Intertidal Oyster Reef Mapping and Population Analysis in West Galveston Bay, Texas
Abstract
1. Introduction
2. Materials and Methods
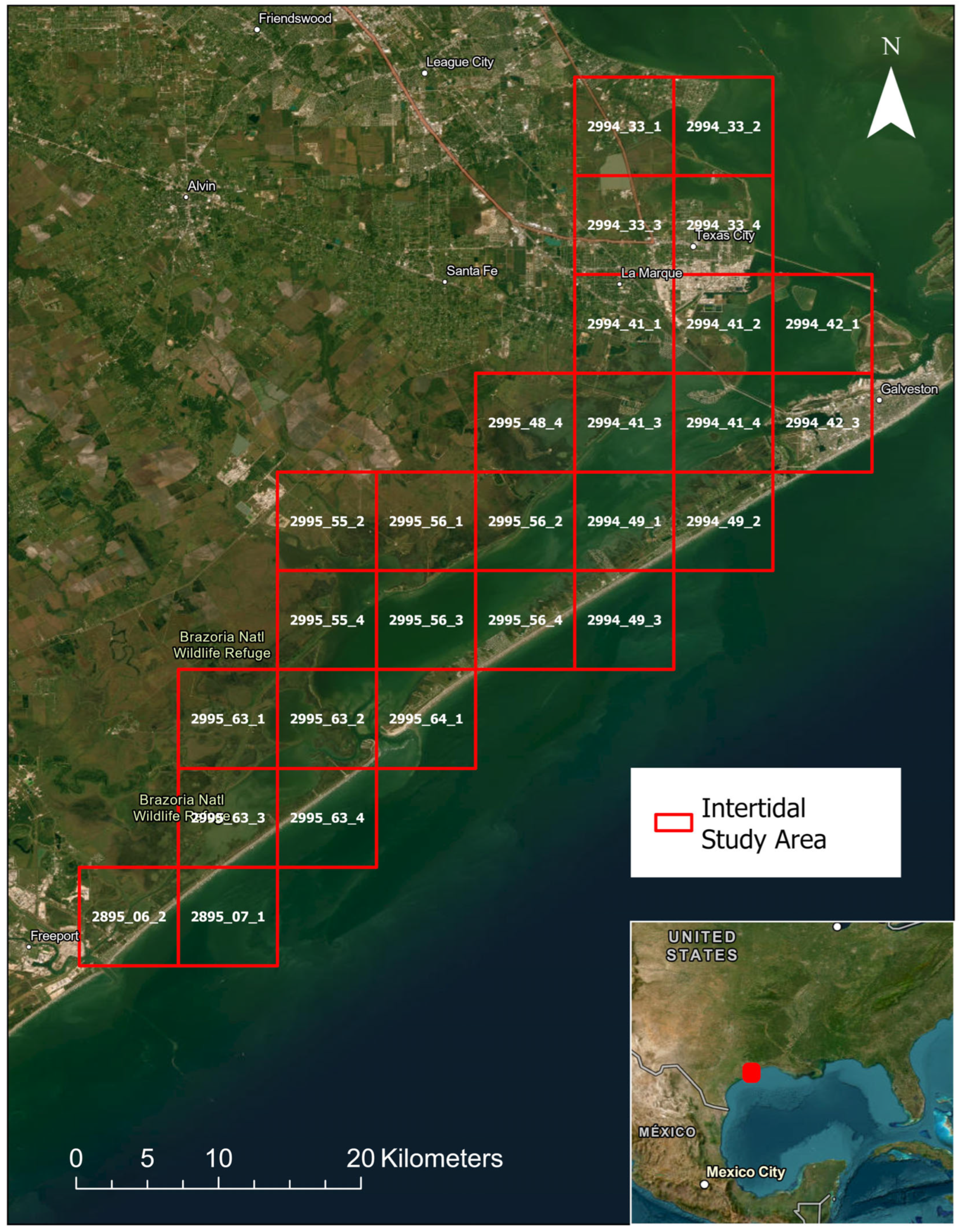
2.1. Study Site
2.2. GIS Analyses
2.3. Ground Truthing of GIS Models
2.4. Reef Characteristics and Oyster Population Structure
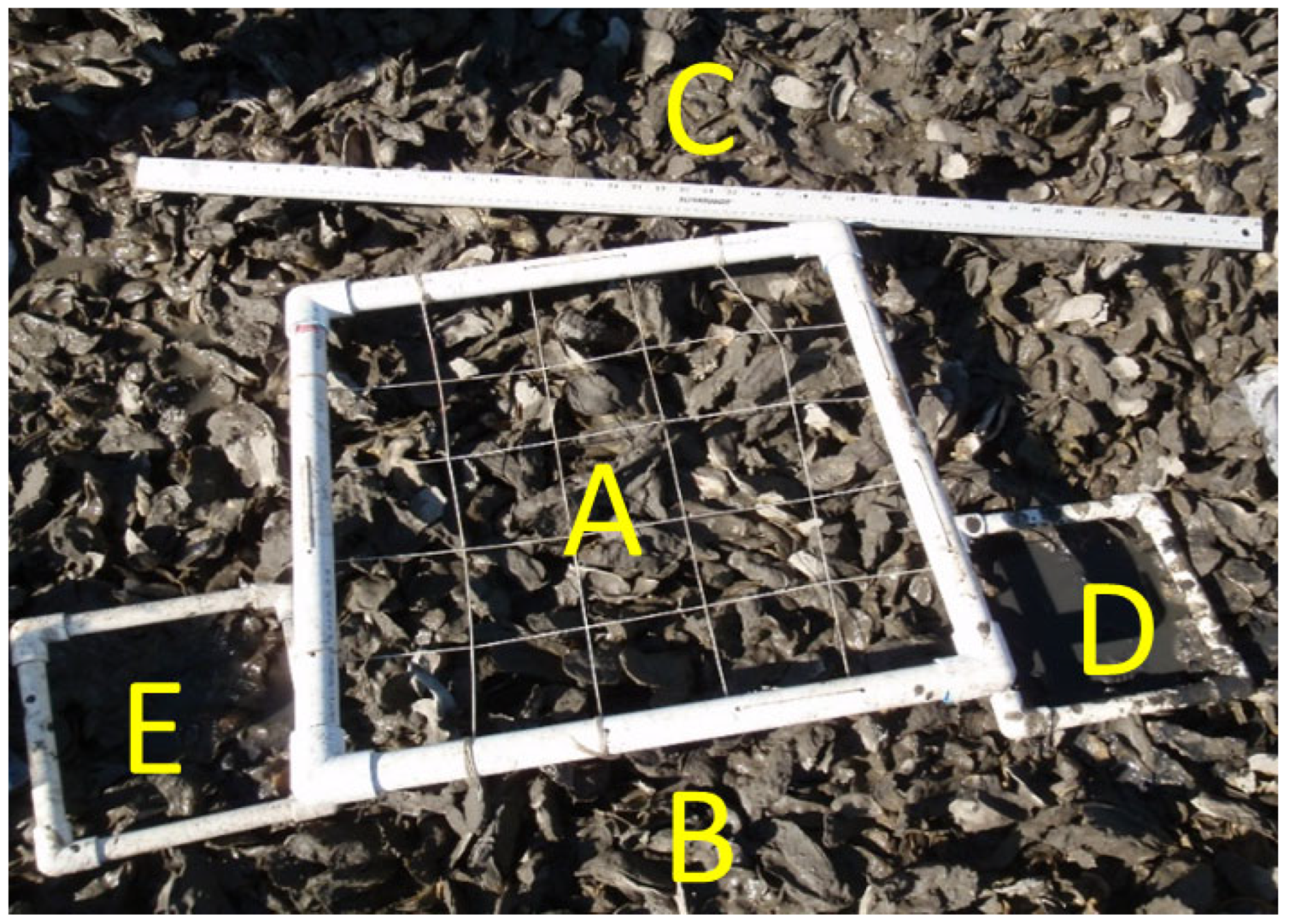
2.4.1. Intertidal Reef Characteristics and Oyster Demography
2.4.2. Oyster Condition Index
2.4.3. Reef Associated Benthic Macrofauna
2.5. Statistical Analysis
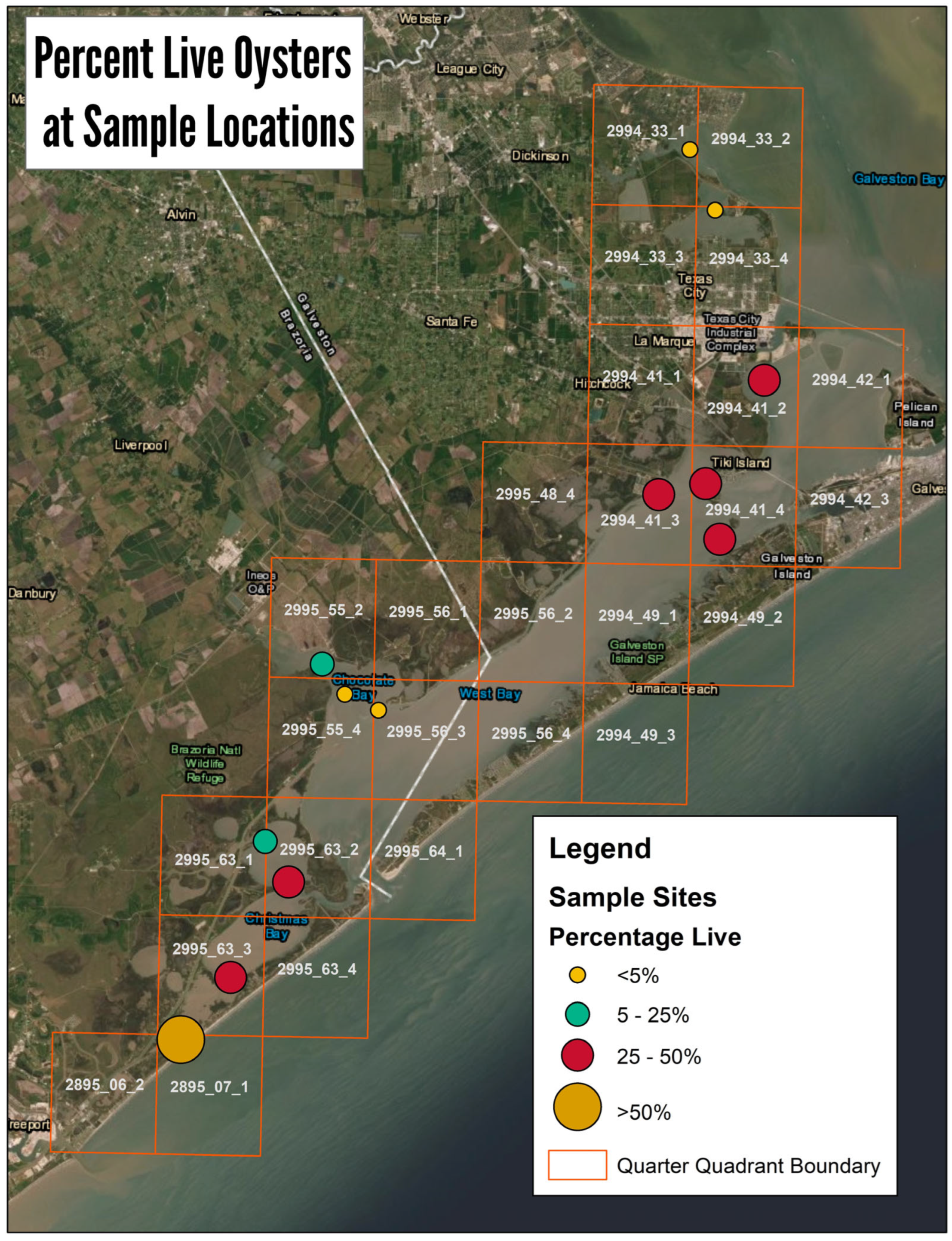
3. Results
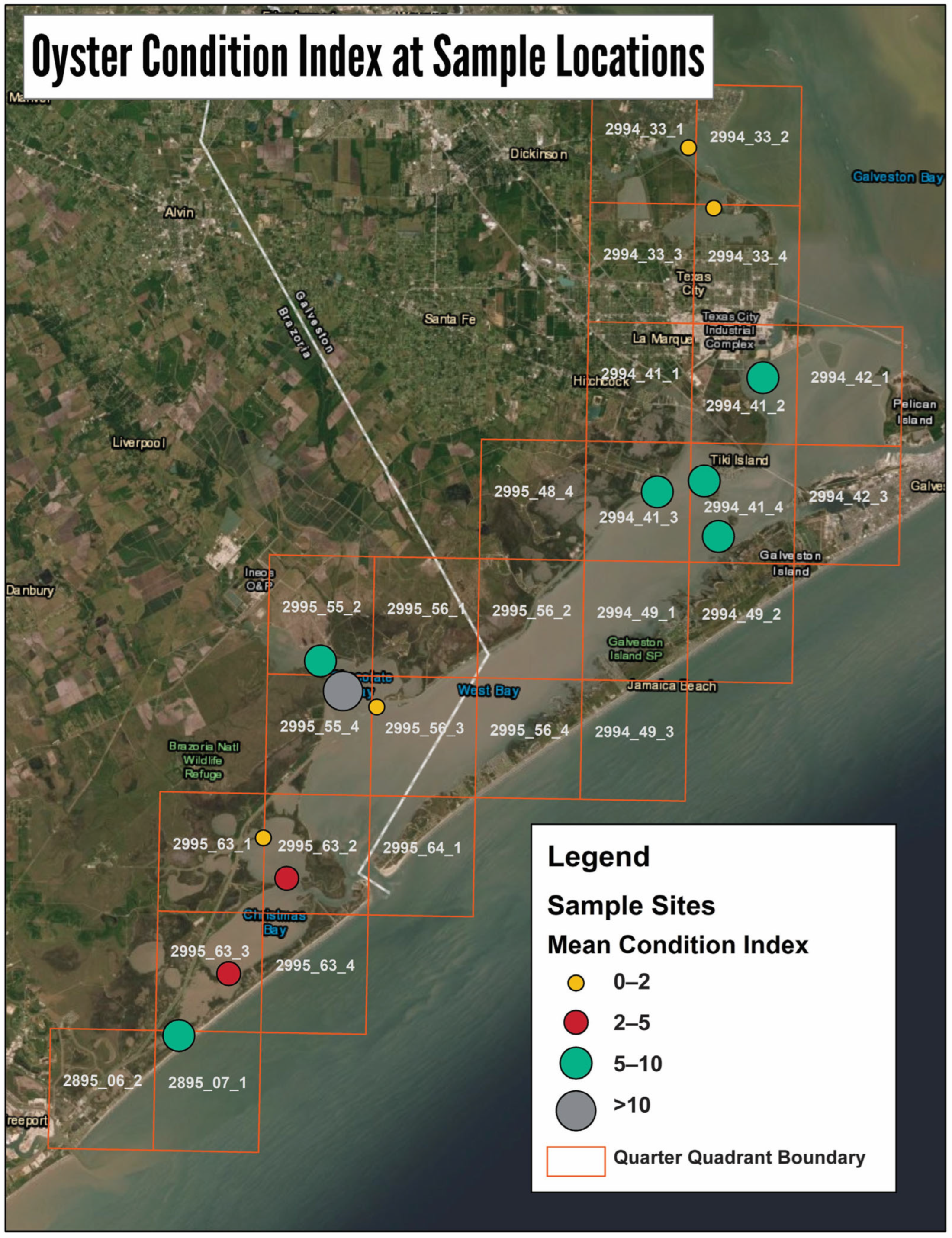
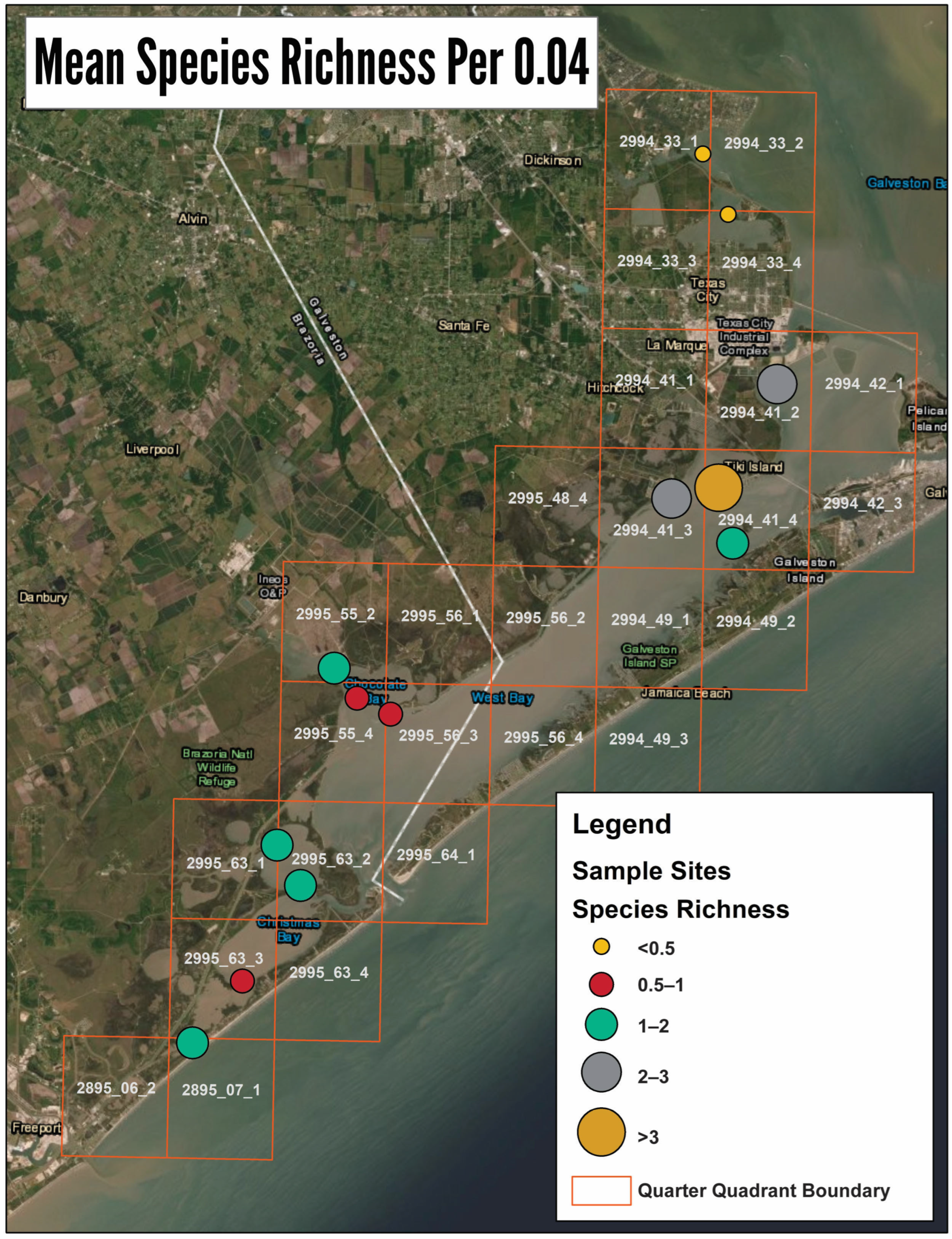
3.1. GIS Analysis and Groud Truthing
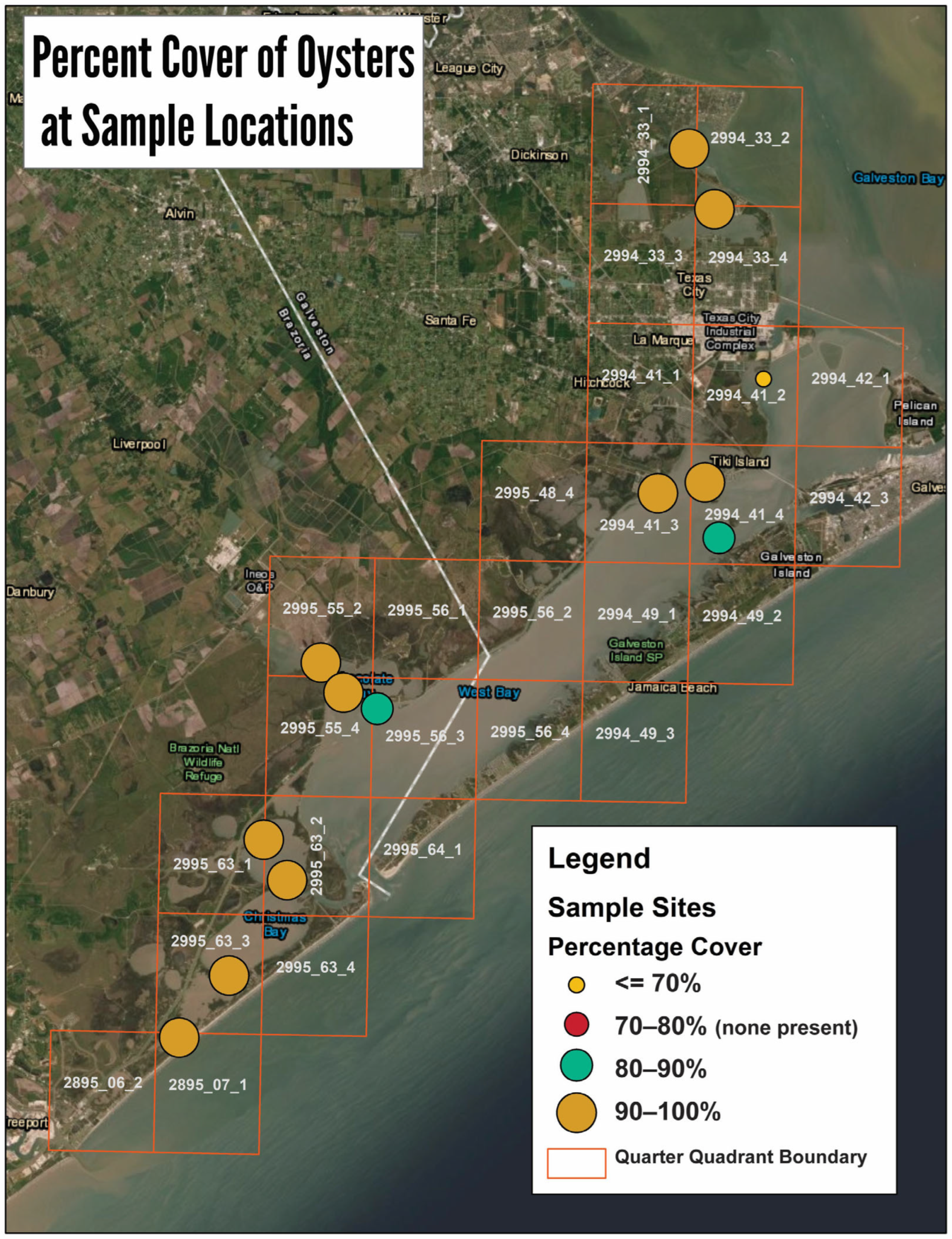
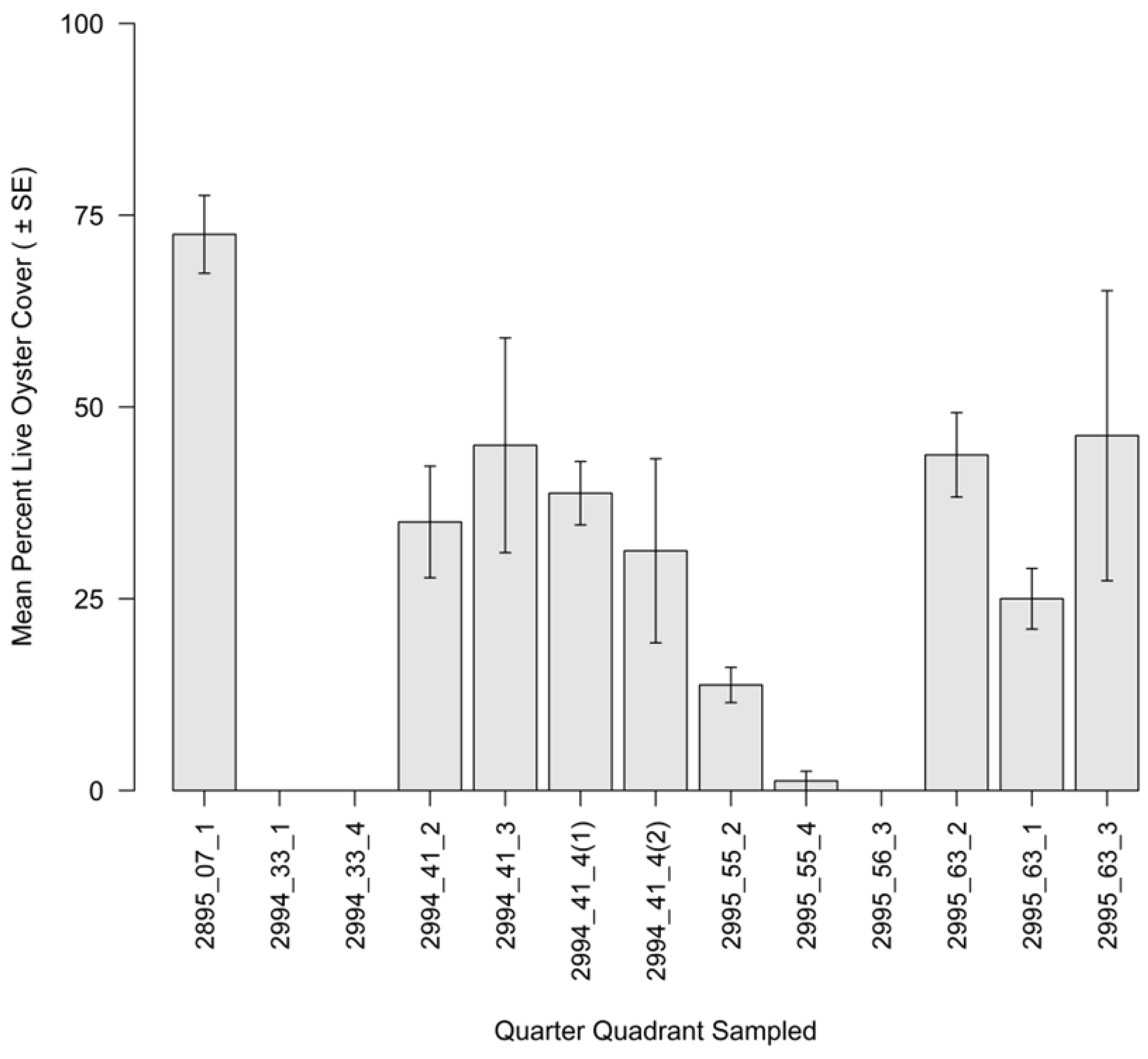
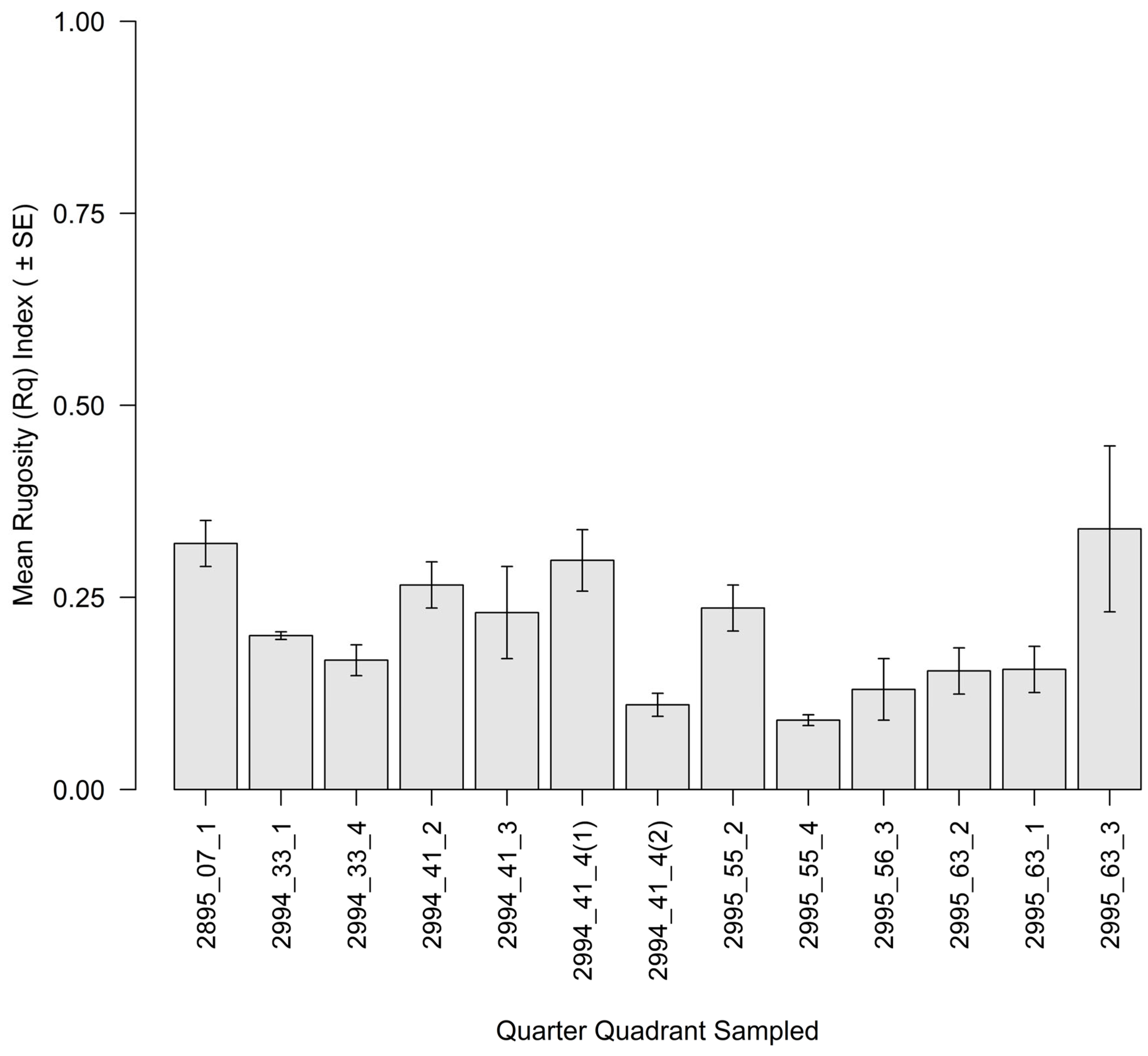
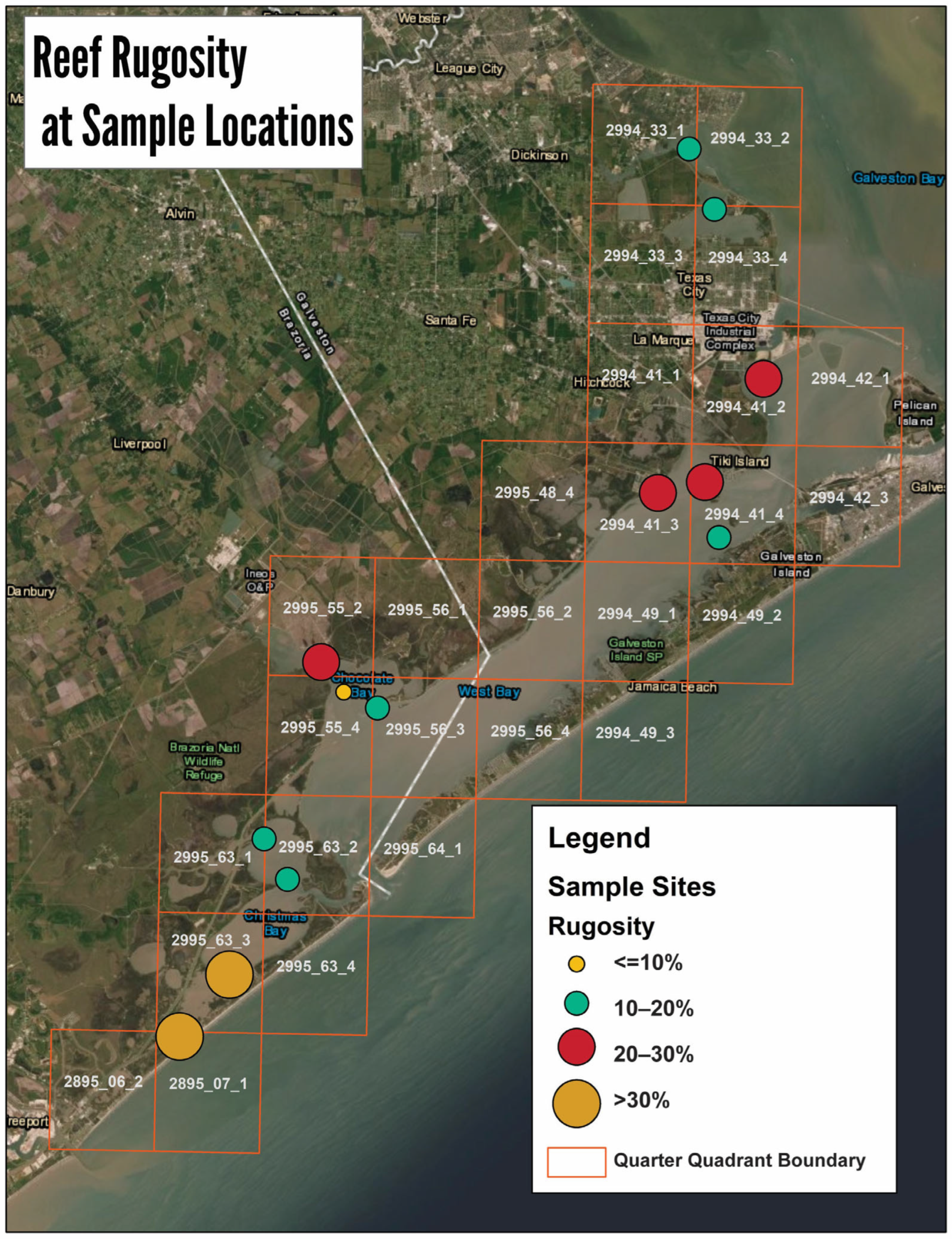
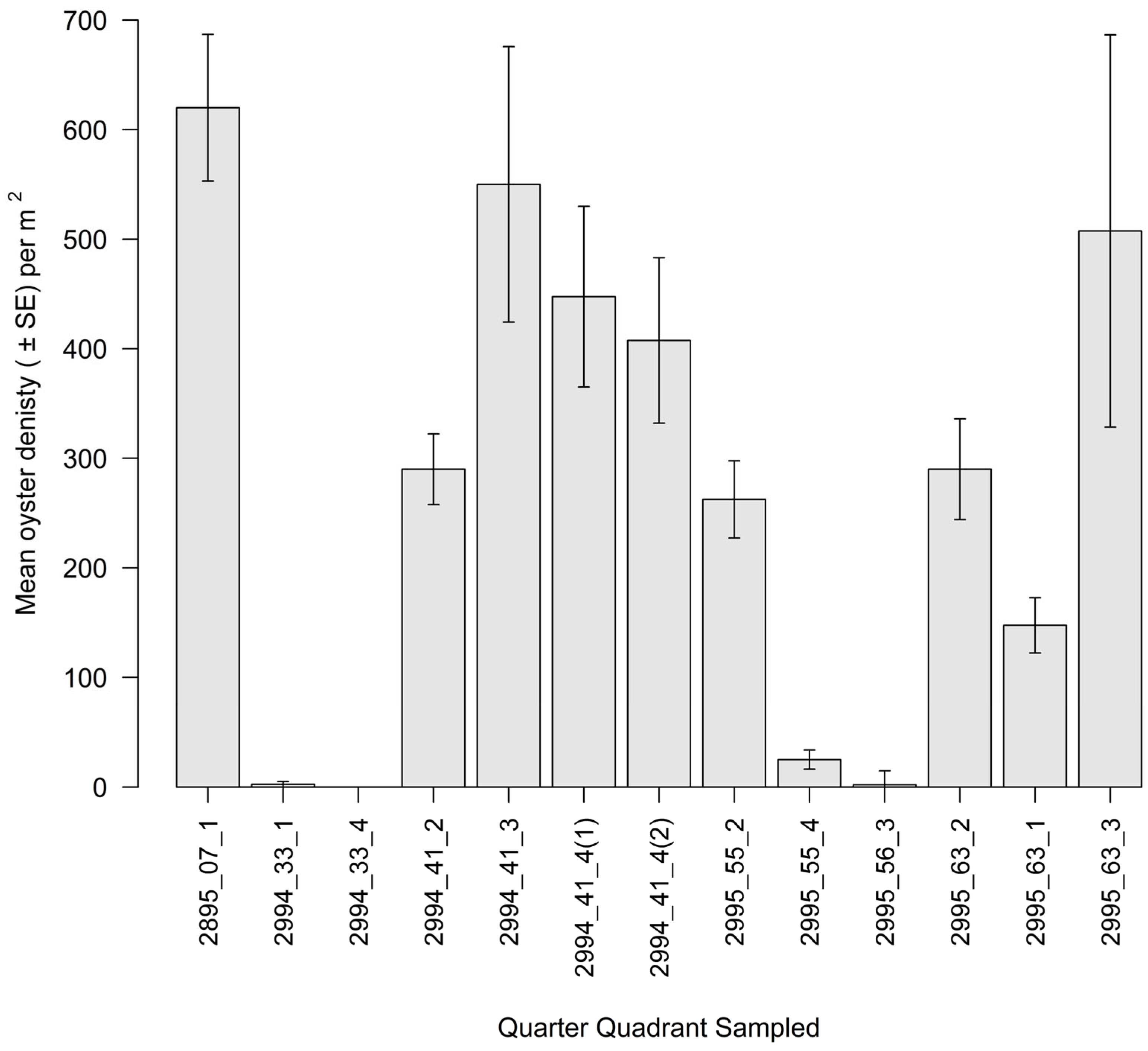
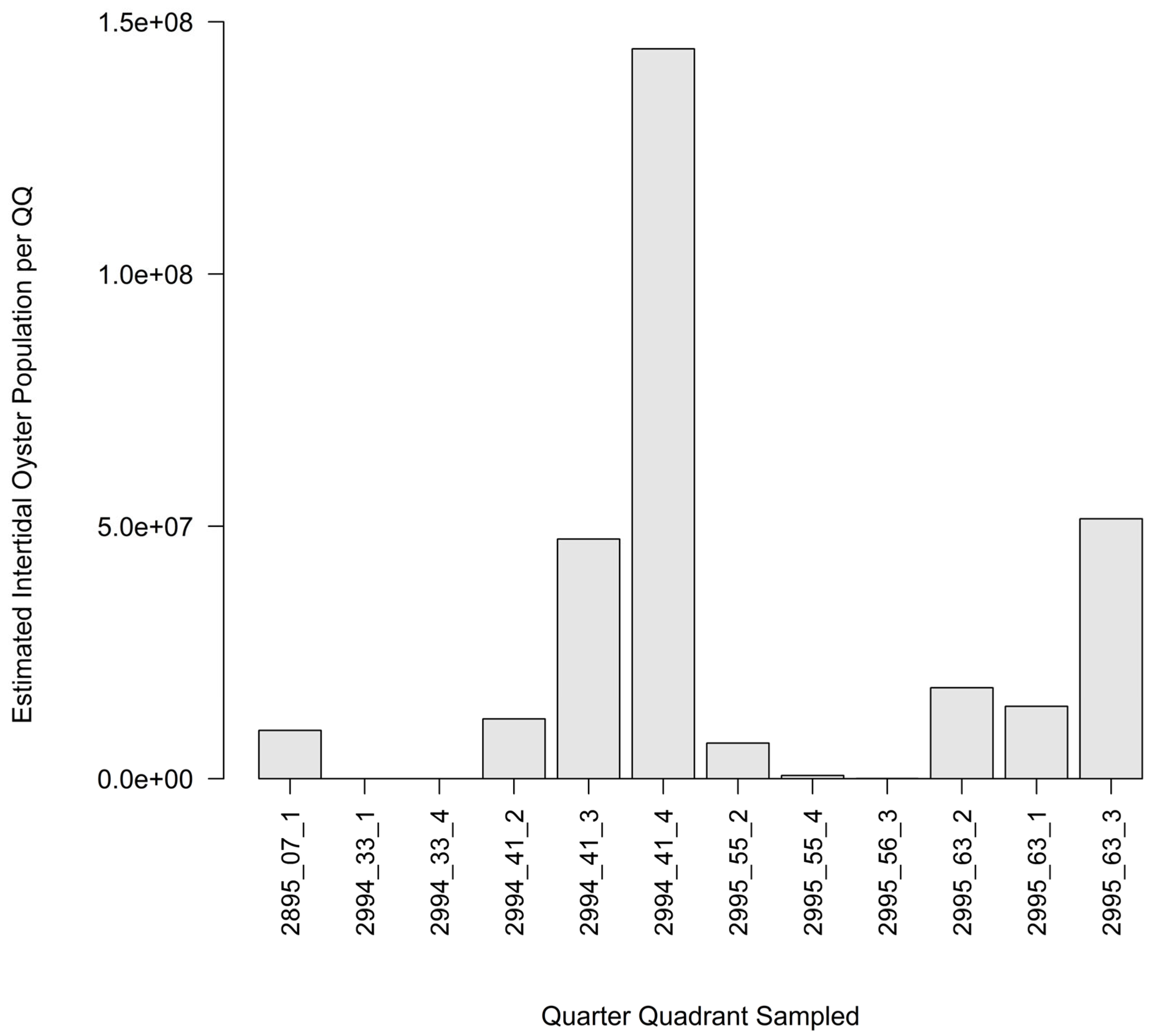
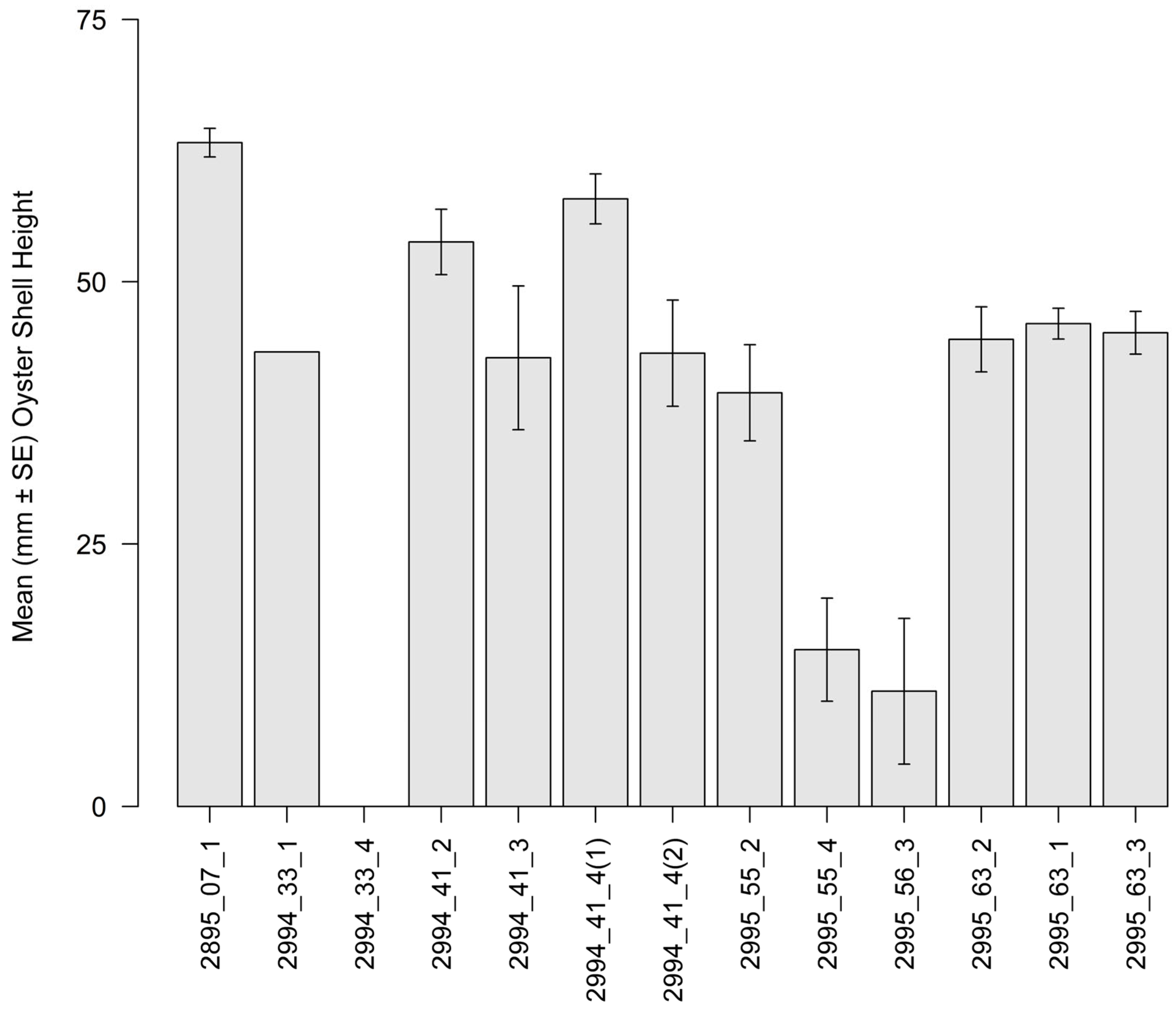
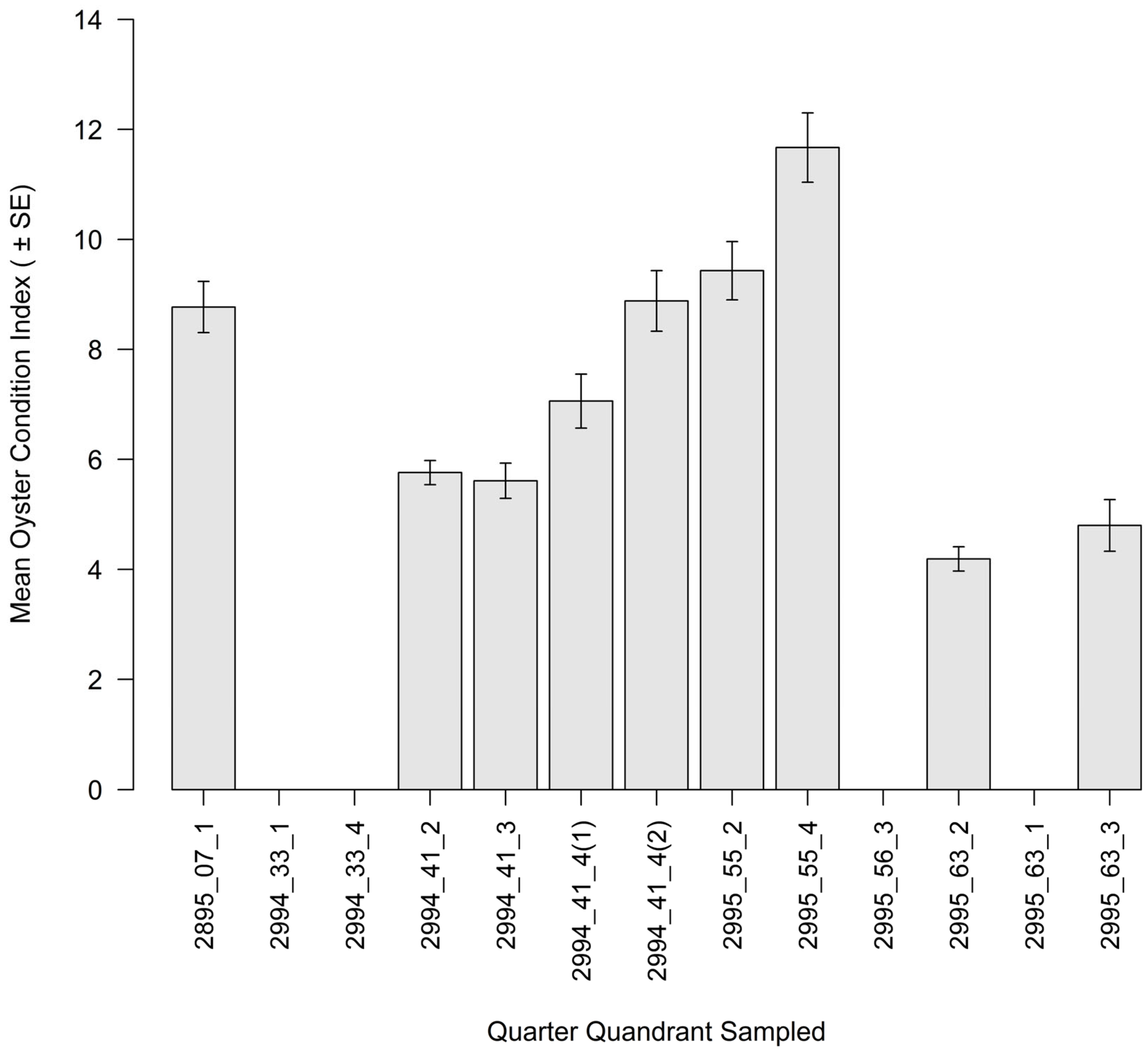
3.2. Intertidal Oyster Reef Characteristics and Oyster Demography
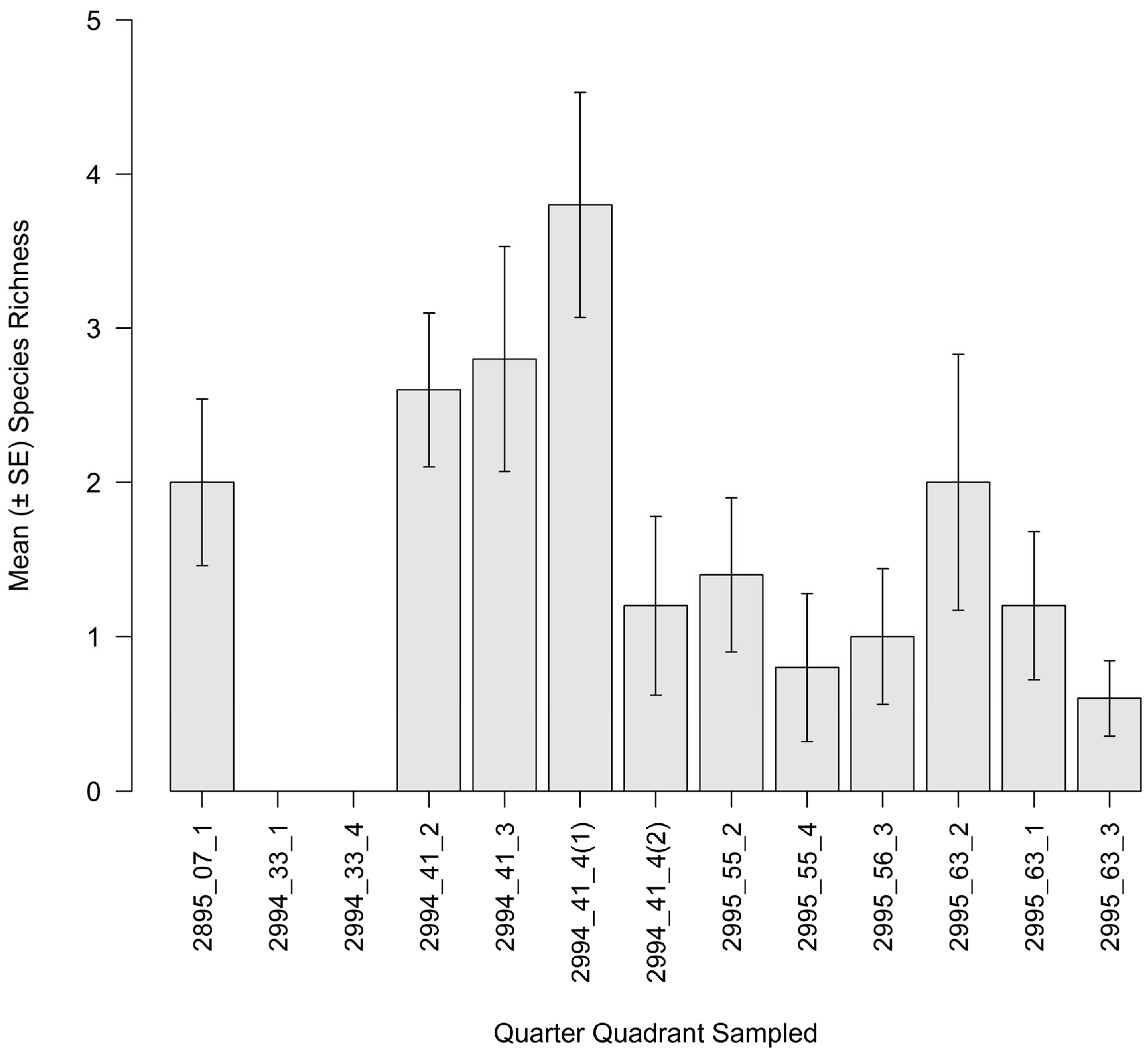
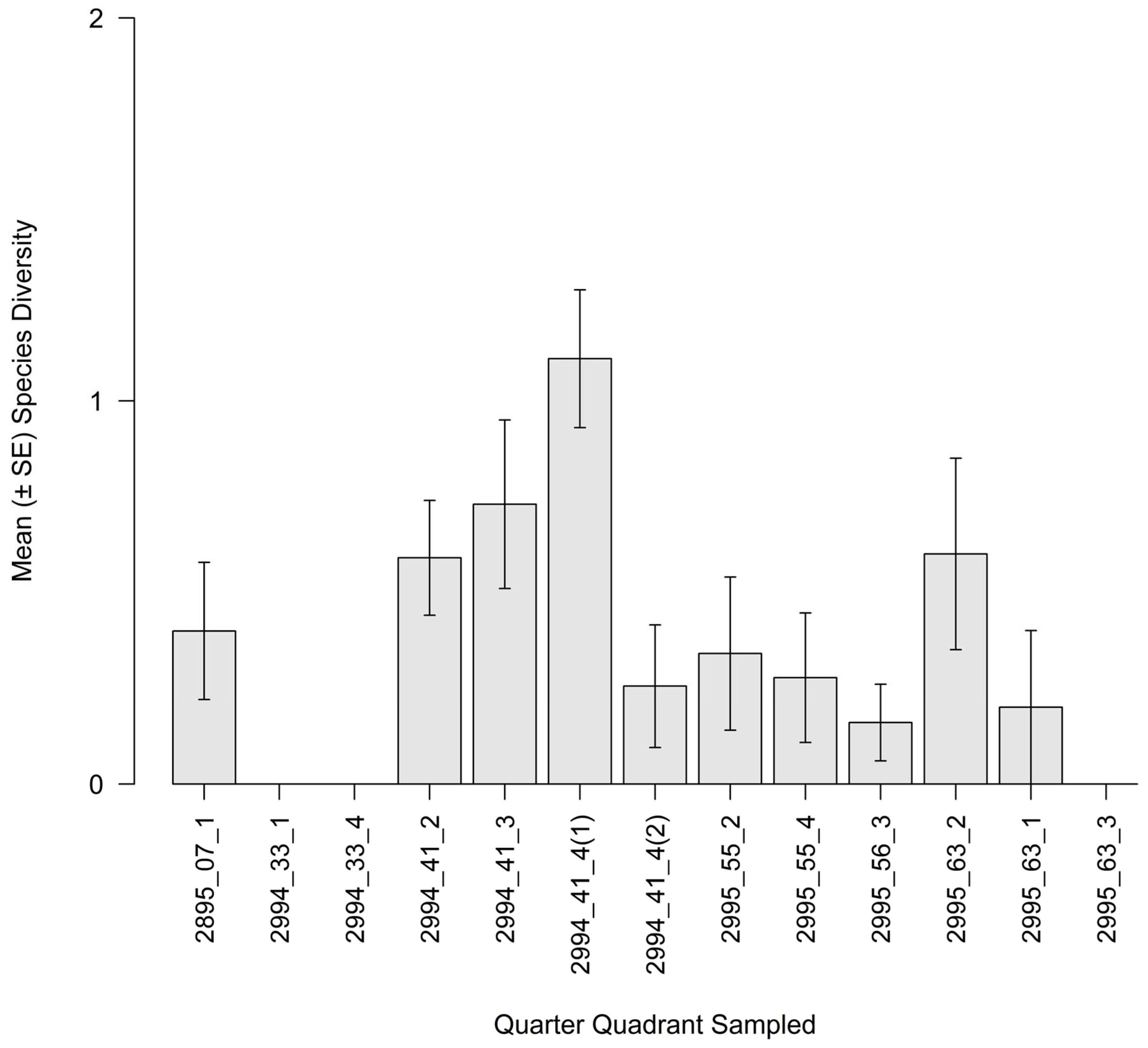
3.3. Intertidal Oyster Reef Associated Benthic Macrofauna (ABM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| QQ Number | Name | Confirmed Reef | Plausible Reef | Not Likely Reef | Not Reef | Area Analyzed |
|---|---|---|---|---|---|---|
| 2895_06_2 | Freeport NE | 90,186.16 | 90,186.16 | |||
| 2895_07_1 | Christmas Point OE S NW | 3174.25 | 12,209.26 | 865.37 | 16,248.88 | |
| 2994_33_1 | Texas City NW | 272.21 | 637.08 | 1027.81 | 1937.10 | |
| 2994_33_2 | Texas City NE | 15,109.90 | 15,109.90 | |||
| 2994_33_3 | Texas City SW | 169.69 | 169.69 | |||
| 2994_33_4 | Texas City SE | 1446.03 | 9918.51 | 417.27 | 2.46 | 11,784.247 |
| 2994_41_1 | Virgina Point NW | 261.28 | 0.86 | 122.94 | 385.08 | |
| 2994_41_2 | Virgina Point NE | 18,474.82 | 22,277.60 | 109,931.12 | 150,683.54 | |
| 2994_41_3 | Virgina Point SW | 2026.99 | 84,266.77 | 104,164.76 | 190,458.51 | |
| 2994_41_4 | Virgina Point SE | 11,485.48 | 326,827.67 | 196,194.97 | 534,508.12 | |
| 2994_42_1 | Galveston NW | 1014.84 | 1014.84 | |||
| 2994_42_3 | Galveston SW | 8218.50 | 8218.50 | |||
| 2994_49_1 | Lake Como NW | 13,393.96 | 13,393.96 | |||
| 2994_49_2 | Lake Como NE | 207.73 | 207.73 | |||
| 2994_49_3 | Lake Como SW | 134.66 | 134.66 | |||
| 2995_48_4 | Hitchcock SE | 897.41 | 897.41 | |||
