Ecological Half-Life of 137Cs in Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Preparation of the Fungal Fruit Bodies Samples
2.3. Radiometry
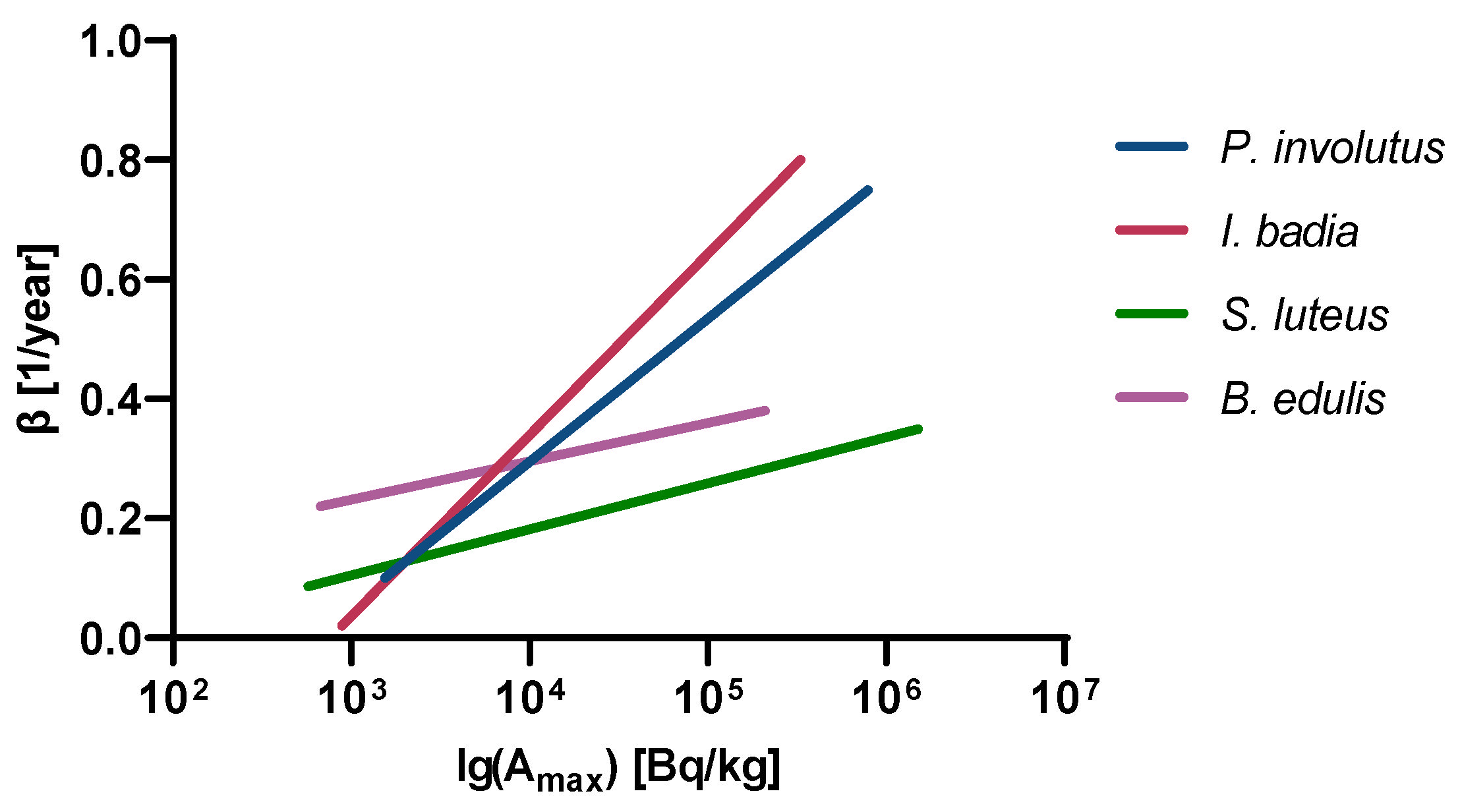
2.4. Calculation of the Ecological Half-Life of 137Cs
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venturi, S. Cesium in Biology, Pancreatic Cancer, and Controversy in High and Low Radiation Exposure Damage—Scientific, Environmental, Geopolitical, and Economic Aspects. Int. J. Environ. Res. Public Health 2021, 18, 8934. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, E.R.; Kolpakov, I.E.; Stepanova, Y.I.; Vdovenko, V.Y.; Naboka, M.V.; Mousseau, T.A.; Mohr, L.C.; Hoel, D.G.; Karmaus, W.J.J. 137-Cesium Exposure and Spirometry Measures in Ukrainian Children Affected by the Chernobyl Nuclear Incident. Environ. Health Perspect. 2010, 118, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.W.; Hutchison-Benson, E.; Svoboda, J. Search for Latitudinal Trends in the Effective Half-Life of Fallout 137Cs in Vegetation of the Canadian Arctic. Can. J. Bot. 1985, 63, 792–796. [Google Scholar] [CrossRef]
- Paller, M.H.; Jannik, G.T.; Baker, R.A. Effective Half-Life of Caesium-137 in Various Environmental Media at the Savannah River Site. J. Environ. Radioact. 2014, 131, 81–88. [Google Scholar] [CrossRef]
- Škrkal, J.; Rulík, P.; Fantínová, K.; Burianová, J.; Helebrant, J. Long-Term 137cs Activity Monitoring of Mushrooms in Forest Ecosystems of the Czech Republic. Radiat. Prot. Dosimetry 2013, 157, 579–584. [Google Scholar] [CrossRef]
- Zibold, G.; Klemt, E. Ecological Half-Times of 137Cs and 90Sr in Forest and Freshwater Ecosystems. Radioprotection 2005, 40, S497–S502. [Google Scholar] [CrossRef]
- AMAP. AMAP Assessment 2002: Radioactivity in the Arctic; AMAP: Tromsø, Norway, 2004; p. 22. ISBN 8279710191. [Google Scholar]
- Bé, M.-M.; Chisté, V.; Dulieu, C.; Browne, E.; Baglin, C.; Chechev, V.; Kuzmenko, N.; Helmer, R.; Kondev, F.; MacMahon, D.; et al. Table of Radionuclides, Cs-137; Monographie BIPM-5; Bureau International des Poids et Mesures: Sèvres, France, 2006; Volume 3, ISBN 92-822-2218-7. [Google Scholar]
