Abstract
Background: Metastatic tumors of the oro-facial tissuesare rare, with an incidence ranging between 1% and 8% of all oral malignant tumors. Generally reported with a peak of incidence in the 5–7th decades but possibly occurring at any age, metastases may represent the first sign of an occult cancer or manifest in patients with an already known history of a primary carcinoma, mostly from the lungs, kidney, prostate, and colon/rectum in males, and the uterus, breast, lung, and ovary in females. In the oro-facial tissues, the most involved sites are the oral mucosa, gingiva/jawbones, tongue, and salivary glands. Methods: A broad and deep literature review with a comprehensive analysis of the existing research on oro-facial metastases from renal-cell carcinoma (RCC) was conducted by searching the most used databases, with attention also paid to the clear-cell histological variant, which is the most frequent one. Results: Among the 156 analyzed studies, 206 cases of oro-facial metastases of renal cancer were found in patients with an average age of 60.9 years (145 males, 70.3%; 61 females, 29.6%). In almost 40% of the cases, metastasis represented the first clinical manifestation of the primary tumor, and 122 were histologically diagnosed as clear-cell renal-cell carcinoma (ccRCC) (59.2%). The tongue was involved in most of the cases (55 cases, 26.7%), followed by the gingiva (39 cases, 18.9%), mandible (35 cases, 16.9%), maxilla (23 cases, 11.1%), parotid gland (22 cases, 10.6%), buccal mucosa (11 cases, 5.3%), lips (7 cases, 3.3%), hard palate (6 cases, 2.8%), soft palate, masticatory space, and submandibular gland (2 cases, 0.9%), and lymph nodes, tonsils, and floor of the mouth (1 case, 0.4%). Among the 122 ccRCCs (84 males, 68.8%; 38 females, 31.1%), with an average age of 60.8 years and representing in 33.6% the first clinical manifestation, the tongue remained the most frequent site (31 cases, 25.4%), followed by the gingiva (21 cases, 17.2%), parotid gland (16 cases, 13.1%), mandibular bone (15 cases, 12.2%), maxillary bone (14 cases, 11.4%), buccal mucosa and lips (6 cases, 4.9%), hard palate (5 cases, 4%), submandibular gland and soft palate (2 cases, 1.6%), and lymph nodes, tonsils, oral floor, and masticatory space (1 case, 0.8%). The clinical presentation in soft tissues was mainly represented by a fast-growing exophytic mass, sometimes accompanied by pain, while in bone, it generally presented as radiolucent lesions with ill-defined borders and cortical erosion. Conclusions: The current comprehensive review collected data from the literature about the incidence, site of occurrence, age, sex, and survival of patients affected by oro-facial metastases from renal-cell carcinoma, with particular attention paid to the cases diagnosed as metastases from clear-cell renal-cell carcinoma, which is the most frequent histological variant. Clinical differential diagnosis is widely discussed to provide clinicians with all the useful information for an early diagnosis despite the effective difficulties in recognizing such rare and easily misdiagnosed lesionsTheir early identification represents a diagnostic challenge, especially when the clinical work-up is limited to the cervico–facial region. Nevertheless, early diagnosis and recently introduced adjuvant therapies may represent the key to better outcomes in such patients. Therefore, general guidelines about the clinical and radiological identification of oro-facial potentially malignant lesions should be part of the cultural background of any dentist.
1. Introduction
The metastatic dissemination of solid tumors may involve the head and neck, including the oral cavity. However, this occurs infrequently, with an incidence rate between 1% and 8% of all oral malignant tumors [1,2,3,4], which peaks in the 5–7th decades [2]. Excluding the malignant tumors of childhood, oro-facial metastases (OFMs) may be the first sign of an occult or still undiagnosed cancer or may manifest during the clinical follow-up of a patient with an already diagnosed primary carcinoma [5,6,7]. Metastases to the oro-facial tissues can involve soft or hard tissues or both synchronously, including the oral mucosa, jawbones, salivary glands, and neck lymph nodes. The most frequent primary localizations, according to the overall incidence rates among the general population, are represented by the lung, kidney, prostate, and colon/rectum in males and the uterus, breast, lung, and ovary in females [1,2,8,9].
It is widely acknowledged that, regardless of the most frequent tissue target of the primary tumor (e.g., bone in the case of prostate cancer), metastatic diffusion also exhibits a preference for certain specific sites in the oro-facial region. In addition, very frequent particular clinical conditions, such as gingival–periodontal tissue inflammation, the presence of removable prosthesis in edentulous individuals, or gingival and alveolar bone remodeling after recent tooth removal, may also have an impact. In fact, in these cases, it has been hypothesized that the reorganization of the area of blood flow caused by inflammation, pressure, or damage from the prosthesis will promote the onset of the disease [10]. Because of their high bone marrow concentration and abundant vascularization, the jawbones, particularly the molar and premolar areas, are frequently affected. Furthermore, metastases may occur in the remaining alveoli after tooth extraction (post-extraction sites), most likely as a result of increased blood flow associated with blood clot development [1,2,8,10].
Renal-cell carcinoma (RCC) represents over 90% of all kidney malignancies in the adult population, making it the most prevalent kind of kidney cancer. It usually affects men and manifests itself around the age of 60 [11,12,13,14,15,16,17]. A number of risk factors have been suggested to favor the development of RCC. These include an elevated body mass index [18], urinary stones in men [19], type 2 diabetes in women [20], chronic liver and kidney illnesses [21], and long-term use of analgesics [22], in addition to environmental variables [23,24]. Patients with localized renal illness treated with nephrectomy often experience recurrence in around 25% of instances, and one third of patients develop locoregional or distant metastases. RCC usually metastasizes to the liver, brain, lungs, regional lymph nodes, and bones [25]. There are very few descriptions of localization to the oro-facial tissue in the literature. RCC metastases are very uncommon in this area, primarily affecting the tongue, gingiva, and maxillary bones in that order [26,27].
There are several histologic subtypes of RCC. The most common types, comprising 90% of cases, are chromophobe RCC (chRCC) (5%) and papillary RCC (pRCC) (10 to 15%); clear-cell RCC (ccRCC) accounts for 70% of cases [28,29]. Though unusual metastatic sites or late metachronous metastases (>10 years) have been reported, and distant metastasis may be the tumor’s initial clinical manifestation, ccRCC has a known propensity to metastasize most frequently via direct invasion of the renal veins and vena cava, followed by hematogenous dissemination to the lungs [30,31].
A true diagnostic conundrum for clinicians and pathologists (primarily because of the rarity of early diagnosis) is presented by the occasional report of metastatic ccRCC to the OFTs [1,2,28,32]. In fact, because of their high glycogen and lipid content, the tumor cells of ccRCC exhibit clear cytoplasmic vacuolization and clearing, mimicking other neoplasms of odontogenic or salivary gland origin that more frequently affect this area [2,28,29,33,34,35,36]. As such, the oral localization of an undetected ccRCC may undoubtedly pose a diagnostic problem, particularly if the cervicofacial region is still the exclusive focus of the clinical work-up [6,7,37,38,39,40]. The present study was proposed to systematically review case reports and case series of RCC’s metastasis to the OFTs. Our primary aim was to perform a comprehensive review of all published cases of ccRCC metastases according to the PRISMA guidelines for systematic review (Figure 1).
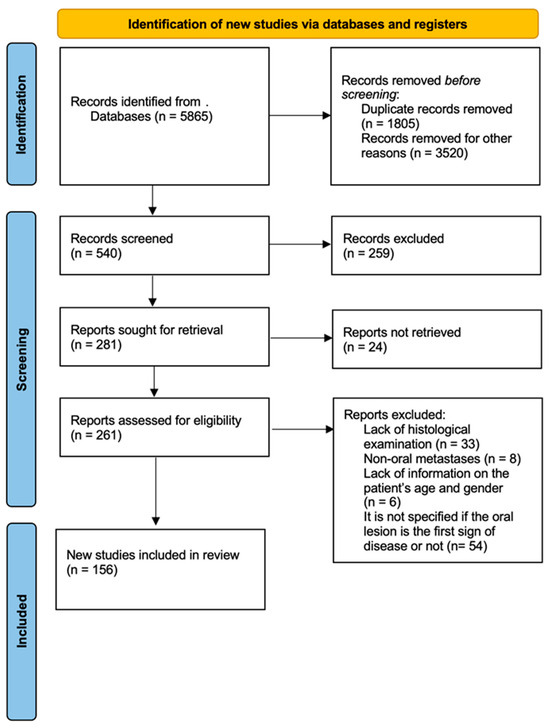
Figure 1.
The PRISMA flow chart for reporting systematic reviews.
