Abstract
Background: In recent years, hyaluronic acid filler for the restoration and increase in buttock volume has been a procedure that has seen increasing success, both thanks to the considerable increase in patient demand and thanks to the improvement of implant techniques and device manufacturing technologies. Aims: The primary objective of this pilot study is to demonstrate the validity of an innovative filler inoculation technique in the upper quadrants of the buttocks and in the supra- and subfascial area in order to optically restore the appearance of a pleasant lumbar lordosis and to lift the upper quadrants with reduction in the infragluteal fold. The secondary objective is to evaluate the safety and efficacy of Sofiderm SubSkin® (Techderm Biological Products Co., Ltd., Hangzhou, China), a highly versatile hyaluronic acid filler, formulated with a rheology suitable for use on the face and body. Patients/Methods: Five female subjects (50–63 years) were subjected to gluteal fillers in the supra- and subfascial areas; the correct positioning of the filler was investigated by means of a 20 Mhz ultrasound probe. Results: All patients obtained a significant improvement in the projection of the upper part of the buttocks. The implantation technique and the optimal rheological properties of the device brought about a natural and well-defined increase in volume, with a projection of the upper part of the buttocks and a consequent lifting of the lower parts and reduction in the length of the infragluteal fold. Conclusions: This study confirmed the efficacy and safety of the cross-linked hyaluronic acid Sofiderm Derm SubSkin® in increasing the projection of the upper part of the buttocks, using an innovative mixed implantation technique, in a sample of selected patients.
1. Introduction
In the majority of the female and male population, the desire to shape one’s body and, in particular, to increase the volume of the breasts and buttocks is now frequent [1]. In recent years, demand has grown considerably; manufacturers and practitioners have worked to meet patient demands, optimizing the technology of filling devices and the safety of injection techniques as much as possible [2].
Before the use of hyaluronic acid (HA), interventions were the exclusive prerogative of surgery, with the use of dedicated prostheses or the use of autologous fat through lipofilling [3].
With the advent of specific HA formulations for buttocks and body reshaping, the procedure has become increasingly noninvasive and less surgical, thus also limiting a good part of the inevitable side effects in a surgical procedure [4].
When planning the intervention, it is essential to consider the ethnic group [5] to which the patient belongs, adapting materials and techniques and considering cultural differences and related aesthetic tastes, to make it easier for the patient to choose the result and not cause them to obtain an appearance different from their desires. In Brazil, highly projected buttocks that are very full, even in the lateral portions, are considered attractive, called a “bubble shape”. In Japan, women consider large buttocks to be extremely vulgar; in Asia, individuals prefer moderately small but well-turned buttocks without lateral fullness. Among Caucasians, full buttocks are favored but not large buttocks, with buttocks without lateral fullness that present an android gluteal shape with a discrete lateral concavity being preferred. In the African group, individuals prefer very large buttocks in every projection.
Buttock remodeling with HA is a minimally invasive procedure indicated for patients presenting poor projection of the upper buttock region, flattening of the superior quadrants, or an insufficiently defined lumbar lordosis [6], all of which can benefit from the lifting and volumizing effect produced by sub- and supra-fascial implantation. The ideal candidate is typically normal-weight, lacking excessive gluteal adiposity, and without significant tissue laxity [7], allowing for a natural enhancement of projection without the risk of exaggerated or disharmonious results. Individuals with already voluminous buttocks, overweight patients, or those with unrealistic expectations, particularly requests for extreme augmentation incompatible with tissue biomechanics or procedural safety, are not good candidates. An essential component of candidate selection is an understanding of cultural aesthetic preferences, which strongly influence the desired outcome, as highlighted in the manuscript (e.g., differences among Brazilian, Caucasian, Asian, and African aesthetic standards).
Patient expectations should be carefully guided: the treatment offers a progressive and natural improvement, which is generally more evident after the first two weeks due to the physiological redistribution of the filler within the anatomical planes. While an immediate enhancement is visible, the best result emerges during short-term follow-up and depends on injected volume, tissue characteristics, and chosen technique. Patients must be aware that this is a temporary procedure, requiring periodic maintenance [8], and that the goal is not to replicate the outcomes of surgical gluteoplasty but to achieve harmonious volumization, improved support of the upper quadrants, and a reduction in infragluteal fold prominence.