| 2995_55_2 | Hoskins Mound NE | 1091.48 | 25,642.14 | 36,076.85 | 62,810.48 | |
| 2995_55_4 | Hoskins Mound SE | 9200.30 | 15,071.44 | 15,270.51 | 39,542,25 | |
| 2995_56_1 | Sea Isle NW | 5020.42 | 5020.42 | |||
| 2995_56_2 | Sea Isle NE | 6880.30 | 6880.30 | |||
| 2995_56_3 | Sea Isle SW | 3220.75 | 10,171.23 | 673.00 | 14,064.97 | |
| 2995_56_4 | Sea Isle SE | 12,907.54 | 12,907.54 | |||
| 2995_63_1 | Christmas Point NW | 3166.00 | 93,807.22 | 0.75 | 96,973.97 | |
| 2995_63_2 | Christmas Point SW | 1991.23 | 99,440.87 | 101,432.10 | ||
| 2995_64_1 | San Luis Pass NW | 37,098.04 | 37,098.04 | |||
| TOTAL | 59,931.98 | 758,197.12 | 687,975.20 | 1186.98 | 1150,291.28 |
| Alitta succinea | Amphipod sp. | Barnacle sp. | Boonea impressa | Brachidontes exustus | Crepidula fornicata | Eurypanopeus depressus | Ischadium recurvum | Panopeus simpsoni | Petrolisthes armatus | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2895_07_1 | 0 | 11 | 0 | 0 | 12 | 0 | 2 | 6 | 1 | 2 |
| 2994_41_2 | 0 | 0 | 4 | 0 | 57 | 1 | 1 | 18 | 0 | 0 |
| 2994_41_3 | 0 | 3 | 0 | 1 | 5 | 3 | 4 | 2 | 0 | 23 |
| 2994_41_4 (1) | 0 | 1 | 0 | 3 | 15 | 2 | 6 | 7 | 0 | 11 |
| 2994_41_4 (2) | 0 | 1 | 0 | 0 | 0 | 0 | 6 | 4 | 0 | 12 |
| 2995_55_2 | 1 | 1 | 0 | 0 | 3 | 0 | 6 | 0 | 0 | 0 |
| 2995_55_4 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| 2995_56_3 | 2 | 14 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 2995_63_1 | 0 | 0 | 0 | 0 | 4 | 0 | 1 | 2 | 0 | 0 |
| 2995_63_2 | 0 | 33 | 0 | 0 | 22 | 0 | 5 | 0 | 1 | 0 |
| 2995_63_3 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 0 |
References
- Vieira, S.; Sroczyńska, K.; Neves, J.; Martins, M.; Costa, M.H.; Adão, H.; Vicente, C.S.L. Distribution Patterns of Benthic Bacteria and Nematode Communities in Estuarine Sediments. Estuar. Coast Shelf Sci. 2023, 291, 108448. [Google Scholar] [CrossRef]
- Suzzi, A.L.; Stat, M.; Gaston, T.F.; Huggett, M.J. Spatial Patterns in Host-Associated and Free-Living Bacterial Communities Across Six Temperate Estuaries. FEMS Microbiol. Ecol. 2023, 99, fiad061. [Google Scholar] [CrossRef] [PubMed]
- Gignoux-Wolfsohn, S.; Ruiz, M.G.; Barron, D.P.; Ruiz, G.; Lohan, K. Bivalve Microbiomes Are Shaped by Host Species, Size, Parasite Infection, and Environment. PeerJ 2024, 12, e18082. [Google Scholar] [CrossRef]
