Bone Drilling: Review with Lab Case Study of Bone Layer Classification Using Vibration Signal and Deep Learning Methods
Abstract
1. Introduction
2. Previous Review Studies on Bone Drilling
| Reference and Publication Year | Content of the Review Paper and Significant | Future Study and Future Direction |
|---|---|---|
| Bertollo and Walsh (2011) [13] |
| Improving drill-bit design for better surgical outcomes and patient recovery. Ultrasonic-assisted drilling reduces axial thrust force and drilling torque. |
| Pandey et al. (2013) [4] |
| Predicting model development for the relationship between drilling force, drill temperature, and surface roughness 1. |
| Ginta et al. (2014) [14] |
| Not available |
| Lee et al. (2018) [15] |
| Not available |
| Timon et al. (2019) [16] |
| Not available |
| Bohra et al. (2019) [17] |
| Acoustic emission (AE) based technique can improve bone surgical quality in micro-drilling and support bone surgery robot systems in the future. |
| Samarasinghe et al. (2020) [23] |
| Improve the prediction model using the force variation based on bone layers. Enhance hand-held drill with intelligent sensors and data acquisition system. |
| Jamil et al. (2020) [24] |
| Robotic bone drilling with multiobjective optimization can reduce thermal and mechanical damage 2. |
| Torun et al. (2020) [25] |
