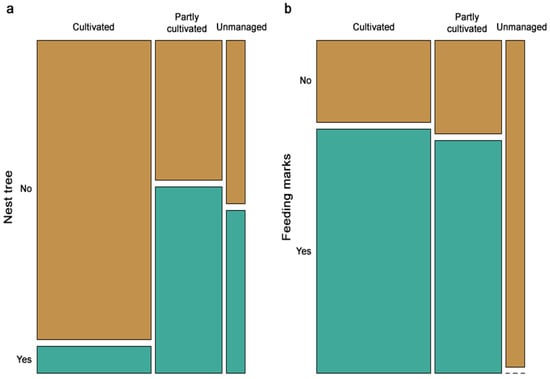
Figure 2.
Mosaic plots showing the frequency of (a) nest trees and (b) feeding marks across cultivated, partly cultivated, and unmanaged sites. The size of each tile corresponds to the proportion of observations in that management category.
The GLM employed to test whether any structural characteristic could predict total woodpecker abundance (summed individuals across all species) showed that none of the measured variables, including mean DBH, basal area, and deadwood volume, emerged as a significant predictor, as residual deviance remained high, and no single factor consistently explained abundance patterns. In contrast, Spearman’s rank correlation revealed that tree diameter variability (standard deviation of DBH) did not correlate significantly with woodpecker diversity (Shannon index) but basal area did (Spearman’s rho = −0.68, p = 0.04). This negative association suggests that sites with a higher basal area may host lower overall species diversity.
Finally, the CCA examining how forest structure variables might shape community composition across sites revealed that distinct forest structural gradients can influence how different woodpecker species partition the habitat (Figure 3). Specifically, the first axis of the CCA, examining how forest structure variables might shape community composition across sites, accounted for approximately 46.6% of the constrained variation, with a strong positive loading for mean DBH and negative loadings of deadwood volume and basal area. Species such as the Lesser spotted and Black woodpeckers aligned more closely with higher DBH stands, whereas the Great spotted and Green woodpeckers were oriented toward stands with greater deadwood volume or higher basal area. The second axis (CCA2) explained an additional 36.4% of the variation, primarily differentiating sites by a gradient of basal area (negative loading) versus log volume (positive loading). The Black woodpecker was positioned in positive side of CCA2, thereby mostly aligning with lower basal area but higher deadwood volume.
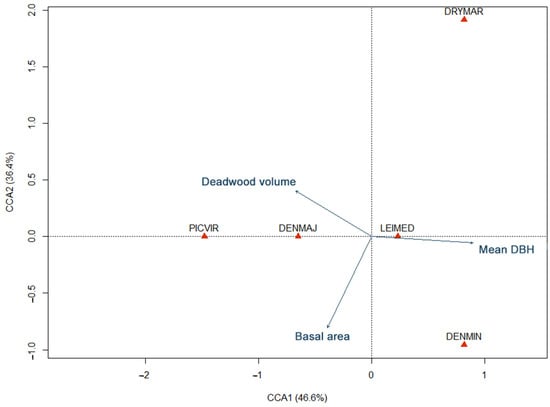
Figure 3.
Canonical correspondence analysis (CCA) biplot relating woodpecker species distributions to forest structural variables (mean DBH, deadwood volume, and basal area). Species scores (filled triangles) indicate how each taxon associates with the main environmental gradients (arrows).
4. Discussion
We examined the influence of forest structure and management intensity on woodpecker community composition and habitat use within Mediterranean chestnut forests. Our findings demonstrate that variations in tree diameter, deadwood volume, and basal area contribute to structuring woodpecker assemblages, while management intensity affects species abundance patterns. Specifically, total woodpecker abundance did not significantly differ among sites, but species-specific responses revealed distinct preferences along structural gradients. The results highlight the importance of maintaining structurally diverse chestnut forests, particularly by retaining large-diameter trees and deadwood elements, to sustain woodpecker populations.