2. Materials and Methods
A comprehensive review of the literature was conducted according to the PRISMA guidelines for systematic reviews, with the intention of providing an overview of the available evidence in reliable databases. The terms “renal metastasis” or “renal metastases” or “clear-cell renal-cell carcinoma” AND “oral” or “Head and Neck” were alternatively used in the search, restricting their presence to the titles of the articles in PubMed, Scopus, Web of Sciences, Google Scholar, and Embase databases, in the period from September 2023 to January 2024. The search was limited to only studies on humans. All kinds of papers were collected, including case reports, case series, reviews of the literature, and systematic reviews of the literature. After applying the keywords to the databases, a total of 5865 results were obtained. Of these, only 156 articles were chosen for inclusion in the present review. The others were removed because the lesions were not confirmed as metastases at the anatomopathological examination, because the metastases were outside the limits of the head and neck, because the genders and ages of the patients were not specified, or because it was not proven whether the oral manifestation was the first sign of disease or not. The reading, selection, and analysis of the articles included in this review were performed by four reviewers (VG, AdA, SC, and MF).
3. Results
In the 156 analyzed studies, 206 cases of oral metastases of renal cancer were found, of which 122 were histologically demonstrated to be ccRCCs (59.2%) at the final diagnosis. The tongue was involved by renal metastases in most of the cases (55 cases, 26.7%), followed by the gingiva (39 cases, 18.9%), mandibular bone (35 cases, 16.9%), maxillary bone (23 cases, 11.1%), parotid gland (22 cases, 10.6%), buccal mucosa (11 cases, 5.3%), lips (7 cases, 3.3%), hard palate (6 cases, 2.8%), soft palate, masticatory space, and submandibular gland (2 cases, 0.9%), and lymph nodes, tonsils, and oral floor (1 case, 0.4%). Of the 206 total cases, 145 were males (70.3%) and 61 were females (29.6%). The average age was 60.9 years. The average male age was 62.2, and the average female age was 57.8. In almost 40% of cases, the development of oral metastasis represented the first clinical manifestation of the primary tumor, which was previously unknown. Data were globally collected and are presented in Table 1, listing the author(s) names, the year of publication, site/sites, histological histotype, sex, age, and occurrence as the first sign of metastatic disease or not, while clinical data in Table 2.

Table 1.
Full list of the selected articles regarding metastases of renal-cell carcinoma to the oral cavity. For each article, the first author, year of publication, site, histological type, gender, age, and eventual presence of the first sign of diseases are described. RCC = renal-cell carcinoma. ccRCC: clear-cell renal-cell carcinoma. pRCC: papillary renal-cell carcinoma.

Table 2.
Data analysis of oro-facial metastases of renal-cell carcinoma. For each site, the total number and percentage of cases are described.
Focusing on the numbers of the most frequent histotype (ccRCC), we found that the tongue was involved in most cases (31 cases, 25.4%), followed by the gingiva (21 cases, 17.2%), parotid gland (16 cases, 13.1%), mandibular bone (15 cases, 12.2%), maxillary bone (14 cases, 11.4%), buccal mucosa and lips (6 cases, 4.9%), hard palate (5 cases, 4%), submandibular gland and soft palate (2 cases, 1.6%) and lymph nodes, tonsils, oral floor, and masticatory space (1 case, 0.8%). It is clear that soft tissues are more affected by ccRCC metastases than hard tissues. Of the 122 total cases, 84 were male (68.8%) and 38 were female (31.1%). The average age was 60.8 years. The average male age was 61.7, and the average female age was 59. In almost 33.6% of cases, the development of oral metastasis was the first clinical manifestation of the primary tumor. Clinical presentations varied depending on the affected tissue: at the level of soft tissues, metastases frequently presented as fast-growing and exophytic masses, accompanied or not by pain; bone metastases radiologically appeared as radiolucent lesions, with ill-defined borders and cortical erosion. In addition, some of them also expanded into the adjacent soft tissues, thus causing submucosal swelling on the gingiva (Table 3).

Table 3.
Metastases of clear-cell renal-cell carcinoma to the oro-facial tissues and clinical radiological presentation. For each article, site, epidemiological features, clinical presentation, and radiological aspects are described.
4. Discussion
4.1. General Considerations
Metastatic tumors from distant organs and tissues to the oro-facial tissues are not encountered frequently. According to the literature, metastatic tumors comprise about only 1% of all oro-facial malignancies [114,155]. Renal-cell carcinoma (RCC) is the most common form of kidney malignancy, accounting for more than 90% of all renal malignancies in the adult population [11]. Distant metastases from RCC are very common and usually multiple to different organs, with a decreasing incidence, respectively, to the lungs (50–60%), bones and liver (30–40%), and head and neck (12–16%) [1,2]. Among the latter, 50% of the metastases were detected in the thyroid, nose, and paranasal sinuses and pharynx [28,33,193]. According to the recent review by Kase AM et al. [194], the statistical data show a five-year survival rate of 70% for patients with regional disease, which drastically decreases to 13% for those showing distant metastases. Such data highlight the importance of the early detection of metastatic lesions, which can be difficult in the absence of signs and/or symptoms of the whole organism. This excludes the oro-facial tissue, the diagnosis of which, conversely, is relatively accessible due to the ease of clinical exploration and/or the frequent use of dental panoramic radiogram and/or CT for dental therapies over one’s lifetime, at least in occidental countries.
Generally, in the oral cavity, large and/or rapidly growing swellings in the tongue and periodontal tissue, as well unpredictable tooth mobility or gingiva-periodontal inflammation (including the peri-implant hard and soft tissues), surely represent clinical signs of possible malignancy (and consequently also of metastatic diffusion), when the most common lesions of benign nature (mainly odontogenic abscess, periodontal or perimplant abscess) have been excluded. The early detection by well-addressed general dentists and their radiological evaluation always need anatomopathological confirmation by hard or soft biopsy, often supported by immunohistochemistry too, which represents the true key for the early and differential diagnosis [195].
Targeted therapies in renal-cell cancer have significantly advanced in recent years, offering more precise and effective treatment options for patients. These therapies often target specific molecules or pathways that play a crucial role in the growth and spread of cancer cells. For example, vascular endothelial growth factor (VEGF) inhibitors and mammalian target of rapamycin (mTOR) inhibitors are commonly used targeted therapies for renal-cell cancer [196,197]. Current treatments are mostly immunotherapy combinations with anti-VEGFR (vascular endothelial growth factor receptor) tyrosine kinase inhibitors (TKIs) [198]. By inhibiting these key pathways, targeted therapies can help slow down disease progression and improve patient outcomes. However, it is important to note that not all patients may respond to these therapies, and resistance can develop over time. Ongoing research efforts are focused on developing novel targeted therapies and combination approaches to overcome resistance mechanisms and improve treatment efficacy for patients with renal-cell cancer.
Therefore, along with the targeted therapies that significantly positively impact both the treatment and prognosis of metastatic RCC patients, early diagnosis certainly plays a key role too, as also reported by The International Metastatic Renal-Cell Carcinoma Database Consortium risk model, which lists it among the risk factors (diagnosis to systemic therapy < 1 year), together with a Karnofsky performance status <80%, corrected calcium > normal, hemoglobin < normal, neutrophil > normal, and platelet count > normal, which may globally help to prognosticate survival in such patients. To date, this model has indicated a median OS of 43.2 months in the group with 0 risk factors, 22.5 months in the group with 1–2 risk factors, and 7.8 months in the risk group exhibiting 3 or more risk factors [199].
This model continues to be used widely today in clinical practice and as a predictive tool for responses to new combinations of immunotherapies. The VEGFR axis has proven to be a key therapeutic target in metastatic RCC, leading to improved outcomes in these risk categories. As translational work has advanced, it has been demonstrated that RCC has a unique immunogenicity that could forever change the treatment landscape
RCC is the third most common malignancy to metastasize to the head and neck region, after lung and breast carcinomas. Oro-facial metastasis is the presenting complaint in 7.5% of patients with RCC [50]. Distant metastases to the oro-facial tissues may involve the jaws, especially the mandible, or the soft tissues, mostly the gingiva and, frequently, the tongue, with a prevalence of 26%, as shown in the current review (Figure 2).
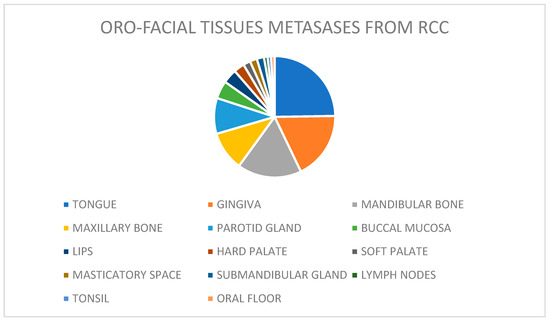
Figure 2.
The prevalence of lesions by site of involvement.