- Carroll, J.M.; Dashiell, R.; Watts, J.C.; Hunter, E.A. Tidal Level Affects the Prevalence and Impacts of Pests and Parasites on Oysters (Crassostrea virginica) on Intertidal Reefs in Georgia, USA. Mar. Biol. 2021, 168, 45. [Google Scholar] [CrossRef]
- LaRoche, R.A.; Doan, T.M.; Hanke, M.H. Habitat Characteristics of Artificial Oyster Reefs Influence Female Oystershell Mud Crab Panopeus simpsoni Rathbun, 1930 (Decapoda: Brachyura: Panopeidae). J. Crustac. Biol. 2022, 42, ruac033. [Google Scholar] [CrossRef]
- Hanke, M.H.; Posey, M.H.; Alphin, T.D. The Effects of Intertidal Oyster Reef Habitat Characteristics on Faunal Utilization. Mar. Ecol. Prog. Ser. 2017, 581, 57–70. [Google Scholar] [CrossRef]
- Hanke, M.H.; Posey, M.H.; Alphin, T.D. The Influence of Habitat Characteristics on Intertidal Oyster Crassostrea virginica Populations. Mar. Ecol. Prog. Ser. 2017, 571, 121–138. [Google Scholar] [CrossRef]
- Smith, T.M.; Hindell, J.S.; Jenkins, G.P.; Connolly, R.M. Seagrass Patch Size Affects Fish Responses to Edges. J. Anim. Ecol. 2010, 79, 275–281. [Google Scholar] [CrossRef]
- Fagan, W.F.; Cantrell, R.S.; Cosner, C. How Habitat Edges Change Species Interactions. Am. Nat. 1999, 153, 165–182. [Google Scholar] [CrossRef]
- Byers, J.E.; Rogers, T.L.; Grabowski, J.H.; Hughes, R.A.; Piehler, M.F.; Kimbro, D.L. Host and Parasite Recruitment Correlated at a Regional Scale. Oecologia 2014, 174, 731–738. [Google Scholar] [CrossRef]
- Byers, J.E.; Grabowski, J.H.; Piehler, M.F.; Hughes, A.R.; Weiskel, H.W.; Malek, J.C.; Kimbro, D.L. Geographic Variation in Intertidal Oyster Reef Properties and the Influence of Tidal Prism. Limnol. Oceanogr. 2015, 60, 1051–1063. [Google Scholar] [CrossRef]
- Peterson, C.; Grabowski, J.; Powers, S. Estimated Enhancement of Fish Production Resulting from Restoring Oyster Reef Habitat: Quantitative Valuation. Mar. Ecol. Prog. Ser. 2003, 264, 249–264. [Google Scholar] [CrossRef]
- Tolley, S.; Volety, A. The Role of Oysters in Habitat Use of Oyster Reefs by Resident Fishes and Decapod Crustaceans. J. Shellfish Res. 2005, 24, 1007–1012. [Google Scholar]
- Harwell, H.D.; Posey, M.H.; Alphin, T.D. Landscape Aspects of Oyster Reefs: Effects of Fragmentation on Habitat Utilization. J. Exp. Mar. Biol. Ecol. 2011, 409, 30–41. [Google Scholar] [CrossRef]
- Grabowski, J.; Powers, S. Habitat Complexity Mitigates Trophic Transfer on Oyster Reefs. Mar. Ecol. Prog. Ser. 2004, 277, 291–295. [Google Scholar] [CrossRef]