- Dementyev, D.; Bolsunovsky, A. A Long-Term Study of Radionuclide Concentrations in Mushrooms in the 30-Km Zone around the Mining-and-Chemical Combine (Russia). Isotopes Environ. Health Stud. 2020, 56, 83–92. [Google Scholar] [CrossRef]
- Oloś, G.; Dołhańczuk-Śródka, A. Effective and Environmental Half-Lives of Radiocesium in Game from Poland. J. Environ. Radioact. 2022, 248, 106870. [Google Scholar] [CrossRef]
- Falandysz, J.; Saniewski, M.; Fernandes, A.R.; Meloni, D.; Cocchi, L.; Strumińska-Parulska, D.; Zalewska, T. Radiocaesium in Tricholoma Spp. from the Northern Hemisphere in 1971–2016. Sci. Total Environ. 2022, 802, 149829. [Google Scholar] [CrossRef]
- Zarubina, N.E.; Burdo, O.S.; Ponomarenko, L.P.; Shatrova, O.V. Two Stages in the Accumulation of 137Cs by Mushroom Suillus Luteus after the Chornobyl Accident. Nucl. Phys. At. Energy 2021, 22, 294–299. [Google Scholar] [CrossRef]
- Zarubina, N. The Influence of Biotic and Abiotic Factors on 137 Cs Accumulation in Higher Fungi after the Accident at Chernobyl NPP. J. Environ. Radioact. 2016, 161, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Katengeza, E.W.; Sanada, Y.; Yoshimura, K.; Ochi, K.; Iimoto, T. The Ecological Half-Life of Radiocesium in Surficial Bottom Sediments of Five Ponds in Fukushima Based on: In Situ Measurements with Plastic Scintillation Fibers. Environ. Sci. Process. Impacts 2020, 22, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Akama, A. Difference of Ecological Half-Life and Transfer Coefficient in Aquatic Invertebrates between High and Low Radiocesium Contaminated Streams. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Baltas, H.; Sirin, M.; Dalgic, G.; Cevik, U. An Overview of the Ecological Half-Life of the 137Cs Radioisotope and a Determination of Radioactivity Levels in Sediment Samples after Chernobyl in the Eastern Black Sea, Turkey. J. Mar. Syst. 2018, 177, 21–27. [Google Scholar] [CrossRef]
- Paller, M.H.; Jannik, G.T.; Fledderman, P.D. Changes in 137Cs Concentrations in Soil and Vegetation on the Floodplain of the Savannah River over a 30 Year Period. J. Environ. Radioact. 2008, 99, 1302–1310. [Google Scholar] [CrossRef]