4.2. Diagnostic Challenges and Clinical Work-Up
The correct diagnosis of metastatic lesions of the oral cavity represents a challenge for clinicians, especially when the patient has no history of malignant diseases. This literature review shows that in 36.4% of cases (77 of 211 patients affected by oral metastases), the development of an oral metastasis is the first clinical manifestation of a primary tumor.
Gingival lesions are more complex to diagnose because of the presence of several benign conditions that may be potentially included among the differential diagnoses (e.g., pyogenic granuloma, peripheral giant cell granuloma, ossifying fibroma, and fibrous hyperplasia), thus frequently leading to a diagnostic delay. However, clinical signs, such as rapid enlargement or invasion of the underlying bone, may support the diagnosis by excluding an inflammatory origin of the lesion [27]. Among the reactive lesions of the gingiva, fibrous hyperplasia is certainly very common, accounting for up to 40% of the mucosal pathologies in a large case series reported and occurring in a wide age range [200].
Additionally, vascular epulis, also called pyogenic granuloma, is a frequently occurring gingival lesion, usually presenting as soft, bright red swelling, with focal ulceration providing a grey/yellow appearance. It is usually related to trauma or chronic irritation and alterations in sex hormone levels (e.g., puberty, pregnancy, use of oral contraceptive drugs, or hormone replacement therapy). Its clinical presentation, along with easily provoked bleeding after trauma, broadens the spectrum of differential diagnosis, including malignant lesions (such as metastasis) and systemic causes of vascular expansion of the gingiva, such as leukemia and granulomatosis with polyangiitis.
The most common peripheral odontogenic tumors most frequently involve the gingiva, peripheral odontogenic fibroma, and peripheral ameloblastoma (PA). Their occurrence in young adults, slow growth, and clinical presentation (mostly as gingival swelling with intact overlying mucosa) represent important criteria for their differential diagnosis of malignancy. Some suspicion may arise with peripheral ameloblastoma, which can have a variable clinical presentation, showing a granular or erythematous surface [201].
Among malignancies with gingival localization, verrucous carcinoma is the most frequent, and its clinical presentation, usually as white plaque or verrucous lesions, helps clinicians diagnose it. Nevertheless, the occurrence of the most aggressive squamous cell carcinoma in the periodontal tissue should also be considered when occurring with a granular or erythematous appearance, often associated with periodontal and bone invasion and related clinical (bleeding, teeth mobility, and pain) and radiological signs (enlargement of the periodontal space and radio-transparencies). Additionally, the AIDS-related type of Kaposi’s sarcoma generally shows gingival manifestation with a reddish appearance (thus mimicking hemangioma, pyogenic granuloma, and giant cell epulis, especially when nodular in appearance) and ulcerated when larger, leading to a differential diagnosis that obviously includes other malignancies. Lastly, the head and neck are the second most common extranodal sites for lymphoma occurrence (11–33%), especially diffuse large B-cell non-Hodgkin lymphoma, with the most common sites affected being the gingiva, mandible, palate, maxilla, and tongue [202,203].
Additionally, the gingiva is frequently affected in patients with acute myeloid leukemia [204]. Although lymphoma and leukemia have nonspecific clinical presentation in the periodontal tissue, they often present with swelling and reddening of the gingival tissues (mimicking gingivitis, periodontitis of different stages, and hyperplastic gingivitis of different etiology when generalized, and pyogenic granuloma or giant cell epulis when swelling is localized), while advanced cases may show signs of malignancy as accompanied by alveolar bone loss and tooth mobility. In such cases, patients frequently have a well-recognized history of generalized/systemic disease, but when still undiagnosed, they represent a challenging situation for clinicians, with the differential diagnoses likely to include several non-neoplastic and neoplastic conditions depending on the extent of the disease at presentation.
The intraosseous presentation of metastasis in the jaw is extremely variable, and consequently, the early diagnosis of jawbone metastasis is the first sign of widespread neoplastic disease and is more difficult than its counterpart in soft tissues. Its frequent association with decayed or unvital teeth or residual root fragments, periodontitis, peri-implant inflammatory conditions, its possible periapical localization, the nonspecificity of its clinical symptoms (pain, anesthesia, paresthesia, swelling, teeth mobility, gingival bleeding, etc.) and the highly variable combination of them, and the nonspecificity of its radiological signs (usually appearing as a radiolucent area with ill-defined borders, but also as a radiopaque or mixed radiopaque–radiolucent lesion mostly when of prostatic origin) make the spectrum of potential differential diagnoses extremely wide [205].
Metastasis occurrence in the major salivary glands, especially the parotid gland (as the most frequent site of inflammatory and neoplastic salivary gland lesions), is also a true diagnostic dilemma, mainly because most patients manifest the metastasis first and undergo parotid surgery before the primary tumor diagnosis and staging. A further complication is the constant increase in its overall incidence, along with its nonspecific characteristics in radiological examination, generally MRI and US [39].
Metastatic disease of the tongue is likely the most challenging situation to diagnose differently, first due to its general rarity reported in the literature, but mainly for the variability in its clinical presentation. It typically remains asymptomatic but alternatively can present as painful hard masses with or without superficial ulceration due to biting trauma. Therefore, its differential diagnosis is very challenging, and histological examination of sample tissue should be performed quickly to define the tumor and its origin. Tongue lesions also frequently require treatment as they may interfere with vital function (swallowing, biting, breathing, or drinking). Treatment generally comprises total or partial surgical excision combined with adjuvant radiotherapy for local and general disease control [184]. It is worth noting the data on ccRCC occurrence in the tongue among the patients listed in the current review, as its incidence was 31 cases (25.4%).
4.3. Pathological Differential Diagnosis and Imaging
Clinical suspicion always needs to be supported by histology and immunohistochemistry to discriminate renal metastases from other lesions characterized by the histologic presence of clear cells. When occurring in major salivary glands, the differential diagnoses of clear-cell neoplasms include mucoepidermoid carcinoma (MEC) and other salivary gland tumors, such as epithelial–myoepithelial carcinoma, oncocytomas, hyalinizing clear-cell carcinoma (HCCC), and acinic cell carcinoma (ACC). All these tumors may display a clear-cell component [28,29,35]. The differential diagnoses of jawbone metastases include some histological types of odontogenic tumors, which may also display clear cells, such as clear-cell ameloblastoma (CCA), calcifying epithelial odontogenic tumor (CEOT), and clear-cell odontogenic carcinoma (CCOC) [8,10,29]. In particular, immunohistochemistry is extremely important to perform differential diagnoses with clear-cell salivary and odontogenic neoplasms [142]. ccRCC consistently expresses positivity for CD10, cytokeratins AE1/AE3, epithelial membrane antigen (EMA), PAX-8, renal-cell carcinoma antigen (RCCAg), and vimentin. Conversely, ccRCC does not express cytokeratin 7, calretinin, CD117, muscle markers (smooth muscle actin, calponin, and myosin), or glial fibrillary acidic protein (GFAP), usually expressed in salivary gland tumors. Regarding odontogenic tumors, cytokeratins AE1/AE3, cytokeratin 7, and EMA are observable in odontogenic carcinoma, while cytokeratins AE1/AE3 and calretinin are observable in ameloblastoma. However, CD10, PAX8, and RCCAg are consistently negative in all salivary glands, and odontogenic tumors show clear-cell features, allowing for certain differential diagnoses with ccRCC metastasis [184]. Furthermore, clear-cell sarcoma of the kidney may be easily ruled out using immunohistochemistry because it is negative for cytokeratins, EMA, and CD10. Consequently, it is important to highlight that the final diagnosis, together with the exclusion of all the possible differential diagnoses, is only made with certainty after histopathological examination. Hence, biopsy is always mandatory, and the role of the anatomical pathologist is vital in the clinical work-up of patients with oral metastases from ccRCC.
Radiology also plays a fundament role in the diagnosis and characterization of renal masses in the early and pretreatment identification of the most frequent histologic subtypes and the staging of metastatic RCCs. CT and MRI are conventionally used as the first choices in RCC characterization and staging, with the latter having the benefits of no radiation exposure and accuracy in the definition of cystic lesions
As recently reported by Bellin et al. in a 2024 update, remarkable advances in the imaging technology of RCC have been recently introduced, including dual-energy CT, photon-counting detector CT, radiomics, and high-resolution multiparametric MRI [206].
An overall aim is to continuously improve diagnostic performance (the detection of tumors at an earlier stage) both in the preoperative assessment of histologic subtypes and the differential diagnoses among malignant and benign lesions, also with a potential reduction in contrast use and radiation exposure. The use of artificial intelligence in the classification, grade, and prognosis of RCC has also shown encouraging results, leading to accurate detection and diagnosis in a reduced time and help in treatment management.