Sofiderm Derm SubSkin® is a filler based on cross-linked HA designed for deep volumetric treatments [9], which is ideal for the treatment of large areas subjected to significant compressive forces such as the buttocks. Its high density, combined with the capacity for stabilization and resistance to deformation (high Gi), makes it particularly suitable [10,11] for sub-fascial injection, where the aesthetic request is to obtain a significant projection of the upper part of the buttocks without creating rigidity or irregularities. This pilot study aims to evaluate the efficacy and safety of the cross-linked HA Sofiderm Derm SubSkin® in increasing the projection of the upper part of the buttocks [12], using a mixed implant technique, in a sample of selected patients.
Anatomy and Causes of Hypotonia of the Gluteus
Some important factors contribute to the formation of a hypotonic gluteus. First of all, posture plays a fundamental role. Pelvic rotation on the anteroposterior axis and incorrect use of the muscles leads to a flattening of the physiological lordosis at the level of the upper quadrants of the gluteus [5], in correspondence with the iliac dimples, two depressions visible in the sacral region. Undoubtedly, a sedentary lifestyle negatively affects the volume of the gluteus; even targeted but incorrect physical exercise can determine an unsatisfactory result. All these factors can be corrected; however, often the patient does not want to engage in physical exercise programs that include long-term goals.
The gluteus are made up of four muscles: the gluteus maximus, gluteus medius, gluteus minimus, and piriformis [13]. The gluteus medius is the anatomical entity that contributes most to the projection, and therefore to the pleasantness of the appearance of the buttocks, according to Caucasian aesthetic criteria. It originates from the gluteal fascia, the iliac crest, the section of the coxal bone between the anterior and posterior gluteus, and the posterior superior iliac spine. The subcutaneous tissue of this region has an average total thickness of 3 cm and is divided into four layers: superficial fat, superficial fascia, deep fat, and deep fascia.
The shape of the fat contributes to both the projection and the rounded shape of the hip. In women, subcutaneous fat tends to be more represented than in men. However, with aging, the total thickness of the subcutaneous tissue increases, regardless of gender or body mass index. Over the years, an increase in the thickness of the deep fat and a reduction in the superficial compartment have been observed.
The trochanteric depression is located under the greater trochanter, located in the lateral gluteus.
The final lower portion of the intergluteal ridge is the point where the buttocks begin to separate from the midline and form the intergluteal fold.
In the so-called ideal gluteal region, this should occur in the lower two-thirds or 3/4 of the gluteus maximus muscle.
The infragluteal fold region is the lower edge of the buttocks. The infragluteal fold is located below it.
2. Materials and Methods
2.1. Enrollment and Exclusion Criteria
The sample consisted of 5 female subjects, aged between 50 and 63 years, who had poor projection of the upper part of the buttocks. The patients were selected for their normal weight and insufficient volume in the area of the upper quadrants of the gluteus, which made treatment with fillers likely to improve the projection and aesthetics of the area.
Exclusion criteria:
- Overweight subjects or subjects with already voluminous buttocks in all projections.
- Pregnant or breastfeeding subjects.
- Subjects with autoimmune diseases or other contraindications to the use of fillers.
- Subjects who had already received HA implants in the buttocks previously.
The filler used in the treatment was Sofiderm Derm SubSkin®, a cross-linked HA that uses MCLPE (Multiphase Cross Linking and Purification Enhancement) technology and contains a concentration of 20 mg/mL of HA [9]. Its density, combined with particles of variable size between 800 and 1800 μm, makes it suitable for deep volumetric treatments. The filler is supplied in 20 mL vials.