Woodpecker abundance varied across sites, with seven species recorded overall and a clear difference in relative species composition between chestnut and oak-dominated stands. Chestnut forests were primarily occupied by the Middle spotted woodpecker and the Green woodpecker, which aligns with previous studies linking these species to mature broadleaf forests with high structural heterogeneity [,,,]. In fact, the association of Middle spotted woodpeckers with older chestnut trees is particularly relevant, as this species depends on larger-diameter trees especially for foraging []. Conversely, oak-dominated sites showed a higher relative abundance of Lesser spotted and Syrian woodpeckers, which suggests that these species may be better adapted to the lower canopy cover and different tree composition of Mediterranean oak stands []. The detection of the White-backed woodpecker within the broader study area, albeit outside chestnut-dominated stands, is ecologically notable, given this species’ strong reliance on mature, unmanaged forests with abundant decaying wood []. Its occurrence near chestnut woodlands may reflect the presence of adjacent mixed forest patches with suitable conditions, such as older oaks and sufficient deadwood. This underscores the role of landscape-level heterogeneity in supporting forest specialists even within anthropogenically modified areas. Similarly, the Syrian woodpecker was recorded in both cultivated and semi-managed orchards, indicating its capacity to exploit edge habitats near human settlements. In fact, in Central Europe, managed orchards and non-forest tree stands were shown to provide key resources for this species [,].
Forest management practices indeed influenced species distribution. Actively managed chestnut orchards were associated with higher relative abundance of Great spotted woodpeckers, possibly reflecting a preference for sites with moderate canopy openness and a mix of tree sizes that provide varied foraging opportunities. In contrast, less intensively managed or abandoned stands supported higher relative abundances of Black woodpeckers, a species known to favor older forests with abundant deadwood and higher canopy cover [,,]. Overall, our results are consistent with studies indicating that woodpecker species richness is generally higher in forests with greater structural complexity and a diversity of tree age classes [,,,,,].
Nest site availability did not significantly differ across management intensities; however, feeding activity was strongly linked to tree diameter, as indicated by the large effect size of DBH on feeding mark occurrence. This suggests that larger trees play a key role in providing foraging resources, potentially supporting higher densities of wood-boring insects, which form a primary food source for many woodpeckers [,,,]. The lack of a significant effect of DBH on nest site frequency may indicate that woodpeckers in the study area rely more on pre-existing cavities in surrounding natural forests rather than excavating new ones on chestnut trees, a pattern observed in managed forests where natural cavity formation is limited []. The negative correlation between basal area and woodpecker diversity further supports the argument that denser stands may be less favorable for diverse woodpecker assemblages. High basal area often corresponds to lower light penetration, fewer understory openings, and reduced deadwood accumulation, all of which can limit foraging and nesting opportunities [,]. This aligns with findings from other temperate forests, where woodpecker diversity tends to be higher in structurally heterogeneous landscapes [,].
As evidenced by our results, DBH, deadwood volume, and basal area were key variables influencing woodpecker species composition. Species such as the Lesser spotted and Black woodpeckers exhibited a preference for stands with larger trees. In contrast, species such as the Great spotted and Green woodpeckers aligned more closely with sites characterized by higher basal area and deadwood volume, reinforcing their association with structurally diverse stands [,,]. Furthermore, the Black woodpecker additionally showed a strong association with high deadwood availability and open forest conditions, which are essential for its foraging behavior [,]. Conversely, the Middle spotted woodpecker appeared in sites with moderate basal area and a mix of tree species, suggesting an affinity for semi-managed chestnut stands where tree age diversity is maintained.
Despite offering valuable insights between woodpecker and forest structure relationships, this study has some limitations. Sample sizes for certain species were relatively low, which may have limited statistical power in detecting finer-scale associations. Additionally, while structural variables were assessed comprehensively, other factors such as insect prey availability and cavity competition were not directly measured, yet they likely influence woodpecker habitat selection [,].