- Saka, A.Z.; Çevik, U.; Bacaksiz, E.; Kopya, A.Í.; Tiraşoǧlu, E. Levels of Cesium Radionuclides in Lichens and Mosses from the Province of Ordu in the Eastern Black Sea Area of Turkey. J. Radioanal. Nucl. Chem. 1997, 222, 87–92. [Google Scholar] [CrossRef]
- Cevik, U.; Celik, N. Ecological Half-Life of 137Cs in Mosses and Lichens in the Ordu Province, Turkey by Cevik and Celik. J. Environ. Radioact. 2009, 100, 23–28. [Google Scholar] [CrossRef]
- Machart, P.; Hofmann, W.; Türk, R.; Steger, F. Ecological Half-Life of 137Cs in Lichens in an Alpine Region. J. Environ. Radioact. 2007, 97, 70–75. [Google Scholar] [CrossRef]
- Monte, L. Evaluation of Radionuclide Transfer Functions from Drainage Basins of Fresh Water Systems. J. Environ. Radioact. 1995, 26, 71–82. [Google Scholar] [CrossRef]
- Ronda, O.; Grządka, E.; Ostolska, I.; Orzeł, J.; Cieślik, B.M. Accumulation of Radioisotopes and Heavy Metals in Selected Species of Mushrooms. Food Chem. 2022, 367, 130670. [Google Scholar] [CrossRef]
- Ernst, A.L.; Reiter, G.; Piepenbring, M.; Bässler, C. Spatial Risk Assessment of Radiocesium Contamination of Edible Mushrooms—Lessons from a Highly Frequented Recreational Area. Sci. Total Environ. 2022, 807, 150861. [Google Scholar] [CrossRef] [PubMed]
- Tagami, K.; Yasutaka, T.; Takada, M.; Uchida, S. Aggregated Transfer Factor of 137Cs in Wild Edible Mushrooms Collected in 2016–2020 for Long-Term Internal Dose Assessment Use. J. Environ. Radioact. 2021, 237, 106664. [Google Scholar] [CrossRef] [PubMed]
- Guido-Garcia, F.; Sakamoto, F.; David, K.; Kozai, N.; Grambow, B. Radiocesium in Shiitake Mushroom: Accumulation in Living Fruit Bodies and Leaching from Dead Fruit Bodies. Chemosphere 2021, 279, 130511. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Suzuki, N.; Ogawa, S.; Ota, Y. Spatial Distribution of 137Cs Concentrations in Mushrooms (Boletus Hiratsukae) and Their Relationship with Soil Exchangeable Cation Contents. J. Environ. Radioact. 2020, 222, 106364. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, Y.; Oomachi, H.; Saito, R.; Kumada, R.; Sasaki, M.; Takatsuki, S. Effects of 137Cs Contamination after the TEPCO Fukushima Dai-Ichi Nuclear Power Station Accident on Food and Habitat of Wild Boar in Fukushima Prefecture. J. Environ. Radioact. 2020, 225, 106342. [Google Scholar] [CrossRef]