For the injection, 16 gauge × 100 mm cannulas were used. The choice of instrumentation and injection strategy plays a central role in achieving a safe, controlled, and aesthetically coherent buttock augmentation with HA [14]. In the presented technique, the authors employed 16 gauge, 100 mm cannulas, a caliber that offers the optimal balance between rigidity, navigational precision, and a sufficiently large lumen to deliver high-viscosity HA fillers for body contouring. This diameter of cannula minimizes the risk of intravascular injection and allows a smooth progression within the subfascial and supramuscular planes, which are essential for building upper-pole projection while maintaining a natural contour while for access to the gluteal area. A No. 11 scalpel blade was used; the decision to use a scalpel to create the access port rather than penetrating with a needle was both technical and safety-driven. It reduced tissue trauma; forcing a large, blunt 16 G cannula through the dermis and fibrous septa may require significant pressure, increasing discomfort and risking sudden, uncontrolled advancement. A pre-formed incision allows for gentle insertion with minimal force and preservation of cannula integrity, as the tip of a long cannula can bend or undergo micro deformations. A clean incision prevents mechanical stress and maintains optimal cannula performance throughout the procedure. To maintain precision of the entry plane, a scalpel incision ensures the access point is placed exactly at the intended angle and depth. This is crucial in buttock augmentation, where the objective is to immediately engage the proper subfascial path without deviating into superficial fat layers. This enhances safety, as creating a small, predictable access reduces the risk of accidental intradermal product deposition or shearing of dermal vessels.
In summary, the micro-incision technique reflects attention to procedural control, patient comfort, and device preservation. These factors contribute to the reproducibility and safety of the methods described in the study.
The lateral access was performed at the trochanteric fossa [15] to reach the deep area near the fascia. The access points were strategically positioned at the trochanteric fossa, a lateral entry site that permits linear advancement toward the upper quadrants while avoiding excessive manipulation of the central aesthetic subunits. This location facilitates a long, controlled trajectory beneath the fascia, enabling even deposition of the product and reducing the risk of contour abnormalities. For medial and superficial refinement, the same entry point can be used or complemented by minimal additional micro-incisions, depending on the need to approximate the medial borders of the buttocks and conceal excessive exposure of the intergluteal cleft.
Regarding injection planes, the technique integrates a dual approach [16]: deep subfascial injection for true projection of the upper buttocks, enhancing the curvature associated with a youthful gluteal silhouette. This plane provides greater structural support and improves filler longevity by anchoring the product beneath a stable anatomical layer.
More superficial injections occur along the medial buttock, designed to soften transitions and improve the central contour without creating visible irregularities. This combination technique ensures both architectural lifting and harmonious shaping of the gluteal mound.
Volume distribution is individualized, as demonstrated in the clinical series, with initial sessions ranging from 200 to 250 mL in most patients and up to 600 mL in selected cases, followed by structured touch-ups tailored to early tissue adaptation. This multilayer, multipass approach proved essential to achieving progressive refinement that becomes particularly visible after 10–14 days, when the tissue–filler interface stabilizes.
The treatment was performed under local anesthesia, with the treated area appropriately disinfected. A subfascial implantation technique was performed for the upper area of the buttocks, using lateral accesses to reach the desired depths. For the more superficial areas, such as the medial one, a less deep technique was applied in order to obtain a smoothing effect and improve the definition of the gluteal contour (Figure 1).
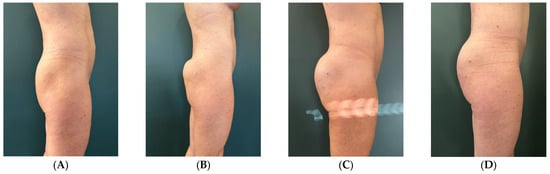
Figure 1.
Subject 1 (A) baseline, (B) right after, (C) 2 weeks after, (D) 2 months after.
During the first visit, each patient received a variable amount of filler, between 200 mL and 250 mL in 4 cases depending on individual needs, and 600 mL in only one subject. The treatment was completed with one or more touch-up sessions at the follow-up visit, with a variable amount of filler between 60 mL and 100 mL (Figure 2).
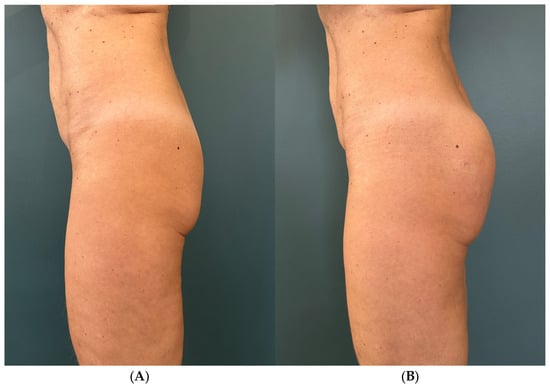
Figure 2.