Our findings underscore the importance of preserving structural diversity within chestnut forests to maintain woodpecker diversity and ecosystem functions. Traditional chestnut management practices, designed for the maintenance of open-canopy orchards for nut production, have been shown to promote structural heterogeneity by favoring the retention of mature trees, thus hosting diverse microhabitats [,,]. While unmanaged or abandoned stands often support structurally complex conditions favorable to many woodpecker species, traditional practices, including selective thinning and retention of old-growth trees, can help sustain key habitat features, ensuring the continued presence of primary cavity nesters [,,]. Moreover, adopting a tree-by-tree approach to rotation age, rather than relying on even-aged systems, offers a multifunctional management pathway that enhances biodiversity conservation. This strategy can also reduce the frequency and intensity of disturbance caused by coppicing, such as soil erosion, compaction, and rutting, particularly in steep terrain []. Furthermore, promoting deadwood retention and maintaining a mix of tree age classes could benefit species that rely on decaying wood for both nesting and foraging. Given the ongoing shifts in land-use practices, including forest abandonment and intensified chestnut cultivation, targeted management strategies should prioritize balancing economic production with biodiversity conservation. Management plans should integrate heterogeneous stand structures, maintain sufficient large-diameter trees, and avoid excessive canopy closure, which may reduce habitat suitability for certain woodpecker species. Additionally, policy frameworks should incorporate biodiversity indicators, such as woodpecker presence, to assess the ecological sustainability of chestnut forestry practices.
Author Contributions
Conceptualization, A.D.; methodology, A.D. and C.G.; validation, N.G., A.D. and A.B.; formal analysis, N.G., A.D. and A.B.; investigation, A.D. and C.G.; data curation, N.G. and A.B.; writing—original draft preparation, N.G., A.D. and A.B.; writing—review and editing, N.G., A.D., C.G. and A.B.; visualization, A.B.; funding acquisition, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hellenic Zoological Society under the “Margarita Metallinou scholarship program 2023–2025”, awarded to A.D.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The researchers would like to thank the Hellenic Zoological Society for their support and contributions. We appreciate their dedication to advancing zoological research in Greece and their commitment to fostering scientific collaboration.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DBH | diameter at breast height |
References
- Jose, S. Agroforestry for ecosystem services and environmental benefits: An overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Baptista, P.; Martins, A.; Tavares, R.M.; Lino-Neto, T. Diversity and fruiting pattern of macrofungi associated with chestnut (Castanea sativa) in the Trás-os-Montes region (Northeast Portugal). Fungal Ecol. 2010, 3, 9–19. [Google Scholar] [CrossRef]
- Venanzi, R.; Picchio, R.; Piovesan, G. Silvicultural and logging impact on soil characteristics in Chestnut (Castanea sativa Mill.) Mediterranean coppice. Ecol. Eng. 2016, 92, 82–89. [Google Scholar] [CrossRef]
- Roces Diaz, J.V.; Varela, E.R.D.; Anta, M.B.; Álvarez, P.Á. Sweet chestnut agroforestry systems in North-western Spain: Classification, spatial distribution and an ecosystem services assessment. For. Syst. 2018, 27, 10. [Google Scholar] [CrossRef]
- Pezzi, G.; Maresi, G.; Conedera, M.; Ferrari, C. Woody species composition of chestnut stands in the Northern Apennines: The result of 200 years of changes in land use. Landsc. Ecol. 2011, 26, 1463–1476. [Google Scholar] [CrossRef]
- Bombelli, A.; Di Paola, A.; Chiriacò, M.V.; Perugini, L.; Castaldi, S.; Valentini, R. Climate change, sustainable agriculture and food systems: The world after the Paris agreement. In Achieving the Sustainable Development Goals Through Sustainable Food Systems; Springer: Berlin/Heidelberg, Germany, 2019; pp. 25–34. [Google Scholar]
- Conedera, M.; Tinner, W.; Krebs, P.; de Rigo, D.; Caudullo, G. Castanea sativa in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 78–79. [Google Scholar]
- EC (European Comission). Interpretation Manual of European Habitats–EUR27; DG Environment, Nature and Biodiversity: Brussels, Belgium, 2007. [Google Scholar]
- Conedera, M.; Krebs, P.; Tinner, W.; Pradella, M.; Torriani, D. The cultivation of Castanea sativa (Mill.) in Europe, from its origin to its diffusion on a continental scale. Veg. Hist. Archaeobotany 2004, 13, 161–179. [Google Scholar] [CrossRef]
- Pezzi, G.; Gambini, S.; Buldrini, F.; Ferretti, F.; Muzzi, E.; Maresi, G.; Nascimbene, J. Contrasting patterns of tree features, lichen, and plant diversity in managed and abandoned old-growth chestnut orchards of the northern Apennines (Italy). For. Ecol. Manag. 2020, 470, 118207. [Google Scholar] [CrossRef]
- FAO. Production / Crops and Livestock Products. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 19 February 2025).