- Posey, M.H.; Alphin, T.D.; Powell, C.M. Use of Oyster Reefs as Habitat for Epibenthic Fish and Decapods. In Oyster Reef Restoration: A Synopsis and Synthesis of Approach; Luckenback, M., Mann, R., Wesson, J., Eds.; Virginia Institute of Marine Science Press: Gloucester Point, VA, USA, 1999; pp. 229–237. [Google Scholar]
- Wernberg, T.; Thomsen, M.S.; Baum, J.K.; Bishop, M.J.; Bruno, J.F.; Coleman, M.A.; Filbee-Dexter, K.; Gagnon, K.; He, Q.; Murdiyarso, D.; et al. Impacts of Climate Change on Marine Foundation Species. Ann. Rev. Mar. Sci. 2024, 16, 247–282. [Google Scholar] [CrossRef]
- Leong, R.C.; Bugnot, A.B.; Ross, P.M.; Erickson, K.R.; Gibbs, M.C.; Marzinelli, E.M.; O’Connor, W.A.; Parker, L.M.; Poore, A.G.B.; Scanes, E.; et al. Recruitment of a Threatened Foundation Oyster Species Varies with Large and Small Spatial Scales. Ecol. Appl. 2024, 34, e2968. [Google Scholar] [CrossRef]
- Whitman, E.; Reidenbach, M. Benthic Flow Environments Affect Recruitment of Crassostrea virginica Larvae to an Intertidal Oyster Reef. Mar. Ecol. Prog. Ser. 2012, 463, 177–191. [Google Scholar] [CrossRef]
- Lenihan, H.S. Physical—Biological Coupling on Oyster Reefs: How Habitat Structure Influences Individual Performance. Ecol. Monogr. 1999, 69, 251–275. [Google Scholar]
- Dame, R.; Alber, M.; Allen, D.; Mallin, M.; Montague, C.; Lewitus, A.; Chalmers, A.; Gardern, R.; Gilman, C.; Kjerfve, B.; et al. Estuaries of the South Atlantic Coast of North America: Their Geographical Signatures. Estuaries 2000, 23, 793–819. [Google Scholar] [CrossRef]
- Anderson, M.J.; Connell, S.D. Predation by Fish on Intertidal Oysters. Mar. Ecol. Prog. Ser. 1999, 187, 203–211. [Google Scholar] [CrossRef]
- Saoud, I.G.; Rouse, D.B. Evaluating Sediment Accretion on a Relic Oyster Reef in Mobile Bay, Alabama. J. Appl. Aquac. 2000, 10, 41–49. [Google Scholar] [CrossRef]
- Southwell, M.W.; Veenstra, J.J.; Adams, C.D.; Scarlett, E.V.; Payne, K.B. Changes in Sediment Characteristics upon Oyster Reef Restoration, NE Florida, USA. J. Coast. Zone Manag. 2017, 20, 1000442. [Google Scholar] [CrossRef]
- Caretti, O.N.; Bohnenstiehl, D.W.R.; Eggleston, D.B. Spatiotemporal Variability in Sedimentation Drives Habitat Loss on Restored Subtidal Oyster Reefs. Estuaries Coasts 2021, 44, 2100–2117. [Google Scholar] [CrossRef]
- Salvador de Paiva, J.N.; Walles, B.; Ysebaert, T.; Bouma, T.J. Understanding the Conditionality of Ecosystem Services: The Effect of Tidal Flat Morphology and Oyster Reef Characteristics on Sediment Stabilization by Oyster Reefs. Ecol. Eng. 2018, 112, 89–95. [Google Scholar] [CrossRef]