- Komatsu, M.; Nishina, K.; Hashimoto, S. Extensive Analysis of Radiocesium Concentrations in Wild Mushrooms in Eastern Japan Affected by the Fukushima Nuclear Accident: Use of Open Accessible Monitoring Data. Environ. Pollut. 2019, 255, 113236. [Google Scholar] [CrossRef]
- Büntgen, U.; Jäggi, M.; Egli, S.; Heule, M.; Peter, M.; Zagyva, I.; Krusic, P.J.; Zimermann, S.; Bagi, I. No Radioactive Contamination from the Chernobyl Disaster in Hungarian White Truffles (Tuber Magnatum). Environ. Pollut. 2019, 252, 1643–1647. [Google Scholar] [CrossRef]
- Pröhl, G.; Ehlken, S.; Fiedler, I.; Kirchner, G.; Klemt, E.; Zibold, G. Ecological Half-Lives of 90Sr and 137Cs in Terrestrial and Aquatic Ecosystems. J. Environ. Radioact. 2006, 91, 41–72. [Google Scholar] [CrossRef]
- Koivurova, M.; Leppänen, A.P.; Kallio, A. Transfer Factors and Effective Half-Lives of 134Cs and 137Cs in Different Environmental Sample Types Obtained from Northern Finland: Case Fukushima Accident. J. Environ. Radioact. 2015, 146, 73–79. [Google Scholar] [CrossRef]
- Koval, G.M.; Shatrova, N.E. The Content of Radionuclides of Accidental Origin in Fungi (Macromycetes) of the Chornobyl Exclusion Zone (in Ukrainian). In Chornobyl. The Exclusion Zone; Naukova Dumka: Kyiv, Ukraine, 2001; pp. 378–407. [Google Scholar]
- Portoghesi, L. European Forest Types (Categories and Types for Sustainable Forest Management Reporting and Policy). Available online: https://www.eea.europa.eu/publications/technical_report_2006_9 (accessed on 10 December 2022).
- Pohrebniak, P.S. Basis of Forest Typology; Publishing House of Academy of Science of USSR: Kyiv, Ukraine, 1955. (In Russian) [Google Scholar]
- Cort, M.; Dubois, G.; Fridman, S.D.; Germenchuk, M.G.; Izrael, Y.A.; Janssens, A.; Jones, A.R.; Kelly, G.N.; Kvasnikova, E.V.; Matveenko, I.I.; et al. Atlas of Caesium Deposition on Europe after the Chernobyl Accident; Office for Official Publications of the European Communities: Luxembourg, 1998; p. 66 (plate No. 19). ISBN 92-828-3140-X. [Google Scholar]