4.4. Summary of Clinico-Epidemiologic Aspects
From the literature over the past 100 years, we identified that the age at diagnosis ranges from 18 to 89 years. Metastases are more common in men than women (145 versus 61 cases, respectively), mirroring the male predominance of RCC more generally. The majority of RCCs are the clear-cell type. Oral metastases from renal-cell carcinoma involve the soft tissues and jawbones almost equally. The most affected sites are the tongue and gingiva (Figure 1, Table 2). A mass or nodule is the most common clinical manifestation, while pain is the most prevalent symptom. In cases where the bone was affected by metastasis, a radiolucent image was the most reported. Any mass present in the oral cavity should be biopsied and analyzed carefully, as metastatic lesions may resemble clinically benign lesions.
5. Conclusions and Future Directions
The current review of the literature confirms the well-recognized data on the low incidence of metastases in the oro-facial tissues and that their occurrence is mostly related to an advanced stage of disease. We found that in almost 40% of cases, metastases to the oro-facial tissues represented the first clinical manifestation of a still unknown clear-cell renal-cell carcinoma. These data are higher than the overall general incidence for all metastases to the head and neck presenting as the first manifestation of an occult malignancy, generally accounting for about 20–35%. Hence, these tumors seem to predilect oro-facial tissues more than others. Moreover, metastases to the head and neck from clear-cell renal-cell carcinoma can occur at any age, and the prognosis is generally poor.
All collected data highlight the importance of early diagnosis, especially for metastases from clear-cell renal-cell carcinoma in the absence of an already known primary tumor (metastases as the first sign of disease), despite the evident difficulties of their identification both via clinical examination and via conventional (first-grade) radiological investigations. Early clinical identification, with consequential histological definition and TNM staging, along with targeted therapies, may be vital to guarantee better outcomes for patients presenting with metastatic clear-cell renal-cell carcinoma.
Regarding future directions for research on oral metastases from renal-cell carcinoma, further investigation into the underlying mechanisms of metastasis development and progression is crucial. Understanding the specific molecular pathways involved could provide insights into potential targeted therapies. However, it is important to acknowledge the limitations of the current study, including the small sample sizes and lack of long-term follow-up data. To translate these findings into clinical settings, the next step would involve conducting larger-scale clinical trials to validate the effectiveness and safety of any potential treatments identified through research on oral metastases from renal-cell carcinoma. Collaborations among researchers, clinicians, and industry partners will be key to moving toward implementing tailored therapeutic strategies for patients in a clinical setting.
Author Contributions
Conceptualization, S.C.; methodology, V.G. and A.L.; validation, C.C. and G.F.; resources, M.F., D.D.V. and G.I.; data curation, V.G., A.d. and A.M.; writing—original draft preparation, S.C. and V.G.; writing—review and editing, S.C. and M.F.; supervision, S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data collected in the current study were downloaded from the following databases: PubMed, Scopus, Web of Sciences, and Google Scholar.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hirshberg, A.; Buchner, A. Metastatic tumours to the oral region. An overview. Eur. J. Cancer Part B Oral Oncol. 1995, 31, 355–360. [Google Scholar] [CrossRef]
- Hirshberg, A.; Berger, R.; Allon, I.; Kaplan, I. Metastatic Tumors to the Jaws and Mouth. Head Neck Pathol. 2014, 8, 463–474. [Google Scholar] [CrossRef] [PubMed]
- McClure, S.A.; Movahed, R.; Salama, A.; Ord, R.A. Maxillofacial Metastases: A Retrospective Review of One Institution’s 15-Year Experience. J. Oral Maxillofac. Surg. 2013, 71, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.-L.; Kang, J.; Wen, Y.-L.; Ying, W.-M.; Yi, J.; Hua, C.-G.; Tang, X.-F.; Wen, Y.-M. Metastatic Tumors to the Oral and Maxillofacial Region: A Retrospective Study of 19 Cases in West China and Review of the Chinese and English Literature. J. Oral Maxillofac. Surg. 2009, 67, 718–737. [Google Scholar] [CrossRef] [PubMed]
- Pastremoli, A. Gingival metastasis, the first clinical sign of a silent kidney carcinoma. A case report. Minerva Stomatol. 1991, 40, 825–828. [Google Scholar]
- Raiss, H.; Duplomb, S.; Tartas, S.; Layachi, M.; Errihani, H. Lingual metastasis as an initial presentation of renal cell carcinoma: A case report. J. Med. Case Rep. 2017, 11, 314. [Google Scholar] [CrossRef]
- Vallalta Morales, M.; Todolí Parra, J.; Cervera Miguel, J.I.; Calabuig Alborch, J.R. Hemiparesia derecha como forma de presentación de carcinoma renal de células claras. Ann. Intern. Med. 2004, 21, 359–360. [Google Scholar] [CrossRef]
- Hirshberg, A.; Leibovich, P.; Buchner, A. Metastatic tumors to the jawbones: Analysis of 390 cases. J. Oral Pathol. Med. 1994, 23, 337–341. [Google Scholar] [CrossRef]
- Hirshberg, A.; Leibovich, P.; Buchner, A. Metastases to the oral mucosa: Analysis of 157 cases. J. Oral Pathol. Med. 1993, 22, 385–390. [Google Scholar] [CrossRef]
- Hirshberg, A.; Leibovich, P.; Horowitz, I.; Buchner, A. Metastatic tumors to postextraction sites. J. Oral Maxillofac. Surg. 1993, 51, 1334–1337. [Google Scholar] [CrossRef]
- Ljungberg, B.; Campbell, S.C.; Cho, H.Y.; Jacqmin, D.; Lee, J.E.; Weikert, S.; Kiemeney, L.A. The Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2011, 60, 615–621. [Google Scholar] [CrossRef]
- Unverzagt, S.; Moldenhauer, I.; Nothacker, M.; Roßmeißl, D.; Hadjinicolaou, A.V.; Peinemann, F.; Greco, F.; Seliger, B. Immunotherapy for metastatic renal cell carcinoma. Cochrane Urology Group, curatore. Cochrane Database Syst. Rev. 2017, CD011673. [Google Scholar] [CrossRef]
- Zerdes, I.; Tolia, M.; Tsoukalas, N.; Mitsis, M.; Kardamakis, D.; Pistevou-Gombaki, K.; Tsekeris, P.; Kyrgias, G. Systemic therapy of metastatic renal cell carcinoma: Review of the current literature. Urol. J. 2019, 86, 3–8. [Google Scholar] [CrossRef]
- Nazha, S.; Tanguay, S.; Kapoor, A.; Jewett, M.; Kollmannsberger, C.; Wood, L.; Bjarnason, G.; Heng, D.; Soulières, D.; Reaume, N.; et al. Use of Targeted Therapy in Patients with Metastatic Renal Cell Carcinoma: Clinical and Economic Impact in a Canadian Real-Life Setting. Curr. Oncol. 2018, 25, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Goebell, P.J.; Staehler, M.; Müller, L.; Nusch, A.; Scheffler, M.; Sauer, A.; Von Verschuer, U.; Tech, S.; Kruggel, L.; Jänicke, M.; et al. Changes in Treatment Reality and Survival of Patients with Advanced Clear Cell Renal Cell Carcinoma—Analyses from the German Clinical RCC-Registry. Clin. Genitourin. Cancer 2018, 16, e1101–e1115. [Google Scholar] [CrossRef] [PubMed]
- De Groot, S.; Redekop, W.K.; Versteegh, M.M.; Sleijfer, S.; Oosterwijk, E.; Kiemeney, L.A.L.M.; Uyl-de Groot, C.A. Health-related quality of life and its determinants in patients with metastatic renal cell carcinoma. Qual. Life Res. 2018, 27, 115–124. [Google Scholar] [CrossRef]