- Uddin, M.J.; Smith, K.J.; Hargis, C.W. Development of Pervious Oyster Shell Habitat (POSH) Concrete for Reef Restoration and Living Shorelines. Constr. Build. Mater. 2021, 295, 123685. [Google Scholar] [CrossRef]
- Piazza, B.P.; Banks, P.D.; La Peyre, M.K. The Potential for Created Oyster Shell Reefs as a Sustainable Shoreline Protection Strategy in Louisiana. Restor. Ecol. 2005, 13, 499–506. [Google Scholar] [CrossRef]
- Morris, R.L.; La Peyre, M.K.; Webb, B.M.; Marshall, D.A.; Bilkovic, D.M.; Cebrian, J.; Mcclenachan, G.; Kibler, K.M.; Walters, L.J.; Bushek, D.; et al. Large-Scale Variation in Wave Attenuation of Oyster Reef Living Shorelines and the Influence of Inundation Duration. Ecol. Appl. 2021, 31, e02382. [Google Scholar] [CrossRef]
- Morris, R.L.; Bilkovic, D.M.; Boswell, M.K.; Bushek, D.; Cebrian, J.; Goff, J.; Kibler, K.M.; La Peyre, M.K.; McClenachan, G.; Moody, J.; et al. The Application of Oyster Reefs in Shoreline Protection: Are We over-Engineering for an Ecosystem Engineer? J. Appl. Ecol. 2019, 56, 1703–1711. [Google Scholar] [CrossRef]
- Graham, P.M.; Palmer, T.A.; Beseres Pollack, J. Oyster Reef Restoration: Substrate Suitability May Depend on Specific Restoration Goals. Restor. Ecol. 2017, 25, 459–470. [Google Scholar] [CrossRef]
- Hanke, M.H.; Bobby, N.; Sanchez, R. Can Relic Shells Be an Effective Settlement Substrate for Oyster Reef Restoration? Restor. Ecol. 2021, 29, 3–6. [Google Scholar] [CrossRef]
- Beck, M.W.; Brumbaugh, R.D.; Airoldi, L.; Carranza, A.; Coen, L.D.; Crawford, C.; Defeo, O.; Edgar, G.J.; Hancock, B.; Kay, M.C.; et al. Oyster Reefs at Risk and Recommendations for Conservation, Restoration, and Management. Bioscience 2011, 61, 107–116. [Google Scholar] [CrossRef]
- Goelz, T.; Vogt, B.; Hartley, T. Alternative Substrates Used for Oyster Reef Restoration: A Review. J. Shellfish Res. 2020, 39, 1–12. [Google Scholar] [CrossRef]
- George, L.M.; De Santiago, K.; Palmer, T.A.; Beseres Pollack, J. Oyster Reef Restoration: Effect of Alternative Substrates on Oyster Recruitment and Nekton Habitat Use. J. Coast. Conserv. 2015, 19, 13–22. [Google Scholar] [CrossRef]
- Coen, L.D.; Luckenbach, M.W. Developing Success Criteria and Goals for Evaluating Oyster Reef Restoration: Ecological Function or Resource Exploitation? Ecol. Eng. 2000, 15, 323–343. [Google Scholar] [CrossRef]