| Signal information and processing to identify different bone densities from motor current, drilling sound, and vibration is one of the future directions. |
| Akhbar and Sulong (2021) [26] |
| A flexible drill design. |
| Jung et al. (2021) [27] |
| Not available |
| Islam et al. (2022) [28] |
| Use more suitable drill bit geometry. Use medical-grade material for the drill bit. |
| Chouhan et al. (2023) [29] |
| Not available |
3. A Brief Review of Medical Training System and Robotic Drilling
4. An Application of Vibration and Machine Learning Methods for Bone Drilling
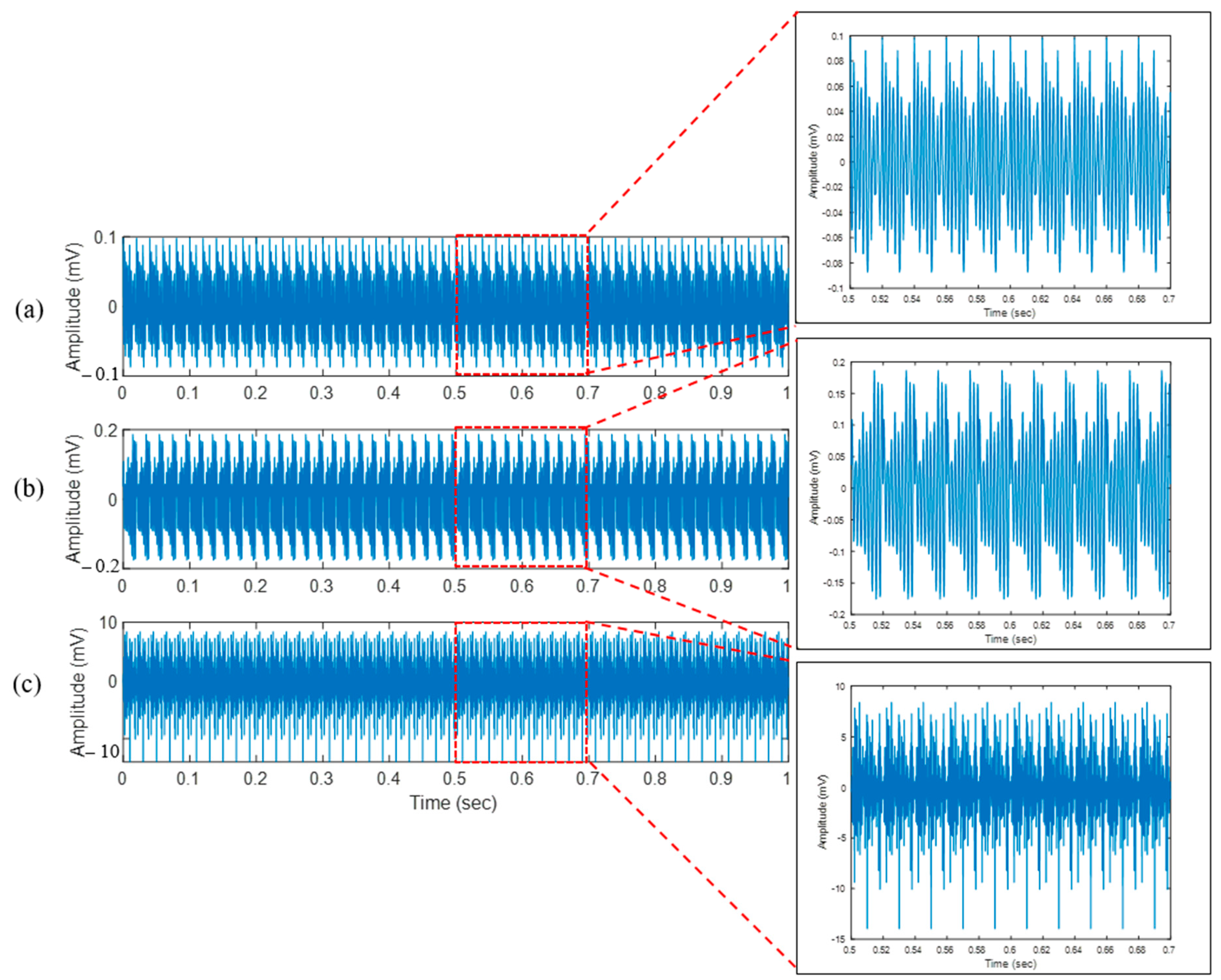
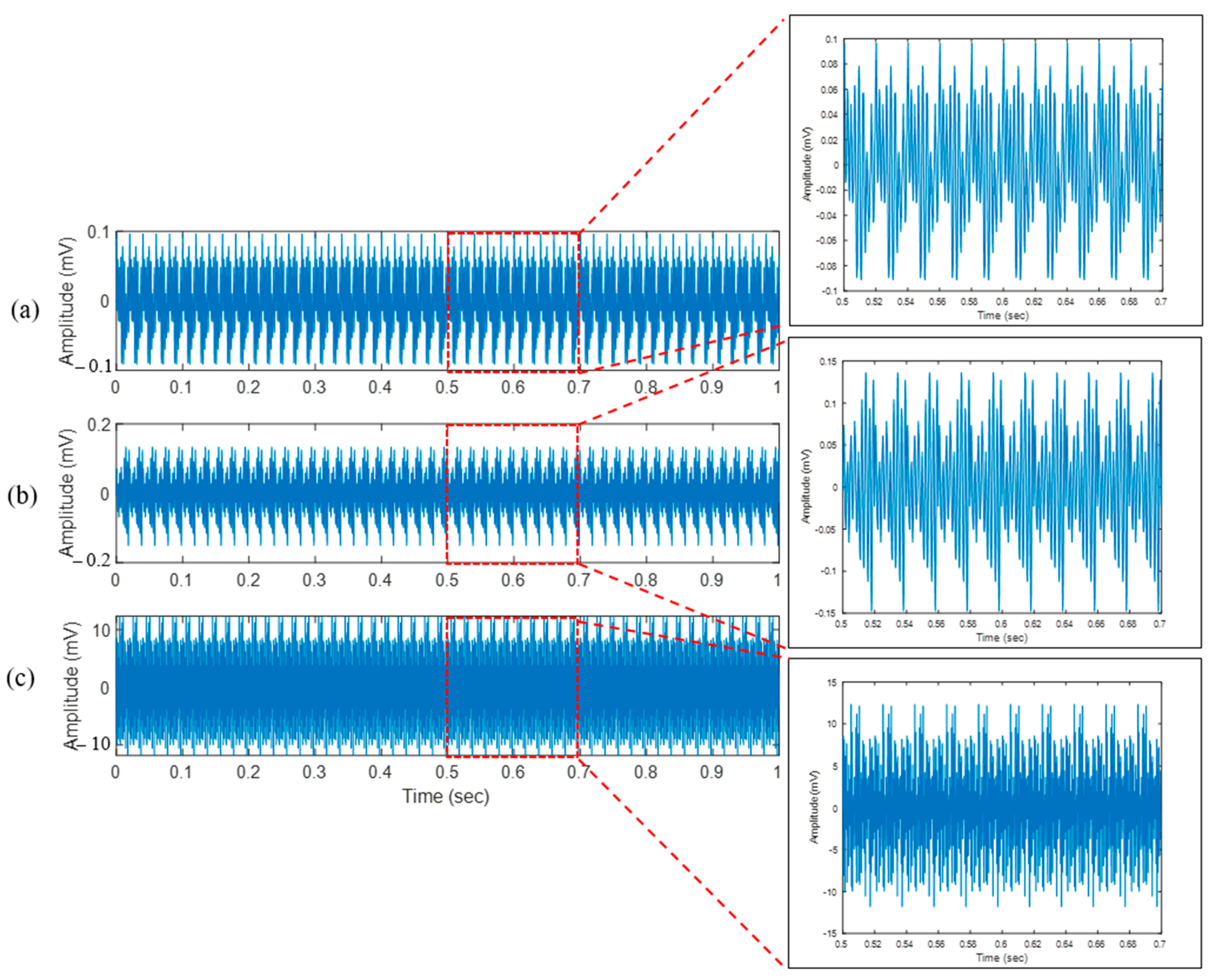