- Atkins, M.B.; Tannir, N.M. Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev. 2018, 70, 127–137. [Google Scholar] [CrossRef]
- Macleod, L.C.; Hotaling, J.M.; Wright, J.L.; Davenport, M.T.; Gore, J.L.; Harper, J.; White, E. Risk Factors for Renal Cell Carcinoma in the VITAL Study. J. Urol. 2013, 190, 1657–1661. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; O’Corragain, O.A.; Edmonds, P.J.; Ungprasert, P.; Kittanamongkolchai, W.; Erickson, S.B. The risk of kidney cancer in patients with kidney stones: A systematic review and meta-analysis. QJM Int. J. Med. 2015, 108, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Joh, H.K.; Willett, W.C.; Cho, E. Type 2 Diabetes and the Risk of Renal Cell Cancer in Women. Diabetes Care 2011, 34, 1552–1556. [Google Scholar] [CrossRef]
- Christensson, A.; Savage, C.; Sjoberg, D.D.; Cronin, A.M.; Frank O’Brien, M.; Lowrance, W.; Nilsson, P.M.; Vickers, A.J.; Russo, P.; Lilja, H. Association of cancer with moderately impaired renal function at baseline in a large, representative, population-based cohort followed for up to 30 years: Cancer. Int. J. Cancer 2013, 133, 1452–1458. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Je, Y.; Cho, E. Analgesic use and the risk of kidney cancer: A meta-analysis of epidemiologic studies. Int. J. Cancer 2014, 134, 384–396. [Google Scholar] [CrossRef]
- Lambe, M.; Lindblad, P.; Wuu, J.; Remler, R.; Hsieh, C.-C. Pregnancy and risk of renal cell cancer: A population-based study in Sweden. Br. J. Cancer 2002, 86, 1425–1429. [Google Scholar] [CrossRef]
- Kabat, G.C.; Silvera, S.A.N.; Miller, A.B.; Rohan, T.E. A cohort study of reproductive and hormonal factors and renal cell cancer risk in women. Br. J. Cancer 2007, 96, 845–849. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.R.; Kroeger, N.; Xie, W.; Lee, J.L.; Knox, J.J.; Bjarnason, G.A.; MacKenzie, M.J.; Wood, L.; Srinivas, S.; Vaishampayan, U.N.; et al. Impact of Bone and Liver Metastases on Patients with Renal Cell Carcinoma Treated with Targeted Therapy. Eur. Urol. 2014, 65, 577–584. [Google Scholar] [CrossRef]
- Ðanić, P.; Ðanić, D.; Macan, D. Tongue metastasis as an initial presentation of renal cell carcinoma. Med. Glas. 2018, 15, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Makos, C.P.; Psomaderis, K. A literature review in renal carcinoma metastasis to the oral mucosa and a new report of an epulis-like metastasis. J. Oral Maxillofac. Surg. 2009, 67, 653–660. [Google Scholar] [CrossRef]
- Pires, F.R.; Azevedo, R.S.; Ficarra, G.; Cardoso, A.S.; Carlos, R.; Kowalski, L.P.; de Almeida, O.P. Metastatic renal cell carcinoma to the oral cavity and clear cell mucoepidermoid carcinoma: Comparative clinicopathologic and immunohistochemical study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, e22–e27. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, E.; Altini, M.; Favia, G. Clear cell tumors of the salivary glands, jaws, and oral mucosa. Semin. Diagn. Pathol. 1997, 14, 203–212. [Google Scholar]
- Lopez-Beltran, A.; Carrasco, J.C.; Cheng, L.; Scarpelli, M.; Kirkali, Z.; Montironi, R. 2009 update on the classification of renal epithelial tumors in adults. Int. J. Urol. 2009, 16, 432–443. [Google Scholar] [CrossRef]
- Eble, J.N.; Sauter, G.; Epstein, L.I.; Sesterhenn, I.A. World Health Organization Classification of Tumours. In Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs; ARC Press: Lyon, France, 2004. [Google Scholar]
- Gobbo, S.; Eble, J.N.; Grignon, D.J.; Martignoni, G.; MacLennan, G.T.; Shah, R.B.; Zhang, S.; Brunelli, M.; Cheng, L. Clear Cell Papillary Renal Cell Carcinoma: A Distinct Histopathologic and Molecular Genetic Entity. Am. J. Surg. Pathol. 2008, 32, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Sangoi, A.R.; Fujiwara, M.; West, R.B.; Montgomery, K.D.; Bonventre, J.V.; Higgins, J.P.; Rouse, R.V.; Gokden, N.; McKenney, J.K. Immunohistochemical Distinction of Primary Adrenal Cortical Lesions From Metastatic Clear Cell Renal Cell Carcinoma: A Study of 248 Cases. Am. J. Surg. Pathol. 2011, 35, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Griffin, N.; Gore, M.E.; Sohaib, S.A. Imaging in Metastatic Renal Cell Carcinoma. Am. J. Roentgenol. 2007, 189, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Corsi, A.; Guerra, F.; Grippaudo, G.; Bosman, C. Oral metastasis of renal cell carcinoma. Report of case and critical evaluation of morphologic features for differential diagnosis. Pathologica 1994, 86, 665–669. [Google Scholar] [PubMed]
- Kumamoto, H.; Yamazaki, S.; Sato, A.; Yamaguchi, T.; Tezuka, F.; Ooya, K. Clear cell odontogenic tumor in the mandible: Report of a case with duct-like appearances and dentinoid induction. J. Oral Pathol. Med. 2000, 29, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.K.; Burkes, E.J.; Chai-U-Dom, O. Radiographic manifestation of clear cell odontogenic tumor. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2000, 89, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Ciorba, A.; Soliani, M.; Di Laora, A.; Valpiani, G.; Bianchini, C.; Stomeo, F.; Merlo, R.; Pelucchi, S. Secondary malignant tumors of the parotid gland: Not a secondary problem! J. Buon 2017, 22, 513–518. [Google Scholar] [PubMed]
- Franzen, A.; Buchali, A.; Lieder, A. The rising incidence of parotid metastases: Our experience from four decades of parotid gland surgery. Acta Otorhinolaryngol. Ital. 2017, 37, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Majewska, H.; Skálová, A.; Radecka, K.; Stodulski, D.; Hyrcza, M.; Stankiewicz, C.; Biernat, W. Renal clear cell carcinoma metastasis to salivary glands—A series of 9 cases: Clinico-pathological study. Pol. J. Pathol. 2016, 1, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Bhattacharya, J.; Ganguly, S. Renal cell carcinoma presenting with oral tongue metastasis: A rare case presentation. J. Cancer Res. Ther. 2013, 9, 117. [Google Scholar] [CrossRef]
- Kalinin, Y.; Correia-Neto, I.J.; Do Nascimento, S.V.; De Branco Gonçaves, V.C.; De Andrade, B.A.B.; Nonaka, C.F.W.; Alves, P.M.; Cunha, J.L.S. Lingual metastasis as the first presentation of clear cell renal cell carcinoma: Report of a rare case clinically mimicking a benign lesion. Oral Oncol. 2023, 137, 106293. [Google Scholar] [CrossRef] [PubMed]
- Nishii, N.; Shimamoto, H.; Ohsako, T.; Yokokawa, M.; Sato, Y.; Ohata, Y.; Kayamori, K.; Ikeda, T.; Harada, H. Renal cell carcinoma metastasis to the maxillary bone successfully treated with surgery after vascular embolization: A case report. J. Med. Case Rep. 2020, 14, 193. [Google Scholar] [CrossRef]
- Zhang, R.; Lee, C.W.; Basyuni, S.; Santhanam, V. Mandibular swelling as the initial presentation for renal cell carcinoma: A case report. Int. J. Surg. Case Rep. 2020, 70, 96–100. [Google Scholar] [CrossRef]
- Jung, S.Y.; Maeng, J.Y.; Lee, H.; Han, J.J.; Kim, S.M.; Myoung, H. Metastasis of Renal Cell Carcinoma to the Mandible. J. Craniofac. Surg. 2023, 34, e334–e336. [Google Scholar] [CrossRef]
- Stojanovic, M.; Krasic, D.; Trajkovic, M.; Petrovic, V. Rare renal cell carcinoma metastasis to mandibular gingiva: A case report and literature review. Niger. J. Clin. Pract. 2020, 23, 1483. [Google Scholar]