- Lux, D.; Kammerer, L.; Rühm, W.; Wirth, E. Cycling of Pu, Sr, Cs, and Other Longliving Radionuclides in Forest Ecosystems of the 30-Km Zone around Chernobyl. Sci. Total Environ. 1995, 173/174, 375–384. [Google Scholar] [CrossRef]
- Orlov, O.O.; Kurbet, T.V.; Kalish, O.B.; Pryshchepa, O.L. Peculiarities of 137Cs Accumulation by Macromycetes in Dry Pinewoods of Ukrainian Polissia. In Proceedings of the Collection of research papers of the Institute for Nuclear Research; Scientific Papers of the Institute for Nuclear Research: Kyiv, Ukraine, 2001; pp. 112–114. ISSN 1606-6723. (In Russian) [Google Scholar]
- Olsen, R.A.; Joner, E.; Bakken, L.R. Soil Fungi and the Fate of Radiocaesium in the Soil Ecosystem. In Transfer of Radionuclides in Natural and Semi-Natural Environment; Desmet, G., Nassimbeni, P., Belli, M., Eds.; Elsevier Applied Science: England, UK, 1990; pp. 657–663. ISBN 1-85166-539-0. [Google Scholar]
- Dahlberg, A.; Nikolova, I.; Johanson, K.-J. Intraspecific Variation in 137Cs Activity Concentration in Sporocarps of Suillus Variegatus in Seven Swedish Populations. Mycol. Res. 1997, 101, 545–551. [Google Scholar] [CrossRef]
- Boonstra, R.; Manzon, R.G.; Mihok, S.; Helson, J.E. Hormetic Effects of Gamma Radiation on the Stress Axis of Natural Populations of Meadow Voles (Microtus Pennsylvanicus). Environ. Toxicol. Chem. 2005, 24, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Song, K.E.; Lee, S.H.; Jung, J.G.; Choi, J.E.; Jun, W.; Chung, J.-W.; Hong, S.H.; Shim, S. Hormesis Effects of Gamma Radiation on Growth of Quinoa (Chenopodium Quinoa). Int. J. Radiat. Biol. 2021, 97, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Belli, M.; Indovina, L. The Response of Living Organisms to Low Radiation Environment and Its Implications in Radiation Protection. Front. Public Heal. 2020, 8, 601711. [Google Scholar] [CrossRef] [PubMed]
- Buldakov, L.A.; Kalistratova, V.S. Radiation Effects on the Organism—Positive Effects; Inform-Atom: San Francisco, CA, USA, 2005; ISBN 5891070421. (In Russian) [Google Scholar]
- Grodzinskiy, D.M. Radiobiology (in Ukrainian); 2nd ed.; Lybid: Kyiv, Ukraine, 2001; ISBN 966-06-0204-9. [Google Scholar]