- Diamandis, S.; Perlerou, C. The mycoflora of the chestnut ecosystems in Greece. For. Snow Landsc. Res. 2001, 76, 499–504. [Google Scholar]
- Konstantinidis, P.; Tsiourlis, G.; Xofis, P.; Buckley, G.P. Taxonomy and ecology of Castanea sativa Mill. forests in Greece. Plant Ecol. 2008, 195, 235–256. [Google Scholar] [CrossRef]
- Remm, J.; Lõhmus, A. Tree cavities in forests–the broad distribution pattern of a keystone structure for biodiversity. For. Ecol. Manag. 2011, 262, 579–585. [Google Scholar] [CrossRef]
- Drever, M.C.; Aitken, K.E.; Norris, A.R.; Martin, K. Woodpeckers as reliable indicators of bird richness, forest health and harvest. Biol. Conserv. 2008, 141, 624–634. [Google Scholar] [CrossRef]
- Menon, T.; Shahabuddin, G. Assessing woodpeckers as indicators of bird diversity and habitat structure in managed forests. Biodivers. Conserv. 2021, 30, 1689–1704. [Google Scholar] [CrossRef]
- Wübbenhorst, J.; Südbeck, P. Woodpeckers as indicators for sustainable forestry. In First Results of a Study in the EU/LIFE–Demonstration Areas Lüneburger Heide und Solling. Demonstration of Methods to Monitor Sustainable Forestry 2001, 1998–2001; EU-LIFE: Barcelona, Spain, 2001. [Google Scholar]
- Ilsøe, S.K.; Kissling, W.D.; Fjeldså, J.; Sandel, B.; Svenning, J.C. Global variation in woodpecker species richness shaped by tree availability. J. Biogeogr. 2017, 44, 1824–1835. [Google Scholar] [CrossRef]
- Martin, K.; Eadie, J.M. Nest webs: A community-wide approach to the management and conservation of cavity-nesting forest birds. For. Ecol. Manag. 1999, 115, 243–257. [Google Scholar] [CrossRef]
- Trzcinski, M.K.; Cockle, K.L.; Norris, A.R.; Edworthy, M.; Wiebe, K.L.; Martin, K. Woodpeckers and other excavators maintain the diversity of cavity-nesting vertebrates. J. Anim. Ecol. 2022, 91, 1251–1265. [Google Scholar] [CrossRef]
- Jusino, M.A.; Hagemeyer, N.D.; Banik, M.T.; Palmer, J.M.; Lindner, D.L.; Smith, M.E.; Koenig, W.D.; Walters, E.L. Fungal communities associated with acorn woodpeckers and their excavations. Fungal Ecol. 2022, 59, 101154. [Google Scholar] [CrossRef]
- Villard, M.A.; Trzcinski, M.K.; Merriam, G. Fragmentation effects on forest birds: Relative influence of woodland cover and configuration on landscape occupancy. Conserv. Biol. 1999, 13, 774–783. [Google Scholar] [CrossRef]
- Carlson, A. The effect of habitat loss on a deciduous forest specialist species: The White-backed Woodpecker (Dendrocopos leucotos). For. Ecol. Manag. 2000, 131, 215–221. [Google Scholar] [CrossRef]
- Basile, M.; Krištín, A.; Mikusiński, G.; Thorn, S.; Żmihorski, M.; Pasinelli, G.; Brockerhoff, E.G. Salvage logging strongly affects woodpecker abundance and reproduction: A meta-analysis. Curr. For. Rep. 2023, 9, 1–14. [Google Scholar] [CrossRef]