4.1. Bone Drilling Vibration
4.2. Applied Machine Learning Methods
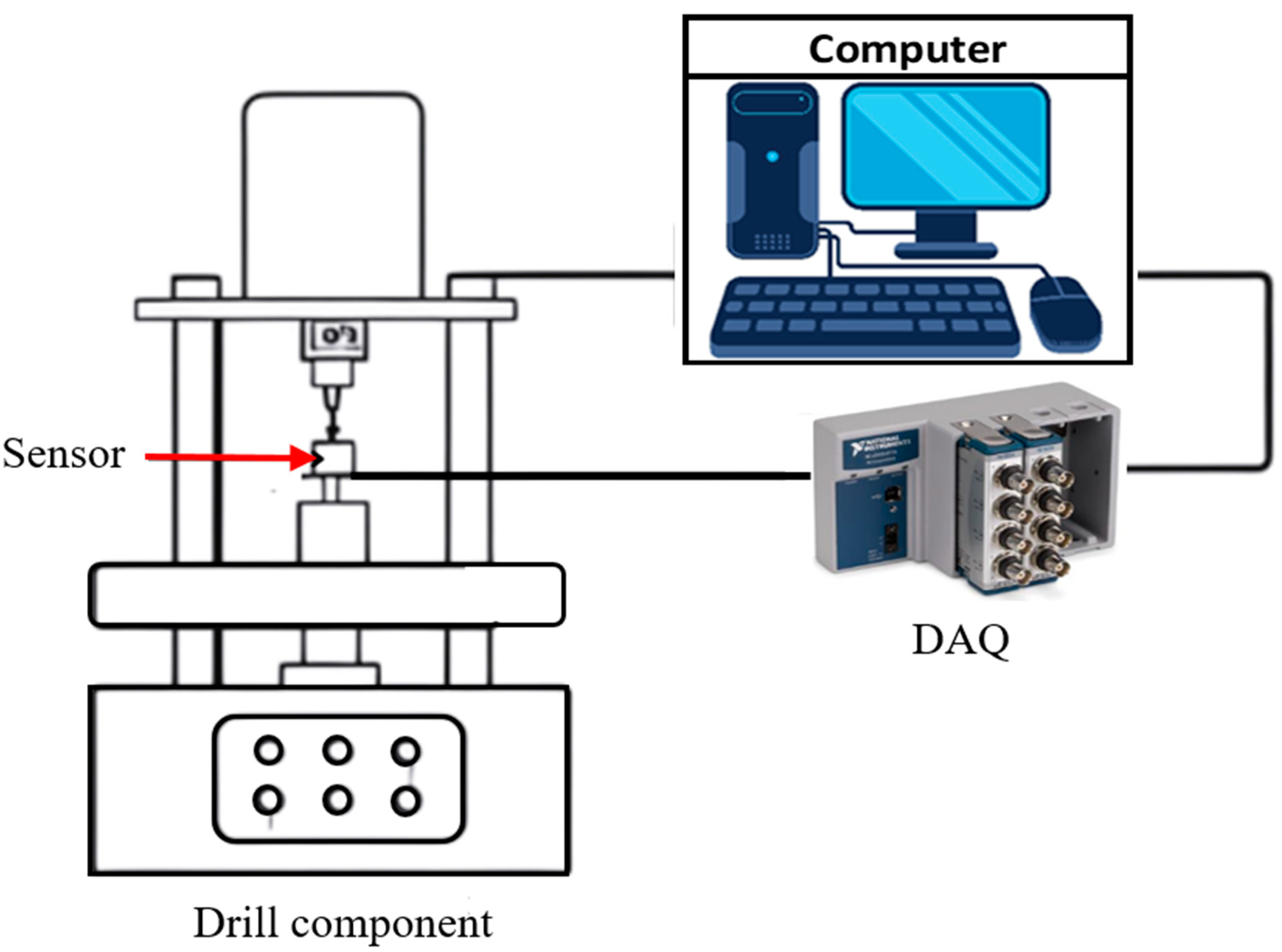
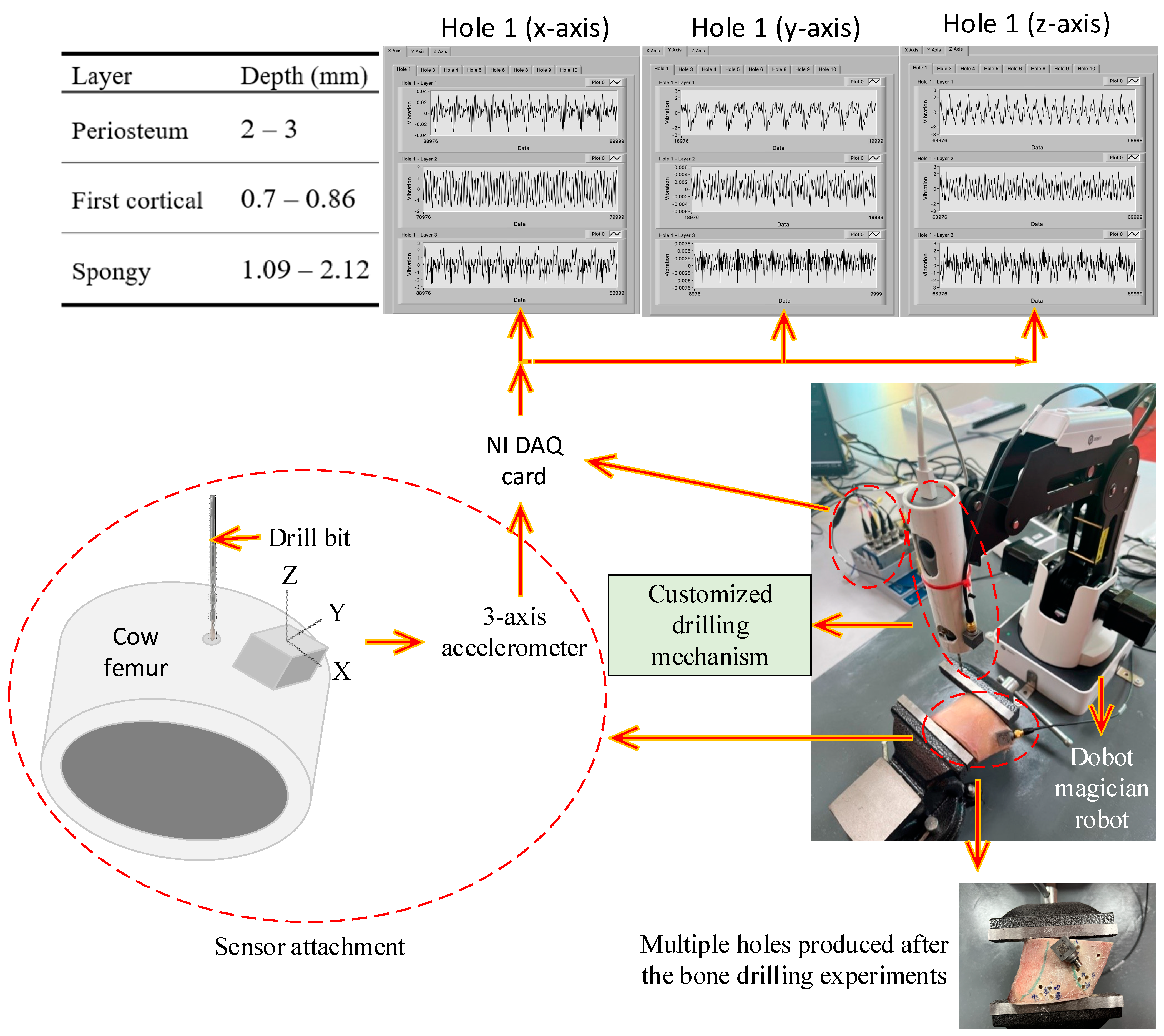
5. Experimental Procedure of Bone Drilling
5.1. Previous Studies
5.2. Bone Drilling Lab Experiment of the Present Study
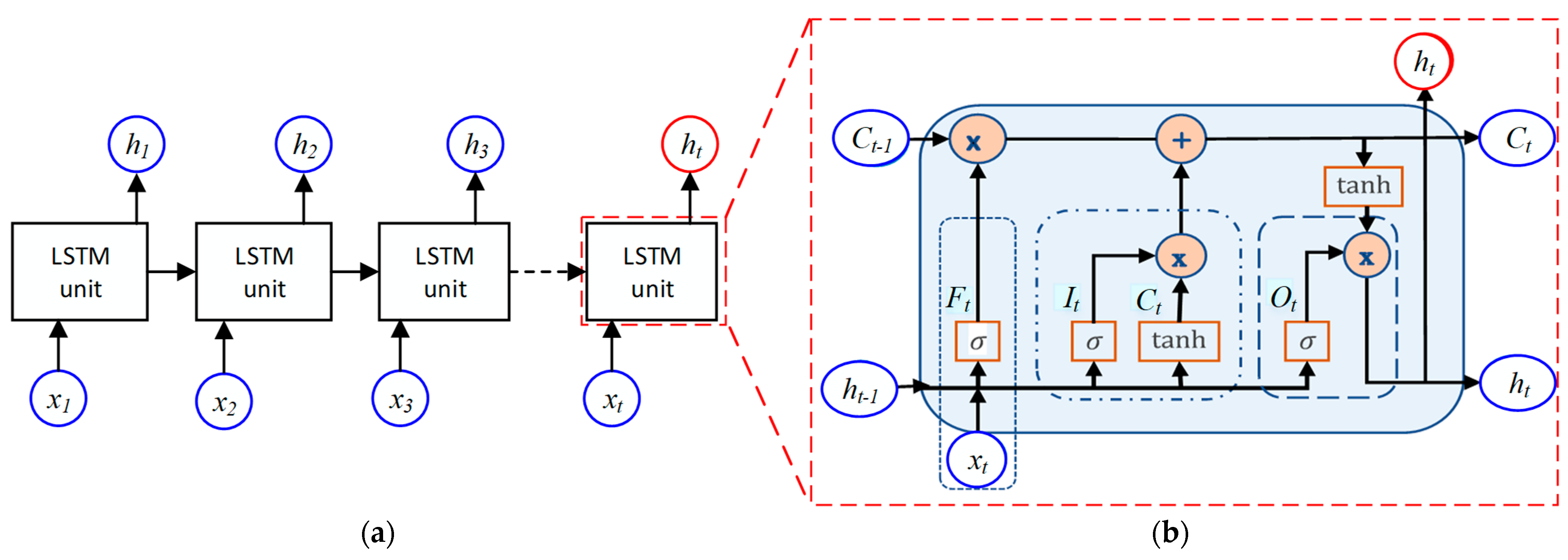
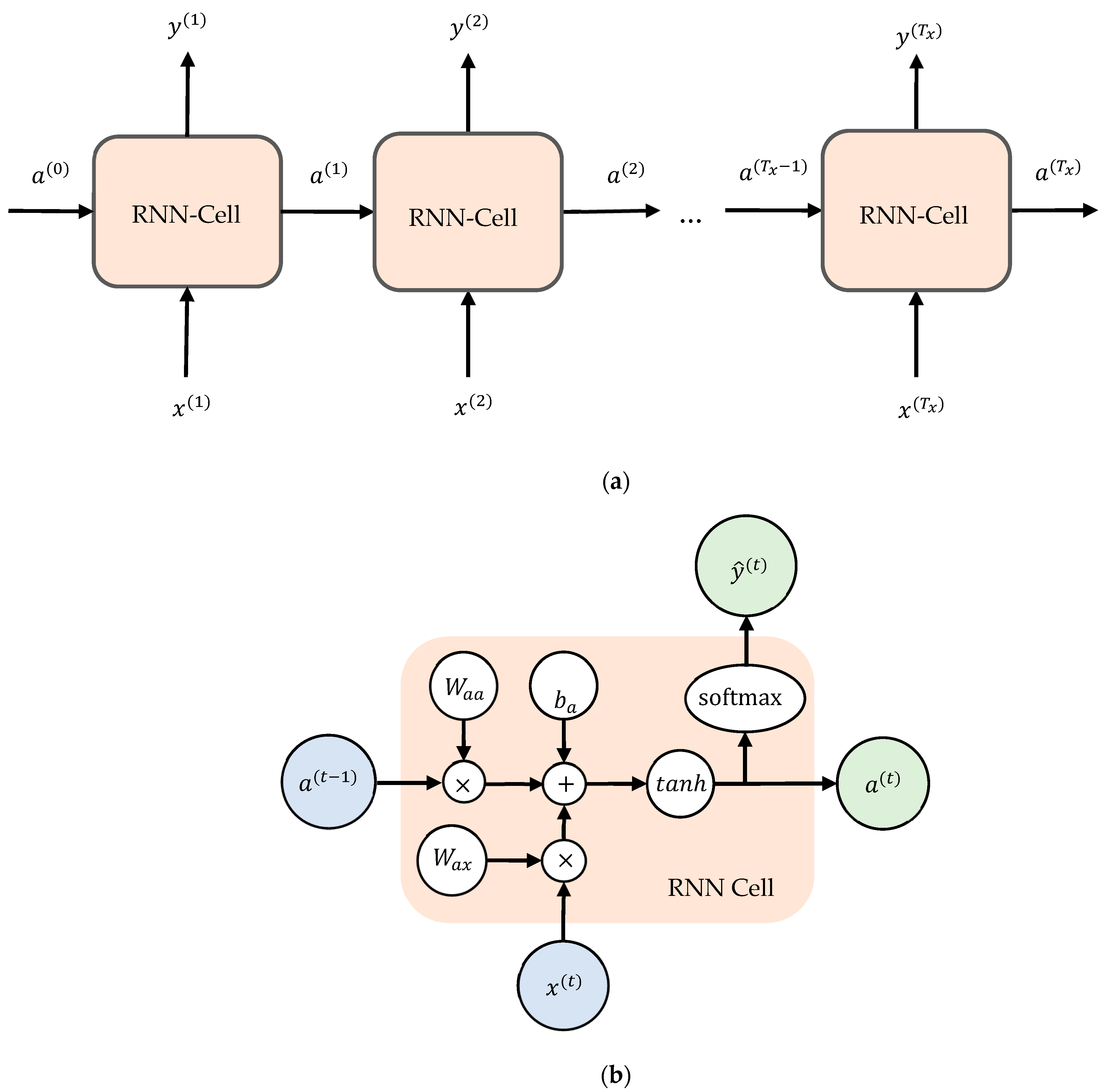
6. Long Term-Short Memory Method
6.1. LSTM Architecture
6.2. LSTM Model
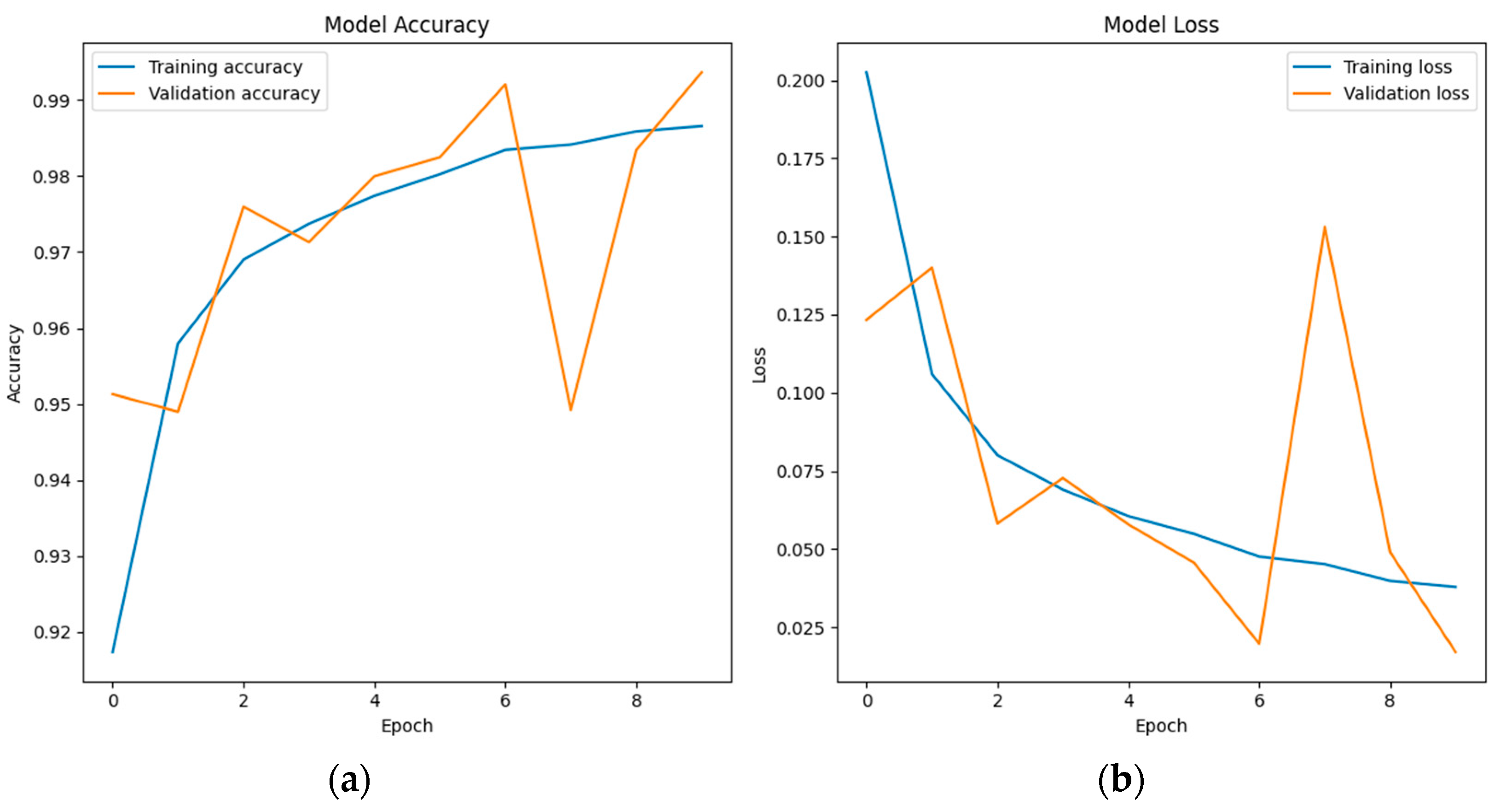
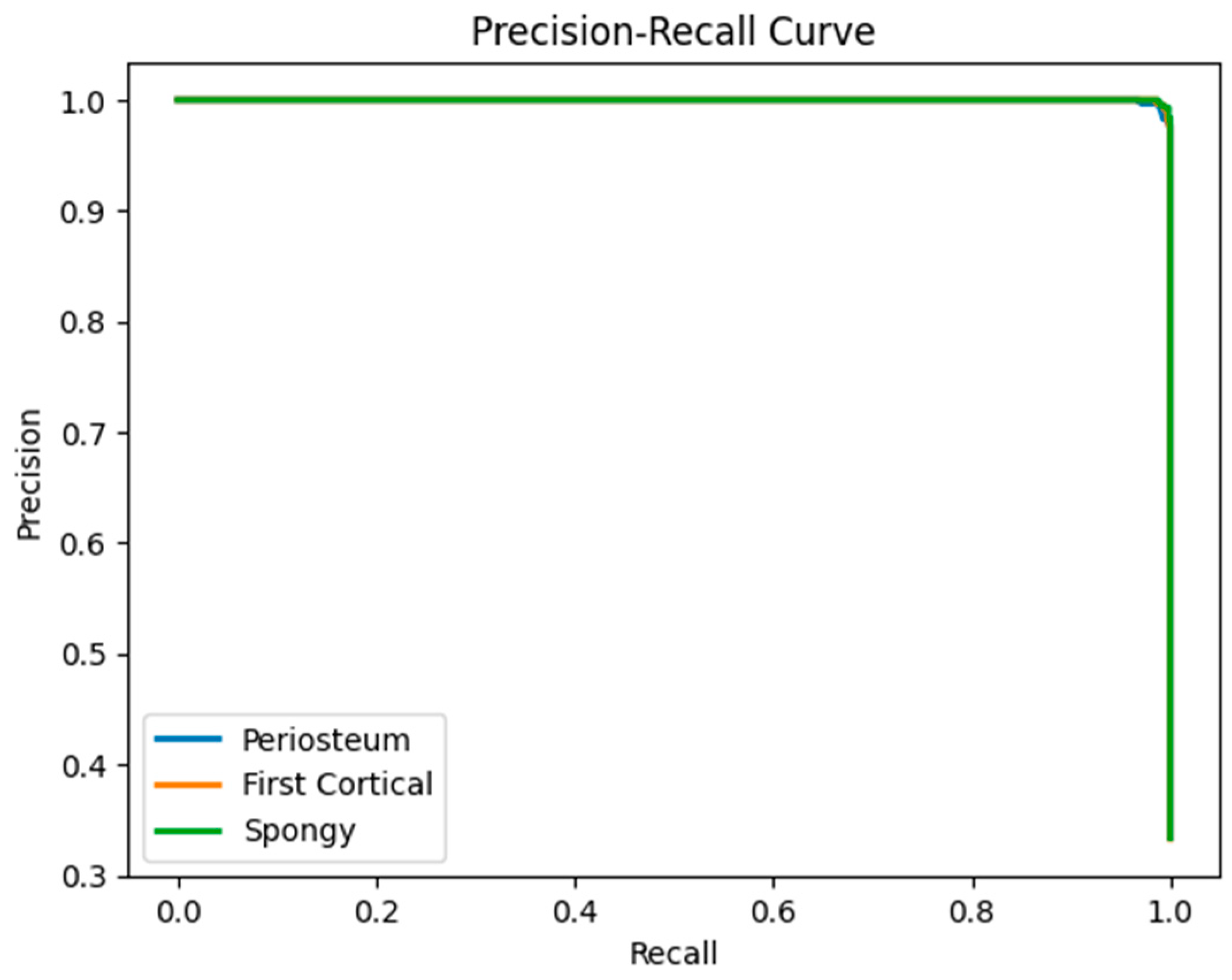
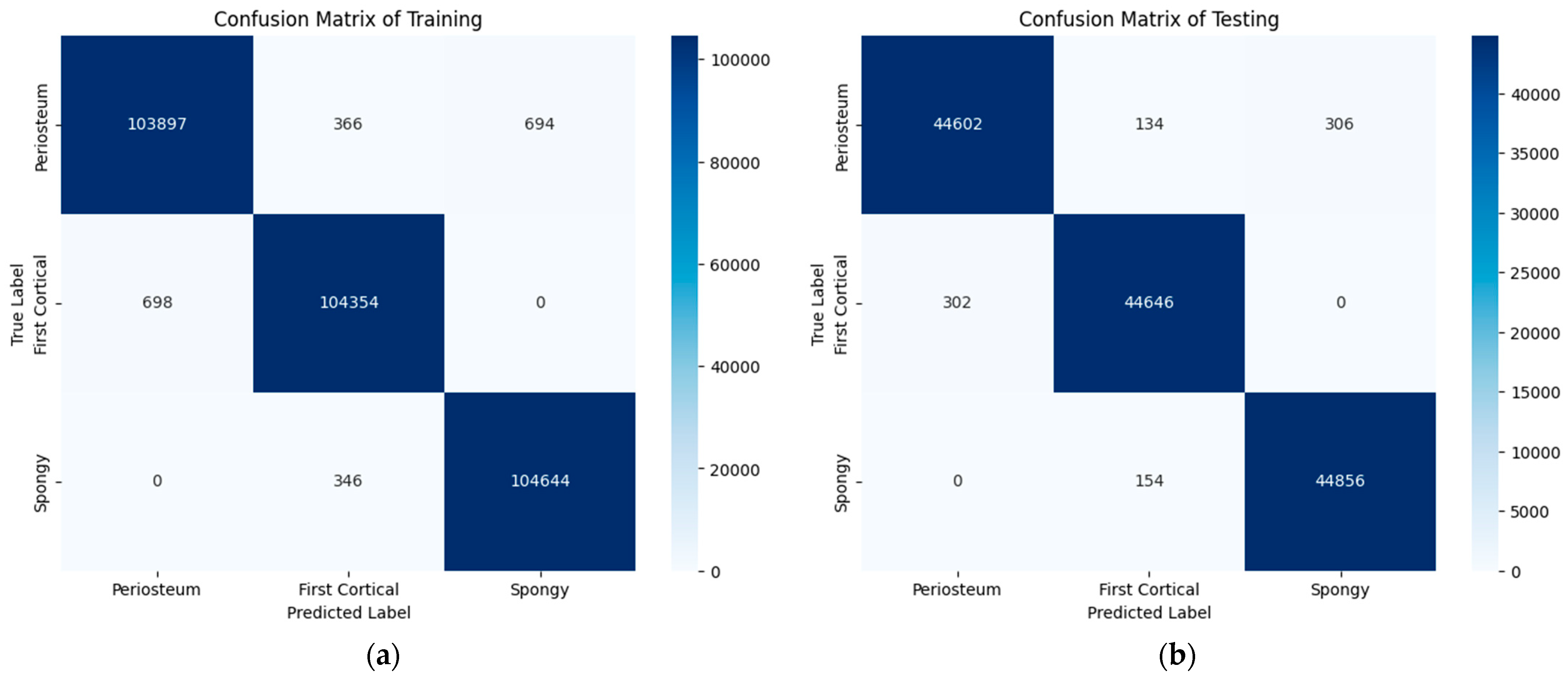
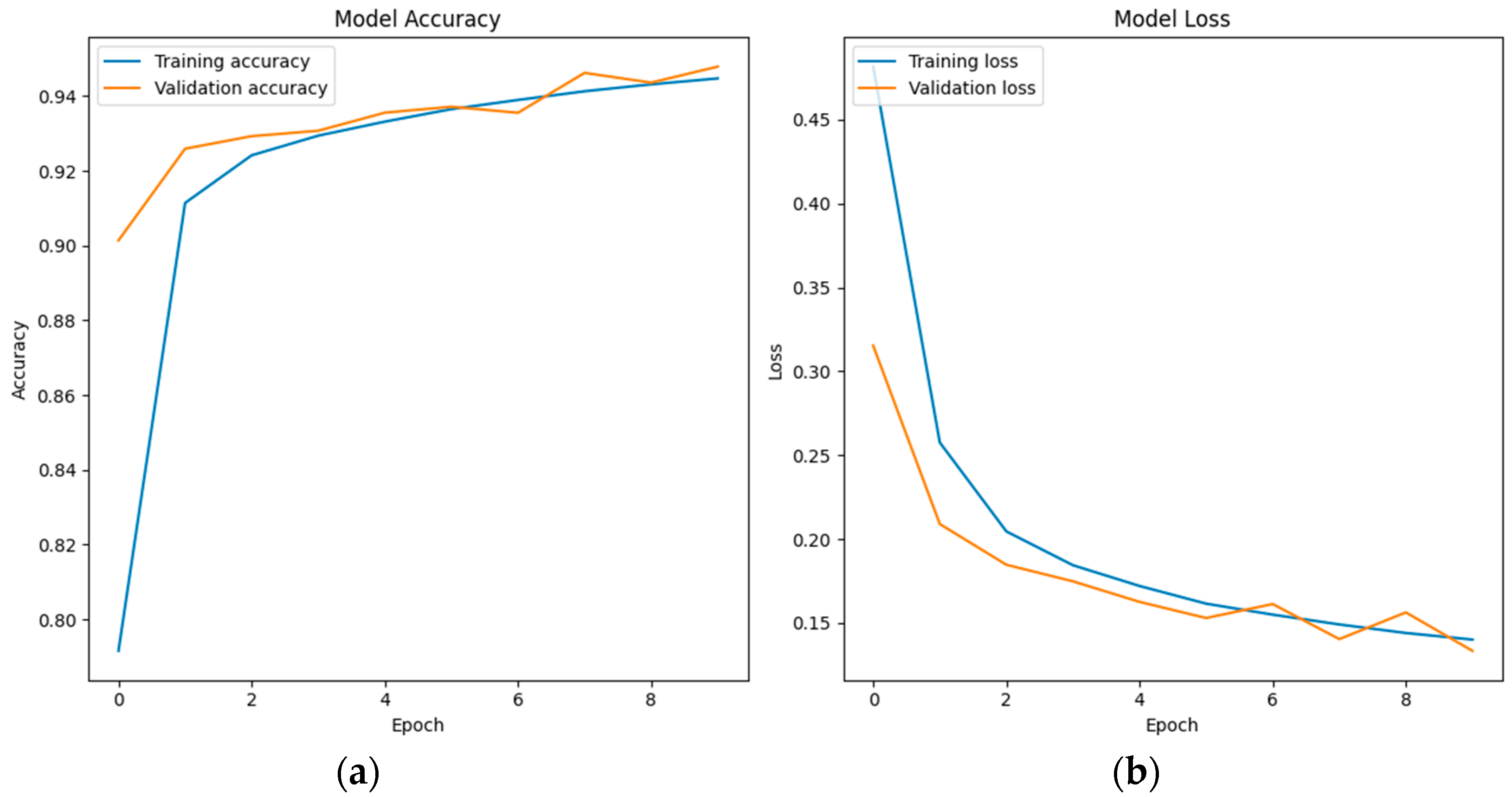
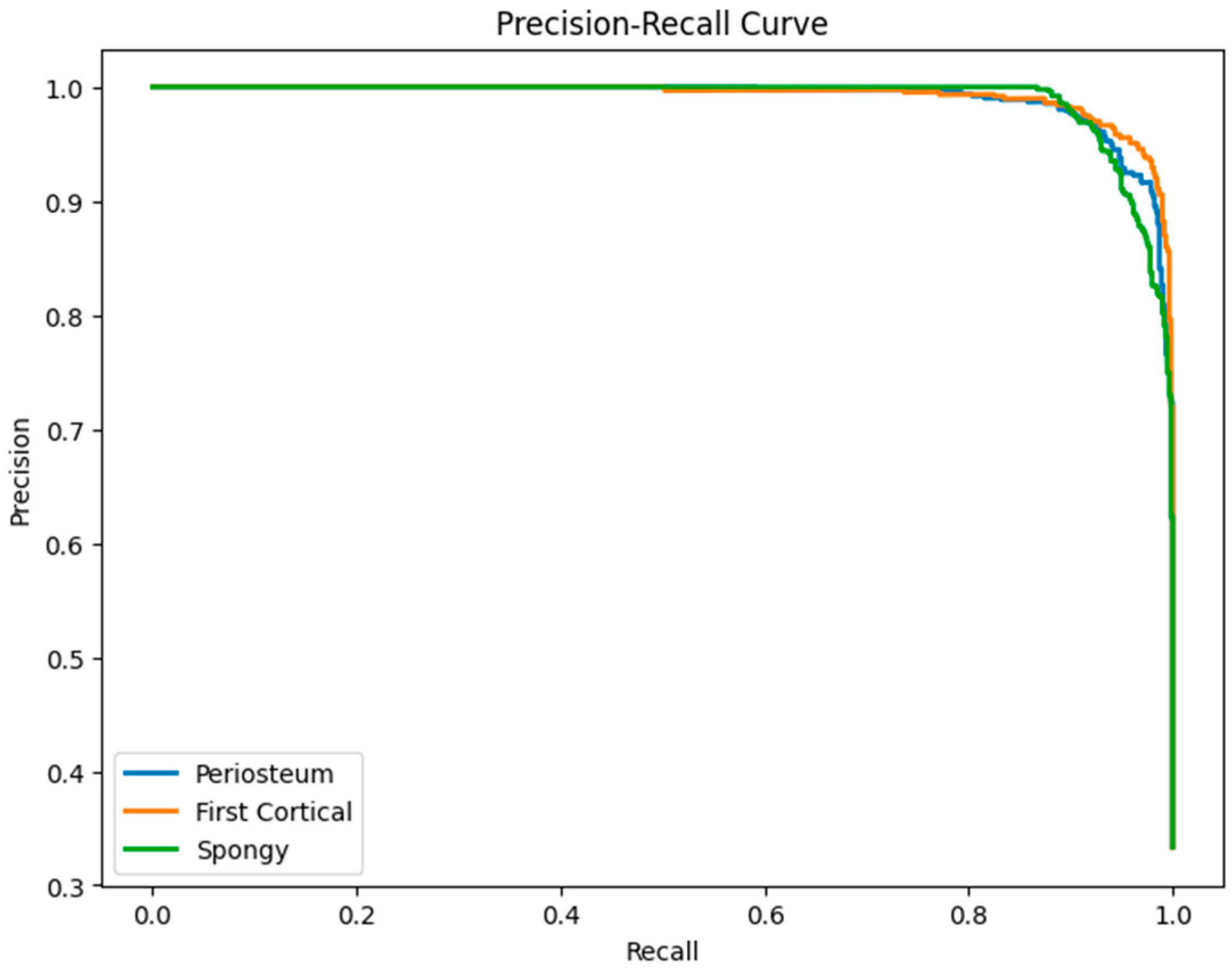
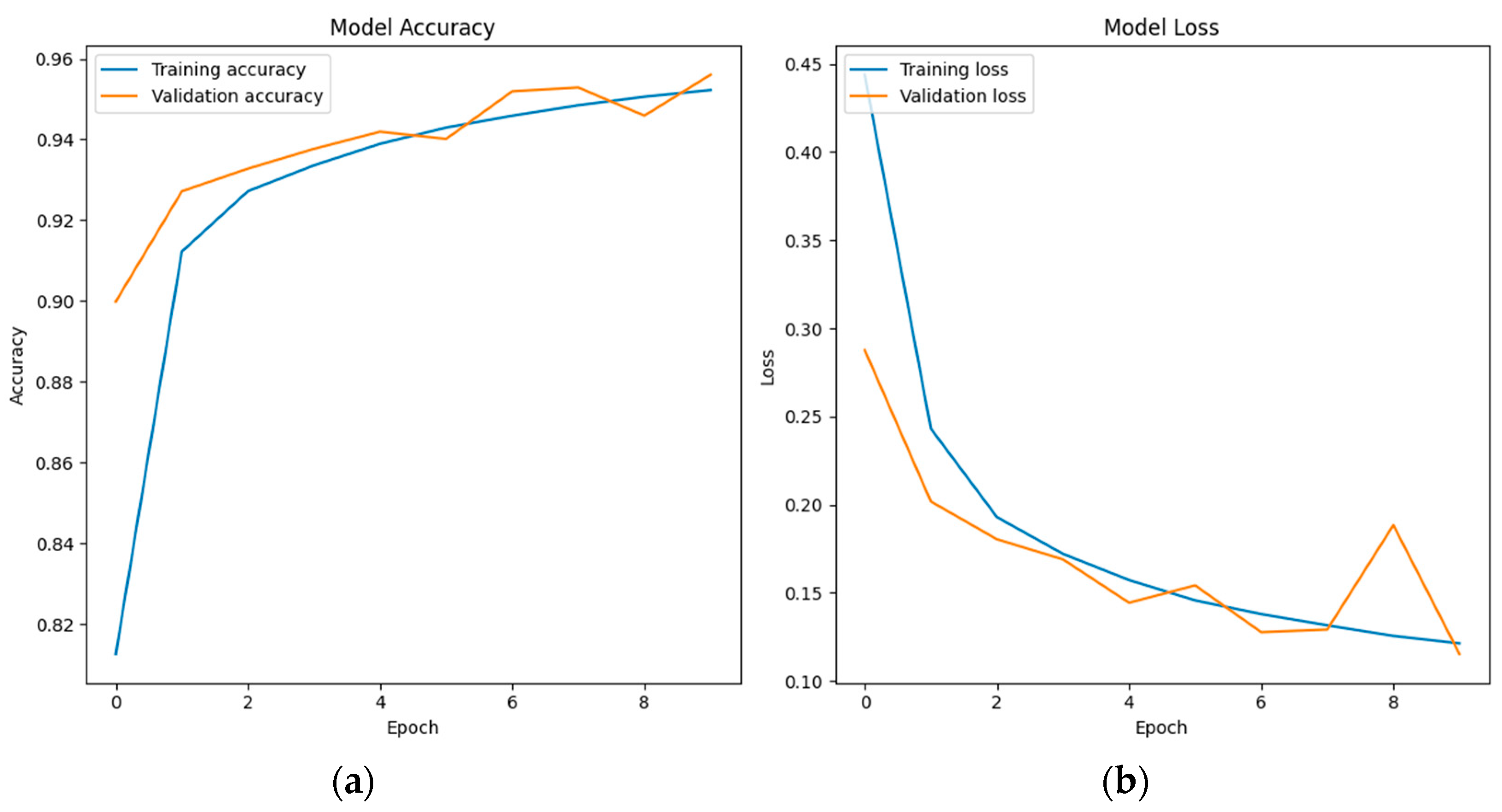