- Powers, S.; Peterson, C.; Grabowski, J.; Lenihan, H. Success of Constructed Oyster Reefs in No-Harvest Sanctuaries: Implications for Restoration. Mar. Ecol. Prog. Ser. 2009, 389, 159–170. [Google Scholar] [CrossRef]
- Grizzle, R.; Ward, K.; Geselbracht, L.; Birch, A. Distribution and Condition of Intertidal Eastern Oyster (Crassostrea virginica) Reefs in Apalachicola Bay Florida Based on High-Resolution Satellite Imagery. J. Shellfish Res. 2018, 37, 1027–1038. [Google Scholar] [CrossRef]
- Zu Ermgassen, P.S.E.; Spalding, M.D.; Grizzle, R.E.; Brumbaugh, R.D. Quantifying the Loss of a Marine Ecosystem Service: Filtration by the Eastern Oyster in US Estuaries. Estuaries Coasts 2012, 36, 36–43. [Google Scholar] [CrossRef]
- Grizzle, R.E.; Brodeur, M.A.; Abeels, H.A.; Greene, J.K. Bottom Habitat Mapping Using Towed Underwater Videography: Subtidal Oyster Reefs as an Example Application. J. Coast. Res. 2008, 24, 103–109. [Google Scholar] [CrossRef][Green Version]
- Windle, A.E.; Puckett, B.; Huebert, K.B.; Knorek, Z.; Johnston, D.W.; Ridge, J.T. Estimation of Intertidal Oyster Reef Density Using Spectral and Structural Characteristics Derived from Unoccupied Aircraft Systems and Structure from Motion Photogrammetry. Remote Sens. 2022, 14, 2163. [Google Scholar] [CrossRef]
- Le Bris, A.; Rosa, P.; Lerouxel, A.; Cognie, B.; Gernez, P.; Launeau, P.; Robin, M.; Barillé, L. Hyperspectral Remote Sensing of Wild Oyster Reefs. Estuar. Coast. Shelf Sci. 2016, 172, 1–12. [Google Scholar] [CrossRef]
- Adams, R.E.; Dijkstra, J.R.; Semme, J.; Smith, B.; Ward, R.W. Mapping and Characterizing Subtidal Oyster Reefs Using Acoustic Techniques, Underwater Videography and Quadrat Counts. Am. Fish. Soc. Symp. 2005, 41, 153–159. [Google Scholar]
- Allen, Y.C.; Wilson, C.A.; Roberts, H.H.; Supan, J. High Resolution Mapping and Classification of Oyster Habitats in Nearshore Louisiana Using Sidescan Sonar. Estuaries 2005, 28, 435–446. [Google Scholar] [CrossRef]
- Espriella, M.C.; Lecours, V.; Camp, E.V.; Andrew Lassiter, H.; Wilkinson, B.; Frederick, P.C.; Pittman, S.J. Drone Lidar-Derived Surface Complexity Metrics as Indicators of Intertidal Oyster Reef Condition. Ecol. Indic. 2023, 150, 110190. [Google Scholar] [CrossRef]