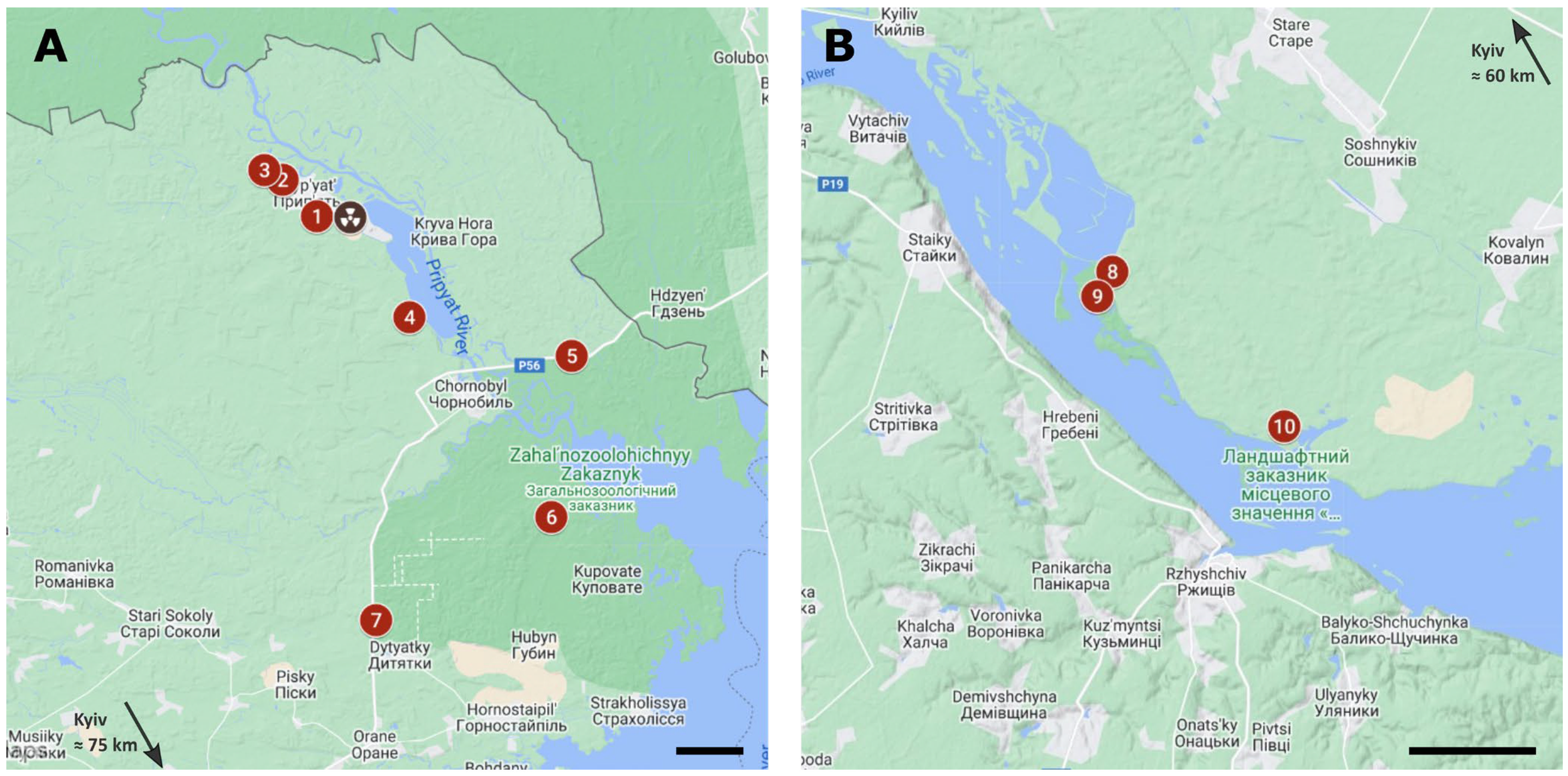

| Sampling Site | GPS Coordinates | Distance from ChNPP | HT (µSv/h) | |
|---|---|---|---|---|
| 1 | Yaniv | 30.06408 E, 51.39017 N | 2.2 km | 10.00 |
| 2 | Prypiat | 30.02982 E, 51.41249 N | 5.2 km | 2.50 |
| 3 | Novoshepelychi | 30.01030 E, 51.41910 N | 6.7 km | 2.00 |
| 4 | Leliv | 30.15856 E, 51.32505 N | 8.5 km | 1.20 |
| 5 | Paryshiv | 30.32473 E, 51.30069 N | 18.8 km | 0.30 |
| 6 | Opachychi | 30.30404 E, 51.197143 N | 25.0 km | 0.25 |
| 7 | Dytyatky | 30.12449 E, 51.13088 N | 29.9 km | 0.25 |
| 8 | Stare | 30.99528 E, 50.06785 N | 159.8 km | 0.18 |
| 9 | Staiky | 30.98756 E, 50.05959 N | 160.6 km | 0.15 |
| 10 | Rzhyshchiv | 31.08317 E, 50.01686 N | 167.5 km | 0.15 |
| Sampling Site | Fungi Species | ||||
|---|---|---|---|---|---|
| S. luteus | B. edulis | I. badia | P. involutus | ||
| 1 | Yaniv | 2.46 (2.27) | |||
| 2 | Prypiat | 3.54 (3.17) | |||
| 3 | Novoshepelychi | 3.14 (2.84) | 1.73 (1.64) | 0.93 (0.90) | 1.16 (1.12) |
| 4 | Leliv | 5.89 (4.92) | |||
| 5 | Paryshiv | 7.76 (6.17) | 3.66 (3.26) | 3.53 (3.16) | 6.68 (5.47) |
| 6 | Opachychi | 4.48 (3.90) | |||
| 7 | Dytyatky | 5.43 (4.60) | 5.01 (4.29) | 3.05 (2.77) | 2.83 (2.59) |
| 8 | Stare | 8.85 (6.84) | 4.61 (4.00) | ||
| 9 | Staiky | 5.94 (4.96) | 7.56 (6.04) | 3.36 (3.02) | 3.35 (3.01) |
| 10 | Rzhyshchiv | 8.21 (6.45) | 2.79 (2.55) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarubina, N.E.; Semak, V.; Burdo, O.S.; Ponomarenko, L.P. Ecological Half-Life of 137Cs in Fungi. Ecologies 2023, 4, 11-19. https://doi.org/10.3390/ecologies4010002
Zarubina NE, Semak V, Burdo OS, Ponomarenko LP. Ecological Half-Life of 137Cs in Fungi. Ecologies. 2023; 4(1):11-19. https://doi.org/10.3390/ecologies4010002
Chicago/Turabian StyleZarubina, Nataliia E., Vladislav Semak, Oleg S. Burdo, and Liliia P. Ponomarenko. 2023. "Ecological Half-Life of 137Cs in Fungi" Ecologies 4, no. 1: 11-19. https://doi.org/10.3390/ecologies4010002
APA StyleZarubina, N. E., Semak, V., Burdo, O. S., & Ponomarenko, L. P. (2023). Ecological Half-Life of 137Cs in Fungi. Ecologies, 4(1), 11-19. https://doi.org/10.3390/ecologies4010002