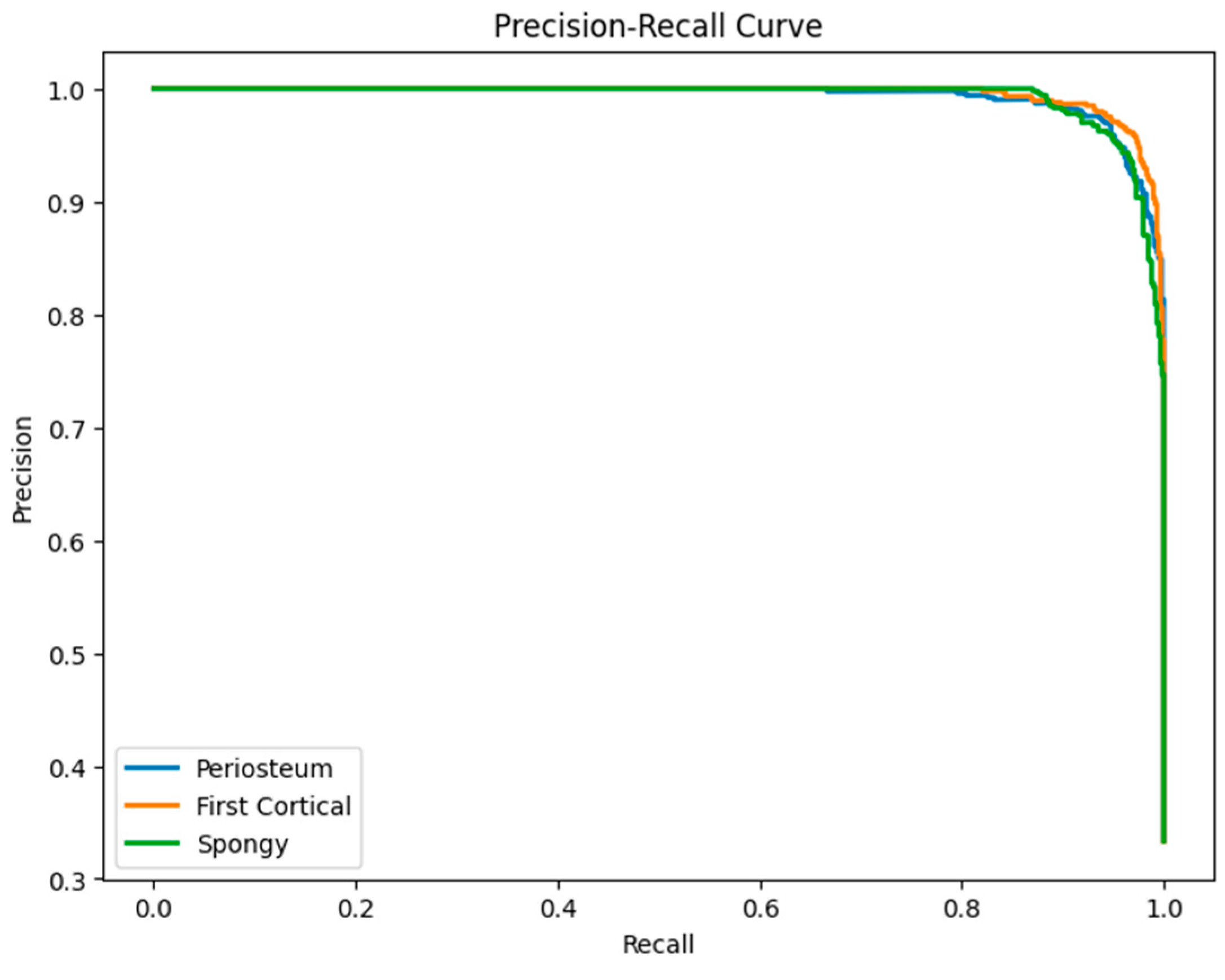
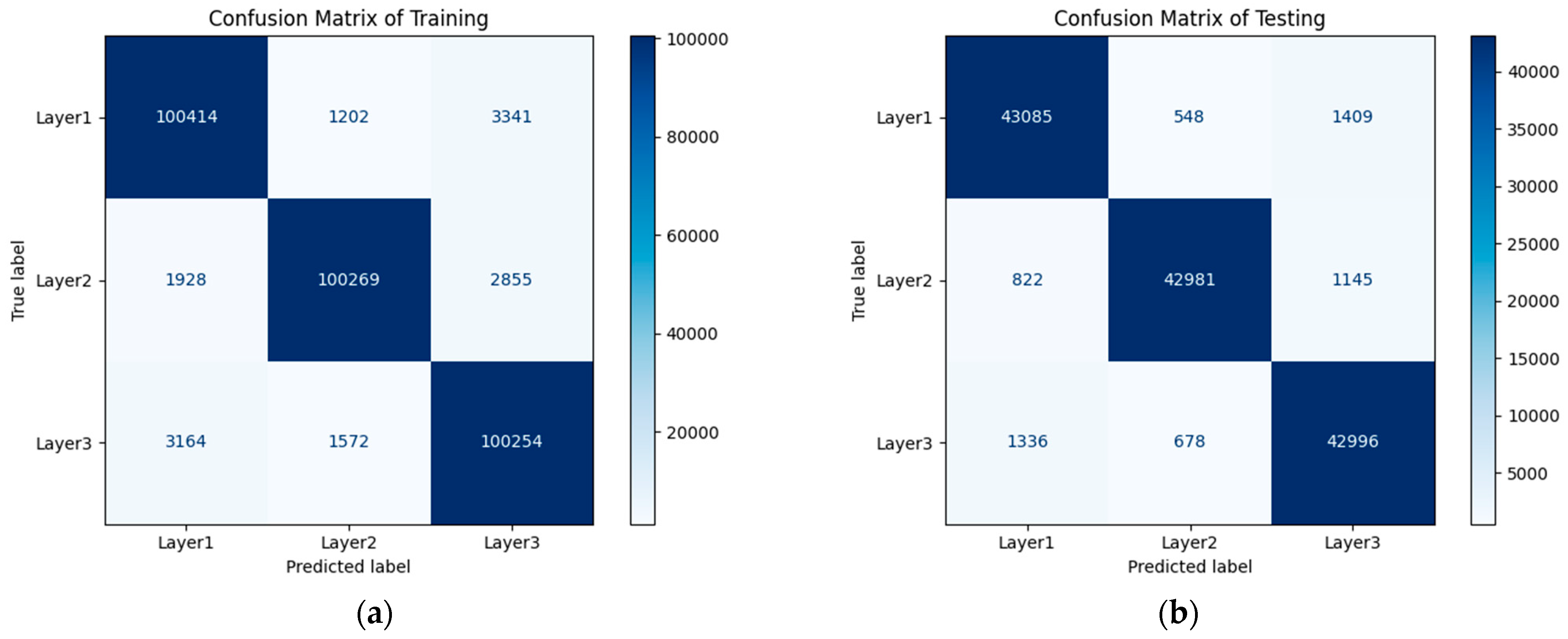
6.3. LSTM Classification Results and Discussion
7. Other Deep Learning (DL) Methods for Performance Comparison with LSTM
7.1. Brief Information of Convolutional Neural Network (CNN)
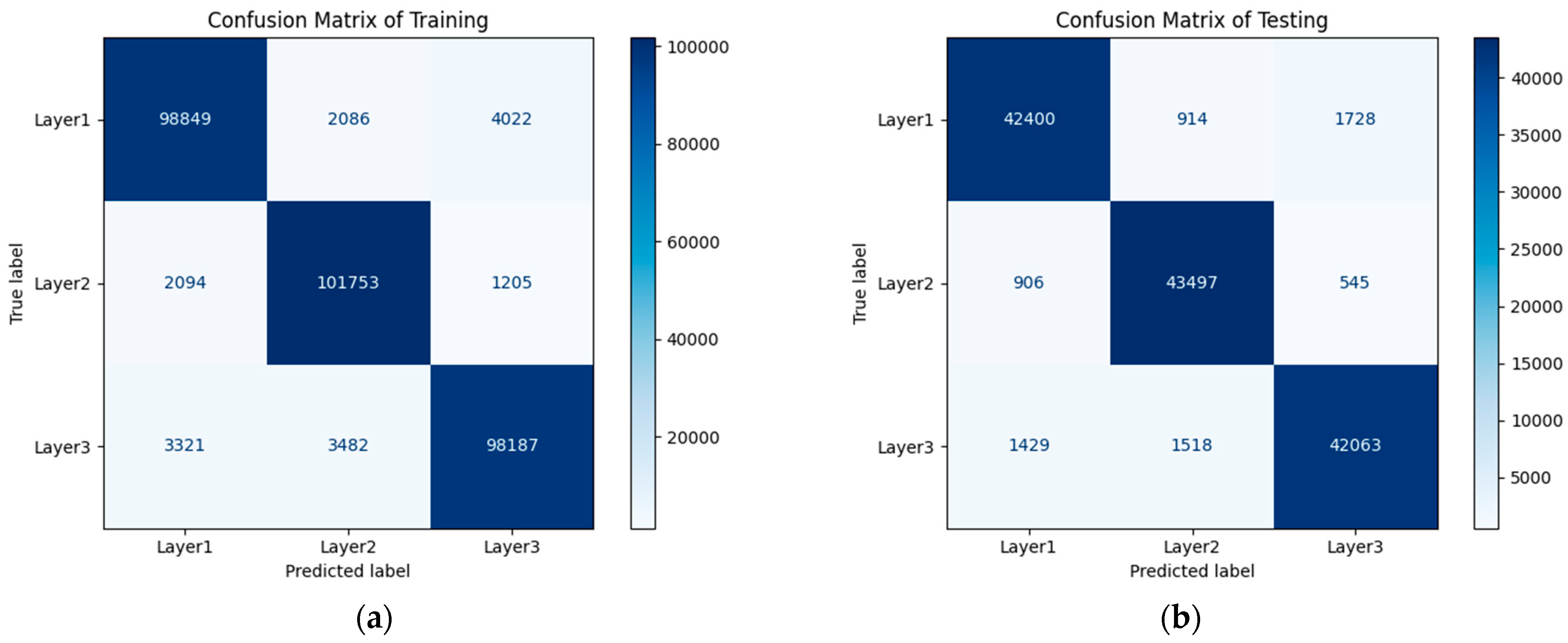
7.2. CNN Classification Results and Discussion
7.3. Brief Information of Recurrent Neural Network (RNN)
7.4. RNN Classification Results and Discussion
8. Conclusions
- •
- With an almost similar DL model development parameters and epoch number, the LSTM shows that it is better than CNN and RNN for vibration data (1D data) of bone layer classification.