Subject 1 (A) baseline, (B) 2 weeks after.
2.2. Follow-Up
All patients were monitored two months after the first treatment. During the visit, the aesthetic improvement was evaluated on a semiquantitative 5-point scale (5 = excellent; 4 = good; 3 = discrete; 2 = mild; 1 = scars/inadequate) by two experts in aesthetic procedures, comparing the baseline picture with the ones immediately after the treatment and at the follow-up visit. Duration of the result was evaluated as volume reduction comparing pictures immediately after treatment and at follow-up on a semiquantitative 5-point scale (5 = no reduction; 4 = slight reduction; 3 = moderate reduction; 2 = marked reduction; 1 = complete loss of volumization).
Also, the aesthetic satisfaction of the patients was evaluated at the end of the follow-up period.
Any side effects were reported.
In the first case of the study, a General Electric Logiq E R9 20 Mhz ultrasound probe was used to check the correct positioning of the filler in the deep sub- and supra-fascial areas and eventual migration phenomena.
No phenomena of product migration were recorded [3], particularly in the areas most commonly affected by this complication, i.e., above the upper quadrants and laterally.
3. Results and Discussion
The amount of filler implanted was customized based on the individual characteristics of each patient:
- Patient 1: 63 years old, normal weight, 30 vials (600 mL). First touch up at 20 days, second at 60 days.
- Patient 2: 50 years old, normal weight, 18 vials (360 mL). First touch up at 10 days, second at 20 days.
- Patient 3: 57 years old, normal weight, 14 vials (280 mL). First touch up at 15 days, second at 30 days.
- Patient 4: 56 years old, normal weight, 16 vials (320 mL). First touch up at 15 days, second at 30 days (Figure 3 and Figure 4).
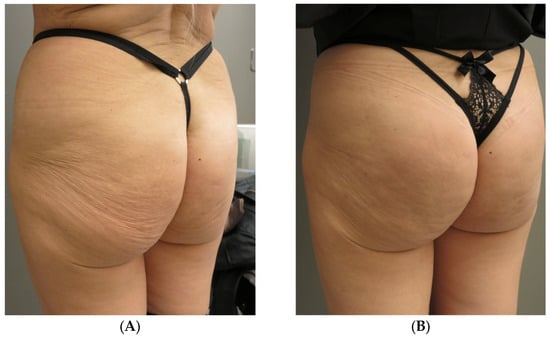 Figure 3. Subject 4, (A) baseline, (B) two weeks later.
Figure 3. Subject 4, (A) baseline, (B) two weeks later.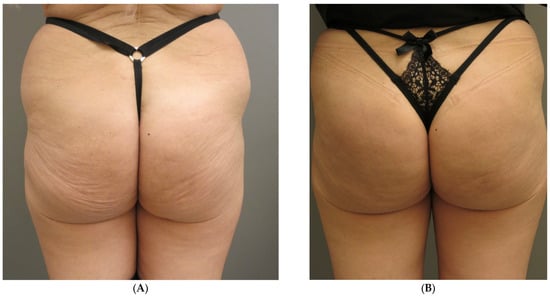 Figure 4. Subject 4, (A) baseline, (B) two weeks after.
Figure 4. Subject 4, (A) baseline, (B) two weeks after. - Patient 5: 55 years old, normal weight, 14 vials (280 mL). First touch up at 60 days.
All patients achieved a significant improvement in the projection of the upper buttocks.
Immediately after the procedure, the average score of the semiquantitative improvement scale was 1.6 SD ± 0.55, whereas at follow-up, it was 4.4 SD ± 0.55.
At the follow-up visit, the loss of volume was evaluated with an average score of 4.6 SD ± 0.55.
All patients expressed a high degree of satisfaction with the aesthetic results obtained.
The 2-month follow-up does not allow for the absolute exclusion of subsequent migration of the implant, nor does it provide absolute guarantees of the duration of the aesthetic correction.