- Li, L.; Friedrich, R.E.; Schmelzle, R.; Donath, K. Metachronous bilateral metastases of renal cell carcinoma to the parotid region. J. Oral Maxillofac. Surg. 2001, 59, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Eynon-Lewis, N.J.; Radcliffe, G.J. Extensive metastatic renal cell carcinoma presenting as facial nerve palsy. J. Laryngol. Otol. 2001, 115, 488–490. [Google Scholar] [CrossRef]
- Park, Y.W.; Hlivko, T.J. Parotid gland metastasis from renal cell carcinoma. Laryngoscope 2002, 112, 453–456. [Google Scholar] [CrossRef]
- Pritchyk, K.M.; Schiff, B.A.; Newkirk, K.A.; Krowiak, E.; Deeb, Z.E. Metastatic Renal Cell Carcinoma to the Head and Neck. Laryngoscope 2002, 112, 1598–1602. [Google Scholar] [CrossRef]
- Göğüş, Ç.; Kiliç, Ö.; Tulunay, Ö.; Tulunay, Ö.; Bedük, Y. Solitary metastasis of renal cell carcinoma to the parotid gland 10 years after radical nephrectomy. Int. J. Urol. 2004, 11, 894–896. [Google Scholar] [CrossRef]
- Torres-Carranza, E.; Garcia-Perla, A.; Infante-Cossio, P.; Belmonte-Caro, R.; Loizaga-Iriondo, J.M.; Gutierrez-Perez, J.L. Airway obstruction due to metastatic renal cell carcinoma to the tongue. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e76–e78. [Google Scholar] [CrossRef]
- Newton, J.R.; O’Donnell, M.; Samuel, P.R. A case of renal cell carcinoma metastasizing to the parotid gland. Otolaryngol. Head Neck Surg. 2007, 136 (Suppl. S4), S65–S67. [Google Scholar] [CrossRef]
- Yoshitomi, I.; Kawasaki, G.; Mizuno, A.; Nishikido, M.; Hayashi, T.; Fujita, S.; Ikeda, T. Lingual metastasis as an initial presentation of renal cell carcinoma. Med. Oncol. 2011, 28, 1389–1394. [Google Scholar] [CrossRef][Green Version]
- Morvan, J.B.; Veyrières, J.B.; Mimouni, O.; Cathelinaud, O.; Allali, L.; Verdalle, P. Clear-cell renal carcinoma metastasis to the base of the tongue and sphenoid sinus: Two very rare atypical ENT locations. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 91–94. [Google Scholar] [CrossRef]
- Balliram, S.; Goetz, L.; Ramsoobhag, K.; Narinesingh, D.; Medford, S.; Naraynsingh, V. Renal Cell Carcinoma Presenting as a Tongue Lesion. J. Oral Maxillofac. Surg. 2012, 70, 1605–1608. [Google Scholar] [CrossRef]
- Serouya, S.M.; Dultz, L.A.; Concors, S.J.; Wang, B.; Patel, K.N. Late Solitary Metastasis of Renal Cell Carcinoma to the Submandibular Gland. J. Oral Maxillofac. Surg. 2012, 70, 2356–2359. [Google Scholar] [CrossRef]
- Wadasadawala, T.; Kumar, P.; Agarwal, J.; Ghosh-Laskar, S. Palliation of dysphagia with radiotherapy for exophytic base tongue metastases in a case of renal cell carcinoma. Indian J. Urol. 2011, 27, 550. [Google Scholar]
- Deeb, R.; Zhang, Z.; Ghanem, T. Metastatic Renal Cell Carcinoma to the Parotid Gland in the Setting of Chronic Lymphocytic Leukemia. Case Rep. Med. 2012, 2012, 265708. [Google Scholar] [CrossRef]
- Özkiris, M.; Kubilay, U.; Sezen, O. Cervical lymph node metastasis in renal cell carcinoma. J. Oral Maxillofac. Pathol. 2011, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, N.; Davis, C.; Barrett, A.W.; Tighe, J.V. Bilateral Asynchronous Renal Cell Carcinoma with Metastatic Involvement of the Tongue. Case Rep. Pathol. 2012, 2012, 729642. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.Y.C.; Chittleborough, T.J.; McCracken, J.A.; Wijeratne, S. Metastatic clear-cell renal carcinoma to the parotid. ANZ J. Surg. 2012, 82, 760–761. [Google Scholar] [CrossRef]
- Mazeron, R.; Fenoll, L.; Mathieu, M.-C.; Dumas, I.; Haie-Meder, C. Brachytherapy for isolated tongue metastasis of renal clear cell carcinoma. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2013, 130, 149–151. [Google Scholar] [CrossRef][Green Version]
- Yanlan, C.; Liping, S.; Shaomin, C.; Zi, L. Metastasis to the parotid region as an initial presentation of renal cell carcinoma: A case report. Oncol. Lett. 2013, 5, 997–999. [Google Scholar] [CrossRef]
- Udager, A.M.; Rungta, S.A. Metastatic renal cell carcinoma, clear cell type, of the parotid gland: A case report, review of literature, and proposed algorithmic approach to salivary gland clear cell neoplasms in fine-needle aspiration biopsies. Diagn. Cytopathol. 2014, 42, 974–983. [Google Scholar] [CrossRef]
- Abbaszadeh-Bidokhty, H.; Motallebnejad, M.; Rajabi-Moghaddam, M. Metastatic Renal cell Carcinoma Presenting as a clear-cell Tumor in Tongue: A Case Report. Iran. J. Otorhinolaryngol. 2014, 26, 185–190. [Google Scholar]
- Kotak, A.; Merrick, G. Presentation of metastatic renal cell carcinoma as a lip lesion. J. Surg. Case Rep. 2014, 2014, rju083. [Google Scholar] [CrossRef]
- Suojanen, J.; Färkkilä, E.; Helkamaa, T.; Loimu, V.; Törnwall, J.; Lindqvist, C.; Hagström, J.; Mesimäki, K. Rapidly growing and ulcerating metastatic renal cell carcinoma of the lower lip: A case report and review of the literature. Oncol. Lett. 2014, 8, 2175–2178. [Google Scholar] [CrossRef]
- Kudva, R.; Nayal, B.; Kantipudi, S.; Ray, S. Metastatic renal cell carcinoma of the buccal mucosa masquerading as a salivary gland neoplasm. J. Oral Maxillofac. Pathol. 2016, 20, 547. [Google Scholar]
- Georgy, J.T.; Mathuram, A.J.; George, A.A.; Chandramohan, J. Renal cell carcinoma presenting as a cutaneous horn and nodules on the gingiva and scalp. BMJ Case Rep. 2017, 2017, bcr-2017-220913. [Google Scholar] [CrossRef] [PubMed]
- Nifosì, G.; Bressand, H.; Nifosì, A.F.; Nifosì, L.; Damseaux, P. Epulis-Like Presentation of Gingival Renal Cancer Metastasis. Case Rep. Oncol. 2017, 10, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Yoon, A.; Vasilyeva, D.; Peters, S.; Philipone, E. Renal cell carcinoma metastatic to the maxillary gingiva: A case report and review of the literature. J. Oral Maxillofac. Pathol. 2018, 22, 102. [Google Scholar] [CrossRef]
- McNattin, R.F.; Dean, J.; Archie, L. Clinical Reports from Memorial Hospital, New York City: A Case of Renal Adenocarcinoma with Unusual Manifestations. Am. J. Cancer 1931, 15, 1570–1576. [Google Scholar]
- Altinel, D.; Etit, D.; Tan, A.; Bayol, Ü.; Bulut, V.; Erdogan, I.G.; Beyhan, R.; Yalçin, Y. Metastatic Renal Cell Carcinoma Initially Presented as a Tongue Mass. Turk. J. Pathol. 2010, 26, 261–263. [Google Scholar]
- Syryło, T.; Syryło, A.; Jurkiewicz, D.; Zieliński, H.; Piętka, T. An upper lip tumour as the presenting symptom of metastatic renal cancer. Otolaryngol. Pol. 2010, 64, 318–319. [Google Scholar] [CrossRef]
- Gil-Julio, H.; Vázquez-Alonso, F.; Fernández-Sánchez, A.J.; Puche-Sanz, I.; Flores-Martín, J.F.; Cózar, J.M. Metastasis of Renal Cell Carcinoma to the Buccal Mucosa 19 Years after Radical Nephrectomy. Case Rep. Oncol. Med. 2012, 2012, 823042. [Google Scholar] [CrossRef]
- Shirazian, S.; Bahrami, N. An oral metastatic carcinoma guiding to discovery of a renal carcinoma: A case report. J. Craniomaxillofacial Res. 2016, 3, 230–234. [Google Scholar]
- Schrag, A.R.; Jordan, F.B. Unusual metastasis from primary hypernephroma. Can. Med. Assoc. J. 1945, 53, 168. [Google Scholar]
- Carmen, B.V.D.; Korbitz, B.C. Oral Metastasis from Hypernephroma. J. Am. Geriatr. Soc. 1970, 18, 743–746. [Google Scholar] [CrossRef]
- Friedlander, A.H.; Singer, R. Renal adenocarcinoma of the kidney with metastasis to the tongue. J. Am. Dent. Assoc. 1978, 97, 989–991. [Google Scholar] [CrossRef]
- Fitzgerald, R.H.; McInnes, B.K.; Manry, H.C. Renal cell carcinoma involving oral soft tissues. J. Oral Maxillofac. Surg. 1982, 40, 604–606. [Google Scholar] [CrossRef]
- Inai, T.; Kagawa, S.; Aga, Y.; Akiyama, K. A renal cell carcinoma with metastasis to the tongue. Hinyokika Kiyo Acta Urol. Jpn. 1987, 33, 1240–1243. [Google Scholar]