- •
- The overall multi-classification accuracy of LSTM, CNN, and RNN, according to the classification report tables, is 0.99, 0.95, and 0.96. This indicates that LSTM is outperformed by CNN and RNN.
- •
- The bone layer classification study based on vibration signals is still developing. This study can be particularly useful in medical procedures in bone drilling, where accurate identification of different bone layers is crucial.
- •
- The future work related to the bone drilling experiment is to generate more datasets and to use other potential methods.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torun, Y.; Malatyali, S. Power Analysis of Robotic Medical Drill with Different Control Approaches. Cumhur. Sci. J. 2020, 41, 527–533. [Google Scholar] [CrossRef]
- Tsai, M.-D.; Hsieh, M.-S.; Tsai, C.-H. Bone Drilling Haptic Interaction for Orthopedic Surgical Simulator. Comput. Biol. Med. 2007, 37, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Pandey, P.M.; Silberschmidt, V.V. Rotary Ultrasonic Bone Drilling: Improved Pullout Strength and Reduced Damage. Med. Eng. Phys. 2017, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.K.; Panda, S.S. Drilling of Bone: A Comprehensive Review. J. Clin. Orthop. Trauma 2013, 4, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lv, Q.; Song, Y.; Zhang, Q. Influence of Parameters on Temperature Rise and Chips Morphology in Low-Frequency Vibration-Assisted Bone Drilling. Med. Eng. Phys. 2022, 103, 103791. [Google Scholar] [CrossRef] [PubMed]
- Pourgiv, S.; Mosavar, A.; Jamshidi, N.; Mohammadi, A. Ultrasonic-Assisted Drilling of Cortical and Cancellous Bone in a Comparative Point of View. Heliyon 2024, 10, e26248. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Babbar, A.; Jain, V.; Gupta, D. Comparative Statement for Diametric Delamination in Drilling of Cortical Bone with Conventional and Ultrasonic Assisted Drilling Techniques. J. Orthop. 2021, 25, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Gupta, V.; Singh, J. Leveraging Ultrasonic Actuation during Inclined Orthopaedic Bone Drilling: An Experimental and Histological Study. Appl. Acoust. 2023, 211, 109520. [Google Scholar] [CrossRef]
- Esen, H.; Yano, K.; Buss, M. Bone Drilling Medical Training System. In The Sense of Touch and Its Rendering; Bicchi, A., Buss, M., Ernst, M.O., Peer, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 45, pp. 245–278. ISBN 978-3-540-79034-1. Springer Tracts in Advanced Robotics. [Google Scholar]
- Esen, H.; Yano, K.; Buss, M. A Virtual Environment Medical Training System for Bone Drilling with 3 DOF Force Feedback. In Proceedings of the 2004 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS) (IEEE Cat. No.04CH37566), Sendai, Japan, 28 September–2 October 2004; IEEE: New York, NY, USA, 2004; Volume 4, pp. 3631–3636. [Google Scholar]
- Esen, H.; Yano, K.; Buss, M. A Control Algorithm and Preliminary User Studies for a Bone Drilling Medical Training System. In Proceedings of the 12th IEEE International Workshop on Robot and Human Interactive Communication, 2003. Proceedings. ROMAN 2003, Millbrae, CA, USA, 2 November 2003; IEEE: New York, NY, USA, 2003; pp. 153–158. [Google Scholar]
- Syamlan, A.; Fathurachman; Denis, K.; Vander Poorten, E.; Pramujati, B.; Tjahjowidodo, T. Haptic/Virtual Reality Orthopedic Surgical Simulators: A Literature Review. Virtual Real. 2022, 26, 1795–1825. [Google Scholar] [CrossRef]
- Bertollo, N.; Robert, W. Drilling of Bone: Practicality, Limitations and Complications Associated with Surgical Drill-Bits. In Biomechanics in Applications; Klika, V., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-969-1. [Google Scholar]
- Ginta, T.L.; Ari-Wahjoedi, B. Cutting Force and Temperature Variation in Bone Drilling—A Review. Adv. Mater. Res. 2013, 845, 934–938. [Google Scholar] [CrossRef]
- Lee, J.; Chavez, C.L.; Park, J. Parameters Affecting Mechanical and Thermal Responses in Bone Drilling: A Review. J. Biomech. 2018, 71, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Timon, C.; Keady, C. Thermal Osteonecrosis Caused by Bone Drilling in Orthopedic Surgery: A Literature Review. Cureus 2019, 11, e5226. [Google Scholar] [CrossRef]
- Bohra, A.; Chandrasekaran, M.; Teyi, N. Bone Drilling Investigation and Possible Research: A State of the Art Review. AIP Conf. Proc. 2019, 2128, 050022. [Google Scholar]
- Alam, K.; Mitrofanov, A.V.; Silberschmidt, V.V. Experimental Investigations of Forces and Torque in Conventional and Ultrasonically-Assisted Drilling of Cortical Bone. Med. Eng. Phys. 2011, 33, 234–239. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, M.; Zhao, Y.; Zhou, G.; Liu, W.; Li, D. Experimental Investigations on Microcracks in Vibrational and Conventional Drilling of Cortical Bone. J. Nanomater. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Den Dunnen, S.; Mulder, L.; Kerkhoffs, G.M.M.J.; Dankelman, J.; Tuijthof, G.J.M. Waterjet Drilling in Porcine Bone: The Effect of the Nozzle Diameter and Bone Architecture on the Hole Dimensions. J. Mech. Behav. Biomed. Mater. 2013, 27, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, Y.; Yang, N.; Yuan, X. Experimental Analysis of Drilling Process in Cortical Bone. Med. Eng. Phys. 2014, 36, 261–266. [Google Scholar] [CrossRef]
- Liao, Z.; Axinte, D.A. On Monitoring Chip Formation, Penetration Depth and Cutting Malfunctions in Bone Micro-Drilling via Acoustic Emission. J. Mater. Process. Technol. 2016, 229, 82–93. [Google Scholar] [CrossRef]
- Samarasinghe, C.; Uddin, M.; Bari, S.; Xian, C. Surgical Bone Drilling: A Review. In Proceedings of the Volume 3: Biomedical and Biotechnology Engineering; American Society of Mechanical Engineers, Salt Lake City, UT, USA, 11 November 2019; p. V003T04A054. [Google Scholar]
- Jamil, M.; Rafique, S.; Khan, A.M.; Hegab, H.; Mia, M.; Gupta, M.K.; Song, Q. Comprehensive Analysis on Orthopedic Drilling: A State-of-the-Art Review. Proc. Inst. Mech. Eng. 2020, 234, 537–561. [Google Scholar] [CrossRef]
- Torun, Y.; Pazarci, O.; Ozturk, A. Current Approaches to Bone-Drilling Procedures with Orthopedic Drills. Cyprus J. Med. Sci. 2020, 5, 93–98. [Google Scholar] [CrossRef]
- Akhbar, M.F.A.; Sulong, A.W. Surgical Drill Bit Design and Thermomechanical Damage in Bone Drilling: A Review. Ann. Biomed. Eng. 2021, 49, 29–56. [Google Scholar] [CrossRef] [PubMed]
- Jung, O.; Lindner, C.; Pantermehl, S.; Barbeck, M. Heat Development During Medical Drilling: Influencing Factors and Examination Methods—Overview and First Results. Vivo 2021, 35, 3011–3017. [Google Scholar] [CrossRef]
- Islam, M.A.; Kamarrudin, N.S.; Daud, R.; Mohd Noor, S.N.F.; Azmi, A.I.; Razlan, Z.M. A Review of Surgical Bone Drilling and Drill Bit Heat Generation for Implantation. Metals 2022, 12, 1900. [Google Scholar] [CrossRef]
- Chouhan, P.S.; Dwivedi, V. Temperature Distribution in Bone Drilling: A Review. Int. J. Res. Publ. Rev. 2023, 4, 2616–2618. [Google Scholar]
- Zahedi, E.; Khosravian, F.; Wang, W.; Armand, M.; Dargahi, J.; Zadeh, M. Towards Skill Transfer via Learning-Based Guidance in Human-Robot Interaction: An Application to Orthopaedic Surgical Drilling Skill. J. Intell. Robot. Syst. 2020, 98, 667–678. [Google Scholar] [CrossRef]