- Cannon, D.J.; Kibler, K.M.; Taye, J.; Medeiros, S.C. Characterizing Canopy Complexity of Natural and Restored Intertidal Oyster Reefs (Crassostrea virginica) with a Novel Laser-Scanning Method. Restor. Ecol. 2023, 31, e13973. [Google Scholar] [CrossRef]
- Wirth, E.E.; Scott, G.I.; Fulton, M.H.; Van Dolah, R.F.; Maier, R.R.; Hadley, N.; Daugomah, J.W.; Key, R.B. In Situ Monitoring of Dredged Material Spoil Sites Using the Oyster Crassostrea virginica. Arch. Environ. Contam. Toxicol. 1996, 30, 340–348. [Google Scholar] [CrossRef]
- Marenghi, F.; Ashton-Alcox, K.; Wong, R.; Reynolds, B.; Ozbay, G. Dredge Efficiency on Natural Oyster Grounds in Delaware Bay and Its Application in Monitoring the Eastern Oyster (Crassostrea virginica) Stock in Delaware, USA. Fish Res. 2017, 186, 292–300. [Google Scholar] [CrossRef]
- Lehnert, R.; Allen, D. Nekton Use of Subtidal Oyster Shell Habitat in a Southeastern US Estuary. Estuaries 2002, 25, 1015–1024. [Google Scholar] [CrossRef]
- Outhwaite, A.; Lebreton, B.; Palmer, T.A.; Beseres Pollack, J. Habitat Provision Differs Across Subtidal Reefs Varying in Location Within the Estuarine Landscape. Estuaries Coasts 2024, 47, 1345–1358. [Google Scholar] [CrossRef]
- Griffitt, J.; Posey, M.H.; Alphin, T.D. Effects of Edge Fragmentation on Oyster Reef Utilization by Transient Nekton. J. Elisha Mitchell Sci. Soc. 1999, 115, 98–103. [Google Scholar]
- Kjelland, M.E.; Piercy, C.D.; Lackey, T.; Swannack, T.M. An Integrated Modeling Approach for Elucidating the Effects of Different Management Strategies on Chesapeake Bay Oyster Metapopulation Dynamics. Ecol. Model. 2015, 308, 45–62. [Google Scholar] [CrossRef]
- Theuerkauf, S.J.; Puckett, B.J.; Eggleston, D.B. Metapopulation Dynamics of Oysters: Sources, Sinks, and Implications for Conservation and Restoration. Ecosphere 2021, 12, e03573. [Google Scholar] [CrossRef]
- Arnold, W.S.; Meyers, S.D.; Geiger, S.P.; Luther, M.E.; Narváez, D.; Frischer, M.E.; Hofmann, E. Applying a Coupled Biophysical Model to Predict Larval Dispersal and Source/Sink Relationships in a Depleted Metapopulation of the Eastern Oyster Crassostrea virginica. J. Shellfish Res. 2017, 36, 101–118. [Google Scholar] [CrossRef]
- Rivera, M. Texas Oyster Industry Tries Comeback after Harvey Devastation. New York Post, 17 July 2018. [Google Scholar]
- Texas Parks and Wildlife Texas Commercial Fishing Regulations Summary. 2024. Available online: https://tpwd.texas.gov/publications/pwdpubs/media/pwd_bk_v3400_0074.pdf (accessed on 10 February 2025).