HA buttock remodeling is generally characterized by a high level of safety and minimal downtime. In the present series, only mild and expected post-treatment effects—such as transient swelling, localized soreness, and occasional bruising—were observed [3], all resolving spontaneously within a few days and requiring, at most, conservative measures such as cold compress application or simple analgesia. Temporary contour irregularities may appear in the early phase but typically improve within 10–14 days as the filler redistributes and integrates naturally into the subfascial and superficial planes. Less frequent issues, such as localized firmness or small nodules, are usually related to transient product accumulation and often soften over time; only rarely is minimal hyaluronidase intervention required. Superficial overfilling or visible product tend to result from injections placed too close to the dermis and can generally be corrected with massage or minor enzymatic adjustment. True complications, including infection or delayed inflammatory reactions, remain uncommon and are usually manageable with antibiotics or short courses of corticosteroids when indicated. Severe adverse events are extremely rare. Among these, arterial embolization is statistically exceedingly uncommon when the procedure is executed correctly, largely due to the use of large blunt cannulas, low-risk injection planes, controlled product delivery, and continuous cannula movement. In the present study no vascular events or filler migration were reported, even in anatomically vulnerable areas. Prompt recognition and immediate management remain crucial in the unlikely event of vascular compromise, but adherence to proper technique makes such occurrences highly improbable.
Overall, the complication profile of HA gluteal augmentation is favorable, with most effects being mild, self-limited, and preventable through precise anatomical knowledge, appropriate instrument selection, and careful execution.
The implantation technique and the optimal rheological properties of the device brought about a natural and well-defined increase in volume, with a projection of the upper part of the buttocks, a consequent lifting of the lower parts, and a reduction in the length of the infragluteal fold.
The results were more visible 2 weeks after treatment, as shown by the improvement of the average score of the semiquantitative improvement scale. In fact, immediately after the procedure, in all subjects, the appearance of the buttocks was less natural, with evident disharmonious accumulations visible especially in profile. After 14 days, the physiological distribution of the substance provided a more natural appearance and consequent appreciation by the enrolled subjects (Figure 2).
Treatment with cross-linked HA Sofiderm Derm SubSkin® has proven to be an effective technique for improving the upper projection of the buttocks, particularly in patients with flattening of this area. The deep implant technique both under and above the fascia has allowed patients to obtain a natural and lasting projection effect, while the superficial technique has contributed to improving the harmony and definition of the medial portions of the buttocks.
The results obtained were very positive, with good filler stability and no migration of the product. The implantation technique was well tolerated by the patients, with a low risk of complications. The volume of filler implanted was adequate to obtain a visible aesthetic improvement, suggesting the need for periodic touch-ups to maintain the results.
The systematic use of ultrasound would certainly increase the safety of the procedure and methodological homogeneity [17].
Careful selection of patients and adaptation of the amount of filler based on individual characteristics were crucial for the success of the treatment. An accurate pre-procedure evaluation is essential to choose the most suitable technique and ensure natural and safe results.
4. Conclusions
The increase in the projection of the upper part of the buttocks with cross-linked HA Sofiderm Sub Skin® through sub- and supra-fascial implantation is a safe and effective procedure to obtain a natural and long-lasting aesthetic improvement. The results obtained in this pilot study were satisfactory for all patients, without significant side effects. This minimally invasive approach could represent a valid alternative to surgical gluteoplasty, with reduced risk and rapid recovery times. Unfortunately, the extremely small sample (five subjects) limits the universality of the results, and the 2-month follow-up is too short to assess long-term stability and device migration. Further studies with larger samples and longer follow-up could help consolidate the results and optimize the technique.