- Ishikawa, J.; Morisue, K.; Imanishi, O.; Kamidono, S. Renal cell carcinoma metastatic to the tongue: A case report. Hinyokika Kiyo Acta Urol. Jpn. 1991, 37, 263–265. [Google Scholar]
- Okabe, Y.; Ohoka, H.; Miwa, T.; Nagayama, I.; Furukawa, M. Renal cell carcinoma metastasis to the tongue. J. Laryngol. Otol. 1992, 106, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, T.; Hasegawa, S.; Nakamura, S.; Tachibana, M.; Jitsukawa, S.; Shiotani, A.; Morinaga, S. Disappearance of Metastatic Renal Cell Carcinoma to the Base of the Tongue after Systemic Administration of Interferon-Alpha. Eur. Urol. 1993, 24, 297–299. [Google Scholar] [CrossRef]
- Ziyada, W.F.; Brookes, J.D.; Penman, H.G. Expectorated tissue leading to diagnosis of renal adenocarcinoma. J. Laryngol. Otol. 1994, 108, 1108–1110. [Google Scholar] [CrossRef] [PubMed]
- Airoldi, M.; Succo, G.; Valente, G.; Cavalot, A.; Gabriele, P.; Bumma, C. Head and Neck Metastases of Renal Cancer after Nephrectomy: A Report of 2 Cases. Tumori J. 1995, 81, 213–214. [Google Scholar] [CrossRef]
- Aguirre, A.; Rinaggio, J.; Diaz-Ordaz, E. Lingual metastasis of renal cell carcinoma. J. Oral Maxillofac. Surg. 1996, 54, 344–347. [Google Scholar] [CrossRef]
- Konya, E.; Hara, Y.; Umekawa, T.; Uejima, S.; Sugiyama, T.; Kurita, T. Two cases of renal cell carcinoma detected by metastasis to another organ. Hinyokika Kiyo Acta Urol. Jpn. 1997, 43, 647–650. [Google Scholar]
- Tomita, T.; Inouye, T.; Shinden, S.; Mukai, M. Palliative radiotherapy for lingual metastasis of renal cell carcinoma. Auris Nasus Larynx 1998, 25, 209–214. [Google Scholar] [CrossRef]
- Navarro, F.; Vicente, J.; Villanueva, M.J.; Sánchez, A.; Provencio, M.; España, P. Metastatic Renal Cell Carcinoma to the Head and Neck Area. Tumori J. 2000, 86, 88–90. [Google Scholar] [CrossRef]
- Mekni, A.; Bouraoui, S.; Touati, S.; el Ouertani, L.; el May, A. Linguinal metastasis from clear cell carcinoma of the kidney. La Tunis. Med. 2002, 80, 570–573. [Google Scholar]
- Kyan, A.; Kato, S.N. Renal cell carcinoma metastatic to the base of tongue: A case report. Hinyokika Kiyo Acta Urol. Jpn. 2004, 50, 791–793. [Google Scholar]
- Huang, H.C.; Chang, K.P.; Chen, T.M.; Wu, K.F.; Ueng, S.H. Renal cell carcinoma metastases in the head and neck. Chang Gung Med. J. 2006, 29, 59–65. [Google Scholar]
- Cochrane, T.; Cheng, L.; Crean, J. Renal Cell Carcinoma: A Rare Metastasis to the Tongue—A Case Report. Dent. Update 2006, 33, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario Regalado, R.; Gallana Álvarez, S.; Creo Martínez, T.; Herce López, J.; Pereira Gallardo, S. Lingual metastasis from renal carcinoma. Rev. Esp. Cir. Oral Maxilofac. 2007, 29, 179–181. [Google Scholar]
- Longo, R.; Baldini, D.; Gasparini, G. An atypical tongue metastasis of renal cell carcinoma in a patient with metachronous hepatocellular carcinoma. Cancer Ther. 2008, 6, 707. [Google Scholar]
- Kella, V.K.N.; Cosgrove, J.M.; Krishnamoorthy, V. Synchronous lingual and thyroid metastasis from renal cell carcinoma. Am. J. Case Rep. 2009, 10, 88–92. [Google Scholar]
- Friedmann, I.; Osborn, D.A. Metastatic Tumours in the Ear, Nose and Throat Region. J. Laryngol. Otol. 1965, 79, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Trinca, A.J.; Willis, R.A. Primary Carcinoma Unsuspected by the Clinician. Med. J. Aust. 1936, 2, 222–227. [Google Scholar]
- Branch, C.; Norton, R. Metastatic hypernephroma of the jaw. N. Engl. J. Med. 1928, 198, 559–561. [Google Scholar] [CrossRef]
- Salman, I.; Langel, I. Metastatic tumors of the oral cavity. Oral Surg. Oral Med. Oral Pathol. 1954, 7, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.A.; Wallenius, K. Metastatic Renal Carcinoma (Hypernephroma) in the Gingiva of the Lower Jaw. Acta Odontol. Scand. 1961, 19, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Cranin, A.N.; Berman, S.; Tucker, N. Renal-cell carcinoma of the mandibular periodontium. Oral Surg. Oral Med. Oral Pathol. 1966, 21, 626–631. [Google Scholar] [CrossRef]
- Buchner, A.; Begleiter, A. Metastatic Renal Cell Carcinoma in the Gingiva Mimicking a Hyperplastic Lesion: Case Report. J. Periodontol. 1980, 51, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Yakata, H.; Kawasaki, T.; Nakajima, T. Metastatic tumours of the mouth and jaws. J. Maxillofac. Surg. 1982, 10, 253–258. [Google Scholar] [CrossRef]
- Fay, J.T.; Weir, G.T. Metastatic renal cell carcinoma from a primary tumor removed 14 years previously. J. Oral Maxillofac. Surg. 1983, 41, 129–132. [Google Scholar] [CrossRef]
- Zohar, Y.; Ben-Tovim, R.; Gal, R.; Laurian, N. Metastatic carcinoma of oral soft tissue. Head Neck Surg. 1985, 7, 484–486. [Google Scholar] [CrossRef]
- Tsianos, E.B.; Karentzos, C.; Papadopoulos, N.E. Metastatic renal cell carcinoma in the gingiva of the maxilla and mandible: Report of a case. J. Oral Maxillofac. Surg. 1987, 45, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Müller-Mattheis, V.; Hagen, M.; Frenzel, H.; Ackermann, R. A rare form of metastasis of renal cell cancer. A case report of intra-oral soft tissue metastasis. Urol. Ausg. A 1989, 28, 355–358. [Google Scholar]
- Hagen, M.; Müller-Mattheis, V.; Frenzel, H.; Fritzemeier, C.U. Intraoral soft tissue metastases of a renal cell carcinoma. Dtsch. Z. Mund- Kiefer- Gesichts-Chir. 1989, 13, 155–160. [Google Scholar]
- Salman, I.; Darlington, C. Rare (unusual) malignant tumors of the jaws. Am. J. Orthod. Oral Surg. 1944, 30, 725. [Google Scholar] [CrossRef]
- Mallett, S.P. A renal-cell metastatic carcinoma involving the mandible and submaxillary gland. Oral Surg. Oral Med. Oral Pathol. 1961, 14, 4–7. [Google Scholar] [CrossRef]
- Meyer, I.; Shklar, G. Malignant tumors metastatic to mouth and jaws. Oral Surg. Oral Med. Oral Pathol. 1965, 20, 350–362. [Google Scholar] [CrossRef]
- Godby, A.F.; Sonntag, R.W.; Cosentino, B.J. Hypernephroma with metastasis to the mandibular gingiva. Oral Surg. Oral Med. Oral Pathol. 1967, 23, 696–700. [Google Scholar] [CrossRef]
- Milobsky, S.A.; Milobsky, L.; Epstein, L.I. Metastatic renal adenocarcinoma presenting as periapical pathosis in the maxilla. Oral Surg. Oral Med. Oral Pathol. 1975, 39, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, M.; Oka, T. Two cases of clear cell carcinoma found in the jaws. Nagoya J. Med. Sci. 1979, 42, 1–6. [Google Scholar] [PubMed]
- Susan, L.P.; Daughtry, J.D.; Stewart, B.H.; Straffon, R.A. Palatal metastases in renal cell carcinoma. Urology 1979, 13, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Yanagihara, N. Renal clear cell carcinoma metastatic to the nose and paranasal sinuses. Laryngoscope 1982, 92, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Pick, J.B.; Wagner, R.M.; Indresano, A.T. Initial appearance of renal cell carcinoma as a metastatic mass in the mandible. J. Am. Dent. Assoc. 1986, 113, 759–761. [Google Scholar] [CrossRef]
- Zachariades, N.; Koumoura, F.; Vairaktaris, E.; Mezitis, M. Metastatic tumors to the jaws: A report of seven cases. J. Oral Maxillofac. Surg. 1989, 47, 991–996. [Google Scholar] [CrossRef]
- Jones, G.M.; Telfer, M.R.; Eveson, J.W. Metastatic renal clear cell carcinoma of the jaws. Two cases illustrating clinical and pathological diagnostic problems. Br. J. Oral Maxillofac. Surg. 1990, 28, 172–175. [Google Scholar] [CrossRef]