- Carter, L.J. Galveston Bay: Test Case of an Estuary in Crisis. Science 1970, 167, 1102–1108. [Google Scholar] [CrossRef]
- Salas-Monreal, D.; Anis, A.; Salas-de-Leon, D.A. Galveston Bay Dynamics under Different Wind Conditions. Oceanologia 2018, 60, 232–243. [Google Scholar] [CrossRef]
- Du, J.; Park, K.; Dellapenna, T.M.; Clay, J.M. Dramatic Hydrodynamic and Sedimentary Responses in Galveston Bay and Adjacent Inner Shelf to Hurricane Harvey. Sci. Total Environ. 2019, 653, 554–564. [Google Scholar] [CrossRef]
- Margiotta, A.M.; Shervette, V.R.; Hadley, N.H.; Plante, C.J.; Wilber, D.H. Species-Specific Responses of Resident Crabs to Vertical Habitat Complexity on Intertidal Oyster Reefs. J. Exp. Mar. Biol. Ecol. 2016, 477, 7–13. [Google Scholar] [CrossRef]
- Lawrence, D.R.; Scott, G.I. The Determination and Use of Condition Index of Oysters. Estuaries 1982, 5, 23–27. [Google Scholar] [CrossRef]
- Abbe, G.R.; Albright, B.W. An Improvement to the Determination of Meat Condition Index for the Eastern Oyster Crassostrea virginica (Gmelin 1791). J. Shellfish Res. 2003, 22, 747–752. [Google Scholar]
- Hanke, M.H.; Hargrove, J.M.; Alphin, T.D.; Posey, M.H. Oyster Utilization and Host Variation of the Oyster Pea Crab (Zaops ostreum). J. Shellfish Res. 2015, 34, 281–287. [Google Scholar] [CrossRef]
- Baggett, L.P.; Powers, S.P.; Brumbaugh, R.; Coen, L.D.; DeAngelis, B.; Greene, J.; Hancock, B.; Morlock, S. Oyster Habitat Restoration Monitoring and Assessment Handbook; The Nature Conservancy: Arlington, VA, USA, 2014; 96p. [Google Scholar]
- Keyes, T. Restoration of Offshore Bars and Shell Rakes. In Georgia Department of Natural Resources-Technical Report; Georgia Department of Natural Resources: Atlanta, GA, USA, 2020; 33p. [Google Scholar]
- Manis, J.E.; Garvis, S.K.; Jachec, S.M.; Walters, L.J. Wave Attenuation Experiments over Living Shorelines over Time: A Wave Tank Study to Assess Recreational Boating Pressures. J. Coast. Conserv. 2015, 19, 1–11. [Google Scholar] [CrossRef]
- Sussan, T.T.; Charpentier, C.L. Conditions That Promote Oyster Settlement Coincide with Areas of High Boating Activity in a Developed Coastal Habitat. J. Exp. Mar. Biol. Ecol. 2024, 572, 151989. [Google Scholar] [CrossRef]
- Lipcius, R.N.; Burke, R.P.; McCulloch, D.N.; Schreiber, S.J.; Schulte, D.M.; Seitz, R.D.; Shen, J. Overcoming Restoration Paradigms: Value of the Historical Record and Metapopulation Dynamics in Native Oyster Restoration. Front. Mar. Sci. 2015, 2, 65. [Google Scholar] [CrossRef]
- Luckenbach, M.; Coen, L.; Ross, P.G., Jr.; Stephen, J. Oyster Reef Habitat Restoration: Relationships Between Oyster Abundance and Community Development Based on Two Studies in Virginia and South Carolina. J. Coast. Res. 2005, 64–78. [Google Scholar]
- Coen, L.; Brumbaugh, R.; Bushek, D.; Grizzle, R.; Luckenbach, M.; Posey, M.; Powers, S.; Tolley, S. Ecosystem Services Related to Oyster Restoration. Mar. Ecol. Prog. Ser. 2007, 341, 303–307. [Google Scholar] [CrossRef]
- Schulte, D.M.; Burke, R.P.; Lipcius, R.N. Unprecedented Restoration of a Native Oyster Metapopulation. Science 2009, 325, 1124–1129. [Google Scholar] [CrossRef]
- Hanke, M.H.; Leija, H.; Laroche, R.A.S.; Modi, S.; Culver-Miller, E.; Sanchez, R.; Bobby, N. Localized Placement of Breakwater Reefs Influences Oyster Populations and Their Resilience after Hurricane Harvey. Ecologies 2022, 3, 422–434. [Google Scholar] [CrossRef]
- Hill, J.; Weissburg, M. Habitat Complexity and Predator Size Mediate Interactions between Intraguild Blue Crab Predators and Mud Crab Prey in Oyster Reefs. Mar. Ecol. Prog. Ser. 2013, 488, 209–219. [Google Scholar] [CrossRef]
- Grabowski, J. Habitat Complexity Disrupts Predator-Prey Interactions but Not the Trophic Cascade on Oyster Reefs. Ecology 2004, 85, 995–1004. [Google Scholar] [CrossRef]
- Ziegler, S.L.; Grabowski, J.H.; Baillie, C.J.; Fodrie, F.J. Effects of Landscape Setting on Oyster Reef Structure and Function Largely Persist More than a Decade Post-Restoration. Restor. Ecol. 2017, 26, 933–942. [Google Scholar] [CrossRef]
- Grabowski, J.H.; Baillie, C.J.; Carlyle, R.; Fodrie, F.J.; Gittman, R.K.; Kimbro, D.L.; Sullivan, K.; Baukus, A.; Hughes, A.R.; Powers, S.P.; et al. Fish and Invertebrate Use of Restored vs. Natural Oyster Reefs in a Shallow Temperate Latitude Estuary. Ecosphere 2022, 13, e4035. [Google Scholar] [CrossRef]
- Mercado-Silva, N. Condition Index of the Eastern Oyster, Crassostrea virginica (Gmelin, 1791) in Sapelo Island Georgia-Effects of Site, Position on Bed and Pea Crab Parasitism. J. Shellfish Res. 2005, 24, 121–126. [Google Scholar]
- Hadley, N.H.; Hodges, M.; Wilber, D.H.; Coen, L.D. Evaluating Intertidal Oyster Reef Development in South Carolina Using Associated Faunal Indicators. Restor. Ecol. 2010, 18, 691–701. [Google Scholar] [CrossRef]
- Matias, M.G. Macrofaunal Responses to Structural Complexity Are Mediated by Environmental Variability and Surrounding Habitats. Mar. Biol. 2013, 160, 493–502. [Google Scholar] [CrossRef]
- Hesterberg, S.G.; Duckett, C.C.; Salewski, E.A.; Bell, S.S. Three-Dimensional Interstitial Space Mediates Predator Foraging Success in Different Spatial Arrangements. Ecology 2017, 98, 1153–1162. [Google Scholar] [CrossRef]
- Lunt, J.; Reustle, J.; Smee, D.L. Wave Energy and Flow Reduce the Abundance and Size of Benthic Species on Oyster Reefs. Mar. Ecol. Prog. Ser. 2017, 569, 25–36. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanke, M.H.; Hackney, A.; Heath, S.A. Intertidal Oyster Reef Mapping and Population Analysis in West Galveston Bay, Texas. Ecologies 2025, 6, 36. https://doi.org/10.3390/ecologies6020036
Hanke MH, Hackney A, Heath SA. Intertidal Oyster Reef Mapping and Population Analysis in West Galveston Bay, Texas. Ecologies. 2025; 6(2):36. https://doi.org/10.3390/ecologies6020036
Chicago/Turabian StyleHanke, Marc H., Amanda Hackney, and Susan A. Heath. 2025. "Intertidal Oyster Reef Mapping and Population Analysis in West Galveston Bay, Texas" Ecologies 6, no. 2: 36. https://doi.org/10.3390/ecologies6020036
APA StyleHanke, M. H., Hackney, A., & Heath, S. A. (2025). Intertidal Oyster Reef Mapping and Population Analysis in West Galveston Bay, Texas. Ecologies, 6(2), 36. https://doi.org/10.3390/ecologies6020036