Author Contributions
G.R., practitioner; G.V. and L.C., wrote Introduction; S.M., F.T. and S.G. wrote Materials and Methods; F.T. and A.D.G., ultrasound interpreters; L.N. and S.G., imaging provider; G.P., wrote Results and Conclusions. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by TECHDERM BIOLOGICAL PRODUCTS Co., Ltd., No. 26, Qiannong 4th Road, Ningwei Street, Xiaoshan District, Hangzhou, Zhejiang Province, 311231, China.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the retrospective nature of the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest. The APC was funded by TECHDERM BIOLOGICAL PRODUCTS Co., Ltd.; The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
References
- Atiyeh, B.; Ghieh, F.; Oneisi, A. Safety and Efficiency of Minimally Invasive Buttock Augmentation: A Review. Aesthetic Plast. Surg. 2023, 47, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Mortada, H.; Alkadi, D.; Saqr, H.; Sultan, F.; Alturaiki, B.; Alrobaiea, S.; Aljaaly, H.A.; Arab, K.; Arkoubi, A.Y. Effectiveness and Role of Using Hyaluronic Acid Injections for Gluteal Augmentation: A Comprehensive Systematic Review of Techniques and Outcomes. Aesthetic Plast. Surg. 2023, 47, 2719–2733. [Google Scholar] [CrossRef] [PubMed]
- Dayal, A.; Bhatia, A.; Hsu, J.T. Fat grafting in aesthetics. Clin. Dermatol. 2022, 40, 35–44. [Google Scholar] [CrossRef] [PubMed]
- De Meyere, B.; Mir-Mir, S.; Peñas, J.; Camenisch, C.C.; Hedén, P. Stabilized hyaluronic acid gel for volume restoration and contouring of the buttocks: 24-month efficacy and safety. Aesthetic Plast. Surg. 2014, 38, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Centeno, R.F.; Sood, A.; Young, V.L. Clinical Anatomy in Aesthetic Gluteal Contouring. Clin. Plast. Surg. 2018, 45, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Santorelli, A.; Cerullo, F.; Salti, G.; Avvedimento, S. Gluteal augmentation with hyaluronic acid filler: A retrospective analysis using the BODY-Q scale. Aesthetic Plast. Surg. 2023, 47, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Robles, M.F. Safety and efficacy study of the application of redensified cross-linked hyaluronic acid for filling gluteal volume and cellulite depressions. Aesthetic Plast. Surg. 2024, 48, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Crabai, P.; Marchetti, F.; Santacatterina, F.; Fontenete, S.; Galera, T. Nonsurgical gluteal volume correction with hyaluronic acid: A retrospective study to assess long-term safety and efficacy. Plast. Reconstr. Surg. Glob. Open 2024, 12, e5792. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, L.M.; de Noronha, M.G.O.; Colla, L.A.; Izzo, T.R.; Sigrist, R.; Braz, A. LL body contour technique—A new way of gluteal contouring and augmentation with hyaluronic acid filler. J. Cosmet. Dermatol. 2022, 21, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Issa, M.C.A.; de Noronha, M.G.O.; Colla, L.A.; Izzo, T.R.; Sigrist, R.; Braz, A. Analysis of morphologic and rheological properties of hyaluronic acid gel fillers to body contouring and its clinical correlation. Gels 2025, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Stocks, D.; Sundaram, H.; Michaels, J.; Durrani, M.J.; Wortzman, M.S.; Nelson, D.B. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J. Drugs Dermatol. 2011, 10, 974–980. [Google Scholar] [PubMed]
- Pazzini, R.; Viana, R.; Petrone, G. Long Term Follow-Up in Gluteal Augmentation Using Cross-Linked Hyaluronic Acid: Up to 20 Months Ultrasound Follow-Up. Cosmetics 2024, 11, 194. [Google Scholar] [CrossRef]
- Flack, N.A.; Nicholson, H.D.; Woodley, S.J. A review of the anatomy of the hip abductor muscles, gluteus medius, gluteus minimus, and tensor fascia lata. Clin. Anat. 2012, 25, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Rohrich, R.J.; Bartlett, E.L.; Dayan, E. Practical approach and safety of hyaluronic acid fillers. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2172. [Google Scholar] [CrossRef] [PubMed]
- Iribarren-Moreno, R.; Pradel-Mora, J.J.; Marin, G.; Garza-Cerna, J.A. Hyaluronic Acid Gluteal Augmentation: New Marking and Application Technique. Plast. Reconstr. Surg. Glob. Open 2025, 13, e7252. [Google Scholar] [CrossRef] [PubMed]
- de la Peña, J.A. Subfascial technique for gluteal augmentation. Aesthet. Surg. J. 2004, 24, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Argalia, G.; Reginelli, A.; Molinelli, E.; Russo, A.; Michelucci, A.; Sechi, A.; Marzano, A.V.; Desyatnikova, S.; Fogante, M.; Patanè, V.; et al. High-Frequency and Ultra-High-Frequency Ultrasound in Dermatologic Diseases and Aesthetic Medicine. Medicina 2025, 61, 220. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.