- Mortelliti, A.; Fagiani, S.; Battisti, C.; Capizzi, D.; Boitani, L. Independent effects of habitat loss, habitat fragmentation and structural connectivity on forest-dependent birds. Divers. Distrib. 2010, 16, 941–951. [Google Scholar] [CrossRef]
- Porro, Z.; Chiatante, G.; Bogliani, G. Associations between forest specialist birds and composition of woodland habitats in a highly modified landscape. For. Ecol. Manag. 2020, 458, 117732. [Google Scholar] [CrossRef]
- Fernandez, C.; Azkona, P. Influence of forest structure on the density and distribution of the White-backed Woodpecker Dendrocopos leucotos and Black Woodpecker Dryocopus martius in Quinto Real (Spanish western Pyrenees). Bird Study 1996, 43, 305–313. [Google Scholar] [CrossRef]
- Drever, M.C.; Martin, K. Response of woodpeckers to changes in forest health and harvest: Implications for conservation of avian biodiversity. For. Ecol. Manag. 2010, 259, 958–966. [Google Scholar] [CrossRef]
- Kajtoch, L.; Figarski, T.; Pelka, J. The role of forest structural elements in determining the occurrence of two specialist woodpecker species in the Carpathians, Poland. Ornis Fenn. 2013, 90, 23–40. [Google Scholar] [CrossRef]
- Basile, M.; Asbeck, T.; Pacioni, C.; Mikusiński, G.; Storch, I. Woodpecker cavity establishment in managed forests: Relative rather than absolute tree size matters. Wildl. Biol. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Laiolo, P.; Rolando, A.; Valsania, V. Avian community structure in sweet chestnut coppiced woods facing natural restoration. Rev. D’écologie 2004, 59, 453–463. [Google Scholar] [CrossRef]
- González-Varo, J.P.; López-Bao, J.V.; Guitián, J. Presence and abundance of the Eurasian nuthatch Sitta europaea in relation to the size, isolation and the intensity of management of chestnut woodlands in the NW Iberian Peninsula. Landsc. Ecol. 2008, 23, 79–89. [Google Scholar] [CrossRef]
- Morelli, F.; Python, A.; Pezzatti, G.B.; Moretti, M. Bird response to woody pastoral management of ancient chestnut orchards: A case study from the southern Alps. For. Ecol. Manag. 2019, 453, 117560. [Google Scholar] [CrossRef]
- Miskos, D. PGI Chestnut Paikou: Benefits and Prospects for the Wider Region. Master’s Thesis, Agricultural University of Athens, Athens, Greece, 2023. [Google Scholar]
- Block, W.M. Foraging ecology of Nuttall’s woodpecker. Auk 1991, 108, 303–318. [Google Scholar]
- Badis, M.; Benchana, I.; Hamdi, N. Nest-site selection by Levaillant’s Woodpecker Picus vaillantii in the Aurès Mountains of northeastern Algeria. Ostrich 2023, 94, 60–64. [Google Scholar] [CrossRef]
- Bibby, C.J.; Burgess, N.D.; Hill, D.A.; Mustoe, S.H. Bird Census Techniques, 2nd ed.; Academic Press: London, UK, 2000. [Google Scholar]
- Dudley, J.; Saab, V. A Field Protocol to Monitor Cavity-Nesting Birds; U.S.D.A. Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2003.