- Ghasemloonia, A.; Baxandall, S.; Zareinia, K.; Lui, J.T.; Dort, J.C.; Sutherland, G.R.; Chan, S. Evaluation of Haptic Interfaces for Simulation of Drill Vibration in Virtual Temporal Bone Surgery. Comput. Biol. Med. 2016, 78, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Aussedat, C.; Venail, F.; Marx, M.; Boullaud, L.; Bakhos, D. Training in Temporal Bone Drilling. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2022, 139, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, P.; Caesarendra, W.; Lai, D.T.C.; Surindra, M.D.; Królczyk, G.; Tjahjowidodo, T. A Study of Vibration Signal Feature Extraction for Bone Drilling Layer Classification. In Proceedings of the 2023 8th International Conference on Mechanical Engineering and Robotics Research (ICMERR), Krakow, Poland, 8–10 December 2023; IEEE: New York, NY, USA, 2023; pp. 50–54. [Google Scholar]
- Wang, R.; Bai, H.; Xia, G.; Zhou, J.; Dai, Y.; Xue, Y. Identification of Milling Status Based on Vibration Signals Using Artificial Intelligence in Robot-Assisted Cervical Laminectomy. Eur. J. Med. Res. 2023, 28, 203. [Google Scholar] [CrossRef]
- Caesarendra, W.; Wulandari, P.; Gatnar, K. Bone Drilling Vibration Signal Classification Using Convolutional Neural Network to Determine Bone Layers. In Lecture Notes in Electrical Engineering, Proceedings of the 4th International Conference on Electronics, Biomedical Engineering, and Health Informatics, Surabaya, Indonesia, 28 April 2024; Triwiyanto, T., Rizal, A., Caesarendra, W., Eds.; Springer: Singapore, 2024; Volume 1182, pp. 577–592. [Google Scholar]
- Kong, F.; Lee, Y.-S. Analytical Modeling of Ultrasonic Vibration Assisted Drilling of Bones for Medical Surgical Applications. In Volume 2: Materials; Biomanufacturing; Properties, Applications and Systems; Sustainable Manufacturing, Proceedings of the ASME 2015 International Manufacturing Science and Engineering Conference, Charlotte, NC, USA, 8 June 2015; American Society of Mechanical Engineers: New York, NY, USA, 2015; p. V002T03A008. [Google Scholar]
- Gupta, V.; Pandey, P.M. Experimental Investigation and Statistical Modeling of Temperature Rise in Rotary Ultrasonic Bone Drilling. Med. Eng. Phys. 2016, 38, 1330–1338. [Google Scholar] [CrossRef]
- Pandey, R.K.; Panda, S.S. Genetic Algorithm Based Prediction of an Optimum Parametric Combination for Minimum Thrust Force in Bone Drilling. In Recent Advances in Information and Communication Technology; Boonkrong, S., Unger, H., Meesad, P., Eds.; Springer International Publishing: Cham, Switzerland, 2014; Volume 265, pp. 103–112. ISBN 978-3-319-06537-3. Advances in Intelligent Systems and Computing. [Google Scholar]
- Pandey, R.K.; Panda, S.S. Optimization of Bone Drilling Parameters Using Grey-Based Fuzzy Algorithm. Measurement 2014, 47, 386–392. [Google Scholar] [CrossRef]
- Staroveski, T.; Brezak, D.; Udiljak, T. Drill Wear Monitoring in Cortical Bone Drilling. Med. Eng. Phys. 2015, 37, 560–566. [Google Scholar] [CrossRef]
- Agarwal, R.; Singh, J.; Gupta, V. Prediction of Temperature Elevation in Rotary Ultrasonic Bone Drilling Using Machine Learning Models: An in-Vitro Experimental Study. Med. Eng. Phys. 2022, 110, 103869. [Google Scholar] [CrossRef]
- Agarwal, R.; Singh, J.; Gupta, V. An Intelligent Approach to Predict Thermal Injuries during Orthopaedic Bone Drilling Using Machine Learning. J. Braz. Soc. Mech. Sci. Eng. 2022, 44, 320. [Google Scholar] [CrossRef]
- Agarwal, R.; Gupta, V.; Singh, J.; Jain, V. Prediction of Surface Roughness and Cutting Force Induced during Rotary Ultrasonic Bone Drilling via Statistical and Machine Learning Algorithms. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2022, 236, 11123–11135. [Google Scholar] [CrossRef]
- Torun, Y.; Öztürk, A. A New Breakthrough Detection Method for Bone Drilling in Robotic Orthopedic Surgery with Closed-Loop Control Approach. Ann. Biomed. Eng. 2020, 48, 1218–1229. [Google Scholar] [CrossRef]
- Song, S.; Cheng, X.; Li, T.; Shi, M.; Zheng, G.; Liu, H. Experimental Study of Bone Drilling by Kirschner Wire. Med. Eng. Phys. 2022, 106, 103835. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Gundjian, A.A. Specific Heat of Bone. Med. Biol. Eng. 1976, 14, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Cordioli, G.; Majzoub, Z. Heat Generation during Implant Site Preparation: An in Vitro Study. Int. J. Oral Maxillofac. Implants 1997, 12, 186–193. [Google Scholar] [PubMed]
- Hillery, M.T.; Shuaib, I. Temperature Effects in the Drilling of Human and Bovine Bone. J. Mater. Process. Technol. 1999, 92–93, 302–308. [Google Scholar] [CrossRef]
- Lee, J.; Gozen, B.A.; Ozdoganlar, O.B. Modeling and Experimentation of Bone Drilling Forces. J. Biomech. 2012, 45, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.K.; Panda, S.S. Optimization of Multiple Quality Characteristics in Bone Drilling Using Grey Relational Analysis. J. Orthop. 2015, 12, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sarparast, M.; Ghoreishi, M.; Jahangirpoor, T.; Tahmasbi, V. Experimental and Finite Element Investigation of High-Speed Bone Drilling: Evaluation of Force and Temperature. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 349. [Google Scholar] [CrossRef]
- Alam, K.; Qamar, S.Z.; Iqbal, M.; Piya, S.; Al-Kindi, M.; Qureshi, A.; Al-Ghaithi, A.; Al-Sumri, B.; Silberschmidt, V.V. Effect of Drill Quality on Biological Damage in Bone Drilling. Sci. Rep. 2023, 13, 6234. [Google Scholar] [CrossRef] [PubMed]
- Hochreiter, S.; Schmidhuber, J. Long Short-Term Memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef] [PubMed]
- Darmawahyuni, A.; Nurmaini, S.; Sukemi; Caesarendra, W.; Bhayyu, V.; Rachmatullah, M.N. Firdaus Deep Learning with a Recurrent Network Structure in the Sequence Modeling of Imbalanced Data for ECG-Rhythm Classifier. Algorithms 2019, 12, 118. [Google Scholar] [CrossRef]
- Rofii, A.; Soerowirdjo, B.; Irawan, R.; Caesarendra, W. Utilize the Prediction Results from the Neural Network Gate Recurrent Unit (GRU) Model to Optimize Reactive Power Usage in High-Rise Buildings. Int. J. Robot. Control Syst. 2024, 4, 628–654. [Google Scholar]
- Sabique, P.V.; Pasupathy, G.; Ramachandran, S. A Data Driven Recurrent Neural Network Approach for Reproduction of Variable Visuo-Haptic Force Feedback in Surgical Tool Insertion. Expert Syst. Appl. 2024, 238, 122221. [Google Scholar] [CrossRef]
- Lu, S.; Yang, J.; Yang, B.; Li, X.; Yin, Z.; Yin, L.; Zheng, W. Surgical Instrument Posture Estimation and Tracking Based on LSTM. ICT Express 2024, 10, 465–471. [Google Scholar] [CrossRef]
- Caesarendra, W.; Rahmaniar, W.; Mathew, J.; Thien, A. Automated Cobb Angle Measurement for Adolescent Idiopathic Scoliosis Using Convolutional Neural Network. Diagnostics 2022, 12, 396. [Google Scholar] [CrossRef]
- Permana, S.D.H.; Saputra, G.; Arifitama, B.; Yaddarabullah; Caesarendra, W.; Rahim, R. Classification of Bird Sounds as an Early Warning Method of Forest Fires Using Convolutional Neural Network (CNN) Algorithm. J. King Saud Univ.-Comput. Inf. Sci. 2022, 34, 4345–4357. [Google Scholar] [CrossRef]
- Caesarendra, W.; Triwiyanto, T.; Pandiyan, V.; Glowacz, A.; Permana, S.D.H.; Tjahjowidodo, T. A CNN Prediction Method for Belt Grinding Tool Wear in a Polishing Process Utilizing 3-Axes Force and Vibration Data. Electronics 2021, 10, 1429. [Google Scholar] [CrossRef]
- Kiranyaz, S.; Avci, O.; Abdeljaber, O.; Ince, T.; Gabbouj, M.; Inman, D.J. 1D Convolutional Neural Networks and Applications: A Survey. Mech. Syst. Signal Process. 2021, 151, 107398. [Google Scholar] [CrossRef]
- Lipton, Z.C.; Berkowitz, J.; Elkan, C. A Critical Review of Recurrent Neural Networks for Sequence Learning. arXiv 2015, arXiv:1506.00019. [Google Scholar]
- Huang, H. Building Your Recurrent Neural Network—Step by Step 2018. Available online: https://github.com/Kulbear/deep-learning-coursera/blob/master/Sequence%20Models/Building%20a%20Recurrent%20Neural%20Network%20-%20Step%20by%20Step%20-%20v2.ipynb (accessed on 3 July 2024).