- Ettwein, A.; Korner, P.; Lanz, M.; Lachat, T.; Kokko, H.; Pasinelli, G. Habitat selection of an old-growth forest specialist in managed forests. Anim. Conserv. 2020, 23, 547–560. [Google Scholar] [CrossRef]
- Fernández, J.M.; Lammertink, M. Resource selection and home range size variation of Atlantic Forest woodpecker species: Implications for selective logging and conservation. For. Ecol. Manag. 2023, 548, 121440. [Google Scholar] [CrossRef]
- Paillet, Y.; Pernot, C.; Boulanger, V.; Debaive, N.; Fuhr, M.; Gilg, O.; Gosselin, F. Quantifying the recovery of old-growth attributes in forest reserves: A first reference for France. For. Ecol. Manag. 2015, 346, 51–64. [Google Scholar] [CrossRef]
- Hunter, M. Wildlife, Forests, and Forestry-Principles of Managing Forests for Biological Diversity; CABI: Wallingford, UK, 1990. [Google Scholar]
- Bettinger, P.; Boston, K.; Siry, J.; Grebner, D. Valuing and characterizing forest conditions. In Forest Management and Planning, 2nd ed.; Academic Press: New York, NY, USA, 2017; pp. 21–64. [Google Scholar]
- R CoreTeam. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 29 January 2025).
- Torchiano, M. Package ‘effsize’: Efficient Effect Size Computation. R Package Version 0.8.1. 2020. Available online: https://CRAN.R-project.org/package=effsize (accessed on 29 January 2025).
- Pedersen, E.J.; Miller, D.L.; Simpson, G.L.; Ross, N. Hierarchical generalized additive models in ecology: An introduction with mgcv. PeerJ 2019, 7, e6876. [Google Scholar] [CrossRef]
- Pasinelli, G. Oaks (Quercus sp.) and only oaks? Relations between habitat structure and home range size of the middle spotted woodpecker (Dendrocopos medius). Biol. Conserv. 2000, 93, 227–235. [Google Scholar] [CrossRef]
- Domokos, E.; Cristea, V. Effects of managed forests structure on woodpeckers (Picidae) in the Niraj valley (Romania): Woodpecker populations in managed forests. North-West. J. Zool. 2014, 10, 131606. [Google Scholar]
- Varasteh Moradi, H.; Sepehri Roshan, Z.; Chamanefar, S. Habitat assessment of Green Woodpecker (Picus viridis) in Golestan National Park using classification tree method. J. Anim. Res. (Iran. J. Biol.) 2018, 30, 495–507. [Google Scholar]
- Stański, T.; Stańska, M.; Goławski, A.; Czeszczewik, D. Foraging site selection of the middle spotted woodpecker (Leiopicus medius L.) in primeval oak-lime-hornbeam forest of the Białowieża National Park: Comparison of breeding and non-breeding seasons. Forests 2021, 12, 837. [Google Scholar] [CrossRef]
- Pasinelli, G.; Hegelbach, J. Characteristics of trees preferred by foraging middle spotted woodpecker Dendrocopos medius in northern Switzerland. Ardea 1997, 85, 203–209. [Google Scholar]
- Delahaye, L.; Monticelli, D.; Lehaire, F.; Rondeux, J.; Claessens, H. Fine-scale habitat selection by two specialist woodpeckers occurring in beech and oak-dominated forests in southern Belgium. Ardeola 2010, 57, 339–362. [Google Scholar]
- Czeszczewik, D.; Walankiewicz, W.; Mitrus, C.; Tumiel, T.; Stanski, T.; Sahel, M.; Bednarczyk, G. Importance of dead wood resources for woodpeckers in coniferous stands of the Białowieża Forest. Bird Conserv. Int. 2013, 23, 414–425. [Google Scholar] [CrossRef]
- Kajtoch, Ł. The importance of traditional orchards for breeding birds: The preliminary study on Central European example. Acta Oecologica 2017, 78, 53–60. [Google Scholar] [CrossRef]
- Michalczuk, J. The importance of non-forest tree stand features for protection of the Syrian Woodpecker Dendrocopos syriacus in agricultural landscape: A case study from South-Eastern Poland. Agrofor. Syst. 2020, 94, 1825–1835. [Google Scholar] [CrossRef]
- Garmendia, A.; Cárcamo, S.; Schwendtner, O. Forest management considerations for conservation of black woodpecker Dryocopus martius and white-backed woodpecker Dendrocopos leucotos populations in Quinto Real (Spanish Western Pyrenees). In Forest Diversity and Management; Springer: Berlin/Heidelberg, Germany, 2006; pp. 339–355. [Google Scholar]
- Pirovano, A.R.; Zecca, G. Black Woodpecker Dryocopus martius habitat selection in the Italian Alps: Implications for conservation in Natura 2000 network. Bird Conserv. Int. 2014, 24, 299–315. [Google Scholar] [CrossRef]
- Olano, M.; Aierbe, T.; Beñaran, H.; Hurtado, R.; Ugarte, J.; Urruzola, A.; Vázquez, J.; Ansorregi, F.; Galdos, A.; Gracianteparaluceta, A. Black woodpecker Dryocopus martius (L., 1758) distribution, abundance, habitat use and breeding performance in a recently colonized region in SW Europe. Munibe Cienc. Nat. 2015, 63, 49–71. [Google Scholar] [CrossRef]
- de Gasperis, S.R.; De Zan, L.R.; Battisti, C.; Reichegger, I.; Carpaneto, G.M. Distribution and abundance of hole-nesting birds in Mediterranean forests: Impact of past management patterns on habitat preference. Ornis Fenn. 2016, 93, 100–110. [Google Scholar] [CrossRef]
- Shaw, T.; Scherer-Lorenzen, M.; Müller, S. Forest structural heterogeneity positively affects bird richness and acoustic diversity in a temperate, central European forest. Front. Ecol. Evol. 2024, 12, 1387879. [Google Scholar] [CrossRef]
- Ciudad, C.; Robles, H.; Matthysen, E. Postfledging habitat selection of juvenile middle spotted woodpeckers: A multi-scale approach. Ecography 2009, 32, 676–682. [Google Scholar] [CrossRef]
- Stański, T.; Stańska, M.; Czeszczewik, D. Foraging behaviour of the Great Spotted Woodpecker (Dendrocopos major) in the Białowieża National Park: Comparison of breeding and non-breeding seasons. Ornis Fenn. 2023, 100, 38–50. [Google Scholar] [CrossRef]
- Kumar, R.; Shahabuddin, G.; Kumar, A. Foraging niche differentiation among sympatric woodpecker species in forests of north-western India. Acta Ornithol. 2020, 55, 88–100. [Google Scholar] [CrossRef]
- Damoc, I.; Sahlean, T.; Ion, R.; Ion, M.; Meşter, L.E. Nesting preferences for two woodpecker species (Dendrocopos major and Dendrocopos medius) in Comana Forest, Southern Romania. Trav. Mus. d’Hist. Nat. Grigore Antipa 2014, 57, 35–45. [Google Scholar] [CrossRef]
- Ónodi, G.; Winkler, D. Nest site characteristics of the Great-spotted Woodpecker in a bottomland riparian forest in the presence of invasive tree species. Ornis Hung. 2016, 24, 81–95. [Google Scholar] [CrossRef]
- Martin, M.; Tremblay, J.A.; Ibarzabal, J.; Morin, H. An indicator species highlights continuous deadwood supply is a key ecological attribute of boreal old-growth forests. Ecosphere 2021, 12, e03507. [Google Scholar] [CrossRef]
- Rolstad, J.; Løken, B.; Rolstad, E. Habitat selection as a hierarchical spatial process: The green woodpecker at the northern edge of its distribution range. Oecologia 2000, 124, 116–129. [Google Scholar] [CrossRef]
- Mattioli, W.; Mancini, L.D.; Portoghesi, L.; Corona, P. Biodiversity conservation and forest management: The case of the sweet chestnut coppice stands in Central Italy. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2016, 150, 592–600. [Google Scholar] [CrossRef]
- Gondard, H.; Romane, F.; Regina, I.S.; Leonardi, S. Forest management and plant species diversity in chestnut stands of three Mediterranean areas. In Forest Diversity and Management; Springer: Berlin/Heidelberg, Germany, 2006; pp. 69–82. [Google Scholar]
- Robles, H.; Ciudad, C.; Vera, R.; Olea, P.P.; Purroy, F.J.; Matthysen, E. Sylvopastoral management and conservation of the middle spotted woodpecker at the south-western edge of its distribution range. For. Ecol. Manag. 2007, 242, 343–352. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).