| Full Terminology | Abbreviation |
|---|---|
| Acoustic Emission | AE |
| Artificial Neural Network | ANN |
| Analysis of Variance | ANOVA |
| Computer-Aided Orthopedic Surgery | CAOS |
| Coordinate Measuring Machine | CMM |
| Computer Numerical Control | CNC |
| Convolutional Neural Network | CNN |
| Deep Learning | DL |
| Direct Current | DC |
| Degree of Freedom | DOF |
| Decision Tree | DT |
| Genetic Algorithm | GA |
| Grey Fuzzy Reasoning Grade | GFRG |
| Grey Relation Analysis | GRA |
| Graphical User Interface | GUI |
| Haptic Display | HD |
| Human-Robot Interaction | HRI |
| High-Speed Steel | HSS |
| K-Nearest Neighbors | KNN |
| Laser-Assisted Drilling | LAD |
| Leaning-based Guidance | LbG |
| Long Short-Term Memory | LSTM |
| Mean Absolute Error | MAE |
| Machine Learning | ML |
| Mean Square Error | MSE |
| Medical Training System | MTS |
| National Instrument | NI |
| Proportional Derivative | PD |
| Radial Basis Function Neural Network | RBFNN |
| Random Forest | RF |
| Rotation Per Minute | RPM |
| Root Mean Square Error | RMSE |
| Rotary Ultrasonic Bone Drilling | RUBD |
| Response Surface Methodology | RSM |
| Scanning Electron Microscopic | SEM |
| Support Vector Machine | SVM |
| Ultrasonic-Assisted Drilling | UAD |
| Virtual Environment | VE |
| Virtual Reality | VR |
| Water Jet-Assisted Drilling | WJAD |
| Author (Year) | Data Used in the Machine Learning Method | Machine Learning Method | Machine Learning Is Used for? | The Purpose of Using Machine Learning Method |
|---|---|---|---|---|
| Pandey et al. (2014) [38] | Temperature, force, and surface roughness | Grey relation analysis and fuzzy logic | Optimization | To determine the optimal combination of bone drilling parameters that minimize temperature, force, and surface roughness. |
| Pandey et al. (2014) [39] | Temperature, force, and surface roughness | Grey fuzzy reasoning grade | Classification | To find an optimal value of feed rate (mm/min) and speed (rpm). |
| Staroveski et al. (2015) [40] | Cutting forces, servomotor drive currents, and acoustic emission | Radial basis function neural network | Classification | To develop a drill wear classification model based on a multi-sensor approach. |
| Torun et al. (2020) [44] | Closed-loop signal and force sensor data | K-nearest neighbors and ensemble classifier | Classification | To detect breakthroughs and estimate the condition of the drill bit in robotic bone drilling. |
| Agarwal et al. (2022) [41] | Temperature | K-nearest neighbors Support vector regression Decision trees Random forest | Regression | To introduce different ML predicting methods for the temperature elevations of rotary ultrasonic bone drilling. |
| Agarwal et al. (2022) [42] | Temperature | Multilayer perceptron Lasso regression Ridge regression | Regression | To predict temperature rise during bone drilling. |
| Agarwal et al. (2022) [43] | Surface roughness and cutting force | Ridge regression, lasso regression, support vector regression, multi-linear regression, artificial neural network | Regression | To predict the surface roughness and cutting force during rotary ultrasonic bone drilling. |
| Caesarendra et al. (2024) [35] | Vibration signal | Convolutional neural network | Classification | To classify three bone layers based on vibration signal. |
| Author (Year) | Bone Sample | Brief Experimental Description | The Outcome of the Study |
|---|---|---|---|
| Chen and Gundjian (1976) [46] | Bovine femur | The bovine femur was split into seven thin-disc samples. Each thin-disc sample dimension is approximately 1 mm thick and 3 mm in diameter. | The material characteristic that affects the bone’s maximum temperature when a heat source is present is specific heat. |
| Cordioli and Majzoub (1997) [47] | Bovine cortical femur bone | The bone sample was drilled with a diameter of 2 and 3 mm running at 1500 rpm and 200 N of axial force. | Correlation between drilling depth and maximum temperature. |
| Hillery et al. (1999) [48] | Femur heads, Bovine tibia | The drilling machine was operated from 400 to 2000 rpm with an interval of 200 rpm. The feed rate during the bone drilling was 50 mm/min. | The temperature increased with the increasing depth of the hole. The optimal speed range is between 800 and 1400 rpm with a drill bit diameter of 3.2 mm. |
| Lee et al. (2012) [49] | Bovine femur | Each bone specimen was attached to a drilling dynamometer. The controlled parameters for the drilling time are gauge torque and thrust. | Presented a novel method based on a CNC system for temperature measurement, various thermocouples, and an accurate position. |
| Pandey et al. (2014) [50] | Bovine bone | Using an MTAB 3-axis Flex mill. Temperature data were gathered using a K-type Extech thermocouple and data-gathering software. | The study found that drill diameter had the greatest influence among these variables based on the result of the Taguchi method. |
| Sarparast et al. (2020) [51] | Bovine femur | A high-speed electrical motor with a rotational speed higher than 10,000 rpm was mounted in the lathe machine. High-speed steel (HSS) drill bit that was 2 mm in diameter was selected for the experiment. The lathe machine was run with an increasing feed rate from 10 to 50 mm/min. Single footing load cells and k-type thermocouples are used for force and temperature measurement. | Bone drilling optimum (minimum) temperature was revealed at a rotational speed of 12,000 rpm and feed rate of 50 mm/min. By increasing the feed rate slightly, it increases the process force, which can also lead to the increasing temperature. |
| Alam et al. (2023) [52] | Femoral and tibia bones | A custom-made drilling setup with a feedback control system for force, torque, and temperature was used in drilling tests. Small holes of 1.5 mm in diameter through the bone were produced with rotational speed of 400 rpm and feed rate of 40 mm/min. | Increasing pressure on a worn drill is necessary when drilling passes through the hard cortex of the bone. The torque in bone drilling has a direct relationship with the depth of drilling. Bone temperature was increased when the drill progressed to wear. |
| Layer | RMS of x-Axis Vibration (mV) | RMS of y-Axis Vibration (mV) | RMS of z-Axis Vibration (mV) |
|---|---|---|---|
| Periosteum (layer 1) | 0.04 | 0.04 | 0.04 |
| First cortical (layer 2) | 0.09 | 0.06 | 0.05 |
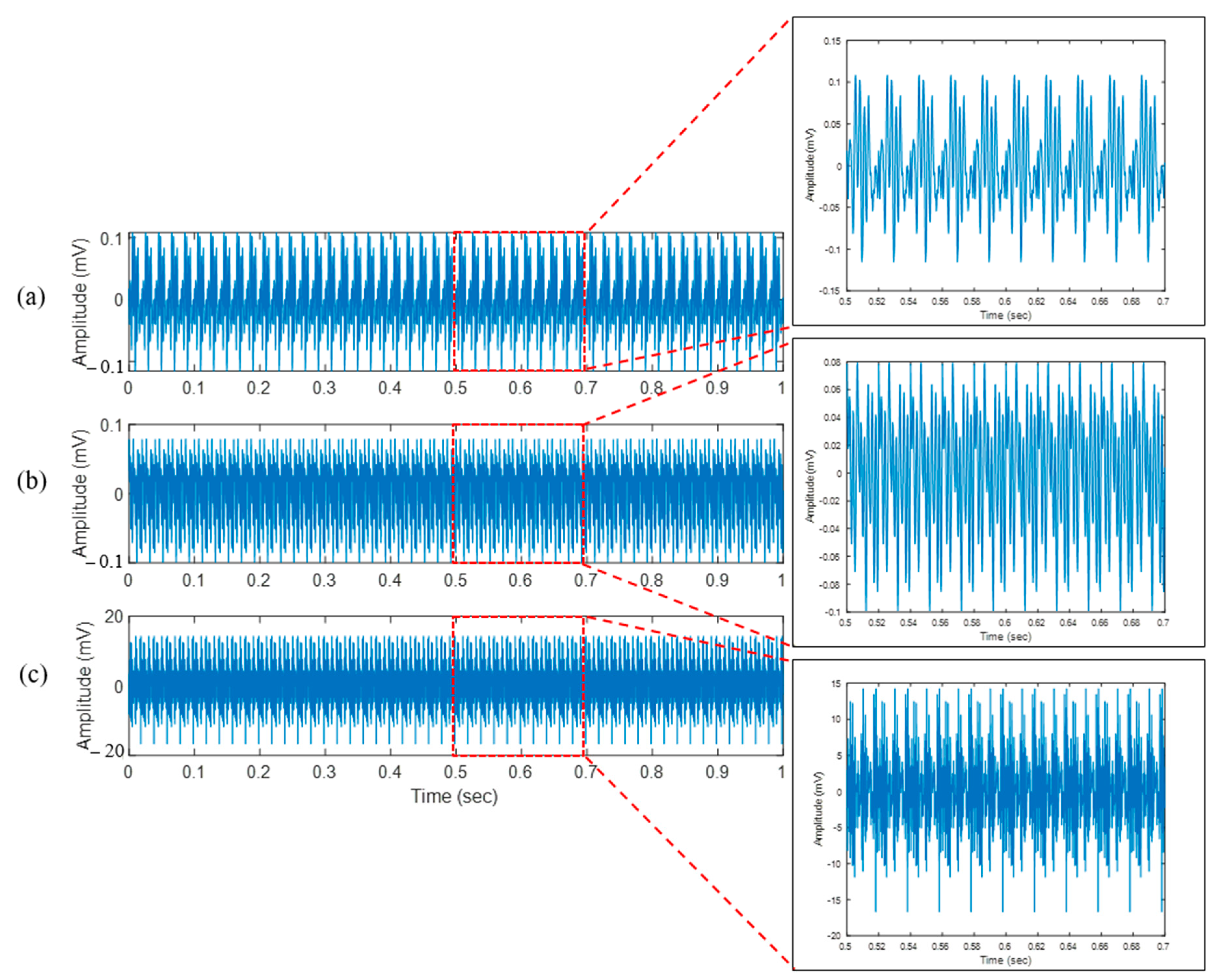
| Spongy (layer 3) | 4.04 | 5.51 | 5.85 |
| Layer (Type) | Output Shape | Param # |
|---|---|---|
| input_1 (InputLayer) | [(None, 3, 1)] | 0 |
| Astm (LSTM) | [(None, 3, 32)] | 4352 |
| lstm_1 (LSTM) | (None, 32) | 8320 |
| flatten (Flatten) | (None, 32) | 0 |
| dense (Dense) | (None, 3) | 99 |
| Total params: 12,771 (49.89 KB) | ||
| Trainable params: 12,771 (49.89 KB) | ||
| Non-trainable params: 0 (0.00 Byte) | ||
| Precision | Recall | F1-Score | Support | |
|---|---|---|---|---|
| Periosteum (layer 1) | 0.99 | 0.99 | 0.99 | 45,042 |
| First cortical (layer 2) | 0.99 | 0.99 | 0.99 | 44,948 |
| Spongy (layer 3) | 0.99 | 1 | 0.99 | 45,010 |
| Accuracy | 0.99 | 135,000 | ||
| Macro avg | 0.99 | 0.99 | 0.99 | 135,000 |
| Weighted avg | 0.99 | 0.99 | 0.99 | 135,000 |
| Precision | Recall | F1-Score | Support | |
|---|---|---|---|---|
| Periosteum (layer 1) | 0.95 | 0.94 | 0.94 | 45,042 |
| First cortical (layer 2) | 0.95 | 0.97 | 0.96 | 44,948 |
| Spongy (layer 3) | 0.95 | 0.93 | 0.94 | 45,010 |
| Accuracy | 0.95 | 135,000 | ||
| Macro avg | 0.95 | 0.95 | 0.95 | 135,000 |
| Weighted avg | 0.95 | 0.95 | 0.95 | 135,000 |
| Precision | Recall | F1-Score | Support | |
|---|---|---|---|---|
| Periosteum (layer 1) | 0.95 | 0.96 | 0.95 | 45,042 |
| First cortical (layer 2) | 0.97 | 0.96 | 0.96 | 44,948 |
| Spongy (layer 3) | 0.94 | 0.96 | 0.95 | 45,010 |
| Accuracy | 0.96 | 135,000 | ||
| Macro avg | 0.96 | 0.96 | 0.96 | 135,000 |
| Weighted avg | 0.96 | 0.96 | 0.96 | 135,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caesarendra, W. Bone Drilling: Review with Lab Case Study of Bone Layer Classification Using Vibration Signal and Deep Learning Methods. Eng 2024, 5, 1566-1593. https://doi.org/10.3390/eng5030083
Caesarendra W. Bone Drilling: Review with Lab Case Study of Bone Layer Classification Using Vibration Signal and Deep Learning Methods. Eng. 2024; 5(3):1566-1593. https://doi.org/10.3390/eng5030083
Chicago/Turabian StyleCaesarendra, Wahyu. 2024. "Bone Drilling: Review with Lab Case Study of Bone Layer Classification Using Vibration Signal and Deep Learning Methods" Eng 5, no. 3: 1566-1593. https://doi.org/10.3390/eng5030083
APA StyleCaesarendra, W. (2024). Bone Drilling: Review with Lab Case Study of Bone Layer Classification Using Vibration Signal and Deep Learning Methods. Eng, 5(3), 1566-1593. https://doi.org/10.3390/eng5030083