- Fandella, A.; Anselmo, G.; Maccatrozzo, L.; Frezza, D.; Marchiori, C. Epistaxis in Renal Carcinoma: Case Report. Scand. J. Urol. Nephrol. 1992, 26, 89. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Sharma, S.D.; Bullock, K.N. An Unusual Case of Renal Cell Carcinoma with Two Rare Metastases. Scand. J. Urol. Nephrol. 1998, 32, 239–240. [Google Scholar] [PubMed]
- Guyot, L.; Sauvant, J.; Menasse, F.; Garcia, S.; Portier, F.; Gola, R. Hemorrhagic Mandibular Metastasis of Renal Origin: Usefulness of Therapeutic Embolization; Presse Medicale: Paris, France, 1983; Volume 28, pp. 1066–1068. [Google Scholar]
- Hönig, J.F. Inheritance of Hippel-Lindau Disease: A Rare Case of Maxillary Bone Metastasis. J. Craniofac. Surg. 2000, 11, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.C.; Gupta, S.; Nagsubramanium, S.; Hasan, S.; Cherry, G. Mandibular metastasis from renal cell carcinoma. A case report. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2001, 12, 77–80. [Google Scholar]
- Heinroth, S.; Bilkenroth, U.; Eckert, A.W.; Maurer, P. Die ossäre Metastase im Oberkiefer als Erstmanifestation eines Nierenzellkarzinoms: Ein Fallbericht. Oral Maxillofac. Surg. 2006, 10, 42–45. [Google Scholar]
- Madison, J.F.; Frierson, H.F. Pathologic quiz case 2. Clear cell carcinoma, consistent with metastatic renal cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 1988, 114, 570–571, 573. [Google Scholar]
- Kishore, M.; Chauhan, D.S.; Dogra, S. Unusual presentation of renal cell carcinoma: A rare case report. J. Lab Physicians 2018, 10, 241–244. [Google Scholar] [CrossRef]
- Abro, C.; Sedhom, R.; Soni, A.; Markowski, M. Cutaneous finger and tongue metastases in renal cell carcinoma. BMJ Case Rep. 2019, 12, e230516. [Google Scholar] [CrossRef]
- Netto, R.; De Freitas Filho, S.A.J.; Cortezzi, W.; Merly, F.; De Andrade, V.M.; Pires, F.R. Metastasis of Renal Cell Carcinoma Causing Significant Facial Asymmetry. Case Rep. Surg. 2019, 2019, 6840873. [Google Scholar] [CrossRef]
- Walsh, M.A.; Quinn, A.J.; Mahesh, B. Case report: Renal cell carcinoma metastasis to the tongue. J. Surg. Case Rep. 2022, 2022, rjac565. [Google Scholar] [CrossRef]
- Mrena, R.; Leivo, I.; Passador-Santos, F.; Hagström, J.; Mäkitie, A.A. Histopathological findings in parotid gland metastases from renal cell carcinoma. Eur. Arch. Otorhinolaryngol. 2008, 265, 1005–1009. [Google Scholar] [CrossRef]
- Aljawad, M.; Alharbi, M.K.; Algahtani, S.M.; Mughallis, H.M.; Almhna, S.M. Metastasis of Clear Cell Renal Cell Carcinoma to the Parotid Gland: A Case Report. Cureus 2023, 15, e43676. [Google Scholar] [CrossRef]
- Migliorelli, A.; Caranti, A.; Manuelli, M.; Bianchini, C.; Ciorba, A.; Pelucchi, S. Clear-Cell Renal Cell Carcinoma Metastasis into Pterygomaxillary Fossa—A Case Report. Ann. Maxillofac. Surg. 2023, 13, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Maschino, F.; Guillet, J.; Curien, R.; Dolivet, G.; Bravetti, P. Oral metastasis: A report of 23 cases. Int. J. Oral Maxillofac. Surg. 2013, 42, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.; Abelardo, E.; Ramachandran, K.; Prabhu, V. Renal cell carcinoma uvula metastasis leading to airway compromise: An unusual site. BMJ Case Rep. 2022, 15, e248098. [Google Scholar] [CrossRef]
- Ludwig, D.C.; Garcia, J.; Chang, O.H.; Closmann, J.J. Metastatic renal cell carcinoma to the mandible: A case report with clinical and histologic findings. Gen. Dent. 2020, 68, 41–44. [Google Scholar]
- Melnick, S.J.; Amazon, K.; Dembrow, V. Metastatic renal cell carcinoma presenting as a parotid tumor: A case report with immunohistochemical findings and a review of the literature. Hum. Pathol. 1989, 20, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Borghi, L.; Bianchini, E.; Ballotta, M.R.; Reale, D. Metastatic renal cell carcinoma presenting as a parotid tumor: A case report. Pathologica 1995, 87, 168–170. [Google Scholar]
- Seijas, B.P.; Franco, F.L.; Sastre, R.M.; García, A.A.; López-Cedrún Cembranos, J.L. Metastatic renal cell carcinoma presenting as a parotid tumor. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 99, 554–557. [Google Scholar] [CrossRef]
- Goel, M.C.; Williams, D.W.; Evans, H.; Roberts, J.G. Lingual metastasis from renal cell carcinoma management and review of the literature. Urol. Int. 2003, 71, 418–421. [Google Scholar] [CrossRef]
- Lenkeit, C.; Bank, J.; Shirazi, M. Renal Cell Carcinoma in the Head and Neck: Case Presentation of a Patient with a Rare Metastatic Pattern. Cureus 2020, 12, e11894. [Google Scholar] [CrossRef]
- Ruiz-Oslé, S.; Prol, C.; Lardies, R.; Gaafar, A.; Barbier, L.; Arruza, A. Renal Cell Carcinoma metastases in the maxillofacial area: Case series. Arch. Esp. Urol. 2017, 70, 732–735. [Google Scholar]
- Schwab, B.; Lee, W.T. Bilateral renal cell carcinoma metastasis in the oral cavity. Am. J. Otolaryngol. 2012, 33, 154–155. [Google Scholar] [CrossRef]
- Erkilic, S.; Keskinruzgar, A.; Bozdag, Z.; Gunhan, O. Metastasis of a Renal Collecting Duct Adenocarcinoma to the Oral Cavity After Tooth Extraction. J. Craniofac. Surg. 2017, 28, e398–e399. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lee, J.I. Metastatic carcinoma of the oral region: An analysis of 21 cases. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e359. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.M.; Pontes, F.S.C.; Miyahara, L.A.N.; Guerreiro, M.Y.R.; de Almeida, M.C.L.; Pontes, H.A.R.; Pinto, D.D.S. Metastatic Renal Cell Carcinoma to the Oral Cavity. J. Craniofac. Surg. 2016, 27, e533–e534. [Google Scholar] [CrossRef] [PubMed]
- Owosho, A.A.; Xu, B.; Kadempour, A.; Yom, S.K.; Randazzo, J.; Ghossein, R.A.; Huryn, J.M.; Estilo, C.L. Metastatic solid tumors to the jaw and oral soft tissue: A retrospective clinical analysis of 44 patients from a single institution. J. Cranio-Maxillofac. Surg. 2016, 44, 1047–1053. [Google Scholar] [CrossRef]
- Nisi, M.; Izzetti, R.; Graziani, F.; Gabriele, M. Renal Cell Carcinoma Metastases to the Oral Cavity: Report of 2 Cases and Review of Literature. J. Oral Maxillofac. Surg. 2020, 78, 1557–1571. [Google Scholar] [CrossRef]
- Lang, E.E.; Patil, N.; Walsh, R.M.; Leader, M.; Walsh, M.A. A case of renal cell carcinoma metastatic to the nose and tongue. Ear Nose Throat J. 2003, 82, 382–383. [Google Scholar] [CrossRef]
- Bućin, E.; Andréasson, L.; Björlin, G. Metastases in the oral cavity. Case reports. Int. J. Oral Surg. 1982, 11, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Marioni, G.; Gaio, E.; Poletti, A.; Derosas, F.; Staffieri, A. Uncommon metastatic site of renal adenocarcinoma: The oral tongue. Acta Otolaryngol. 2004, 124, 197–201. [Google Scholar] [CrossRef] [PubMed]
- van der Waal, R.I.F.; Buter, J.; van der Waal, I. Oral metastases: Report of 24 cases. Br. J. Oral Maxillofac. Surg. 2003, 41, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Miyata, M.; Okabe, K.; Sakashita, H. A case series of 9 tumors metastatic to the oral and maxillofacial region. J. Oral Maxillofac. Surg. 2002, 60, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Morii, T. A case report of metastatic clear cell carcinoma of the oral cavity. Jpn. J. Oral Maxillofac. Surg. 1975, 21, 213–216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sidhu, S.S.; Parkash, H.; Chopra, P. Renal metastatic carcinoma of the mandible. J. Dent. 1982, 10, 103–106. [Google Scholar] [CrossRef]
- Sánchez Aniceto, G.; García Peñín, A.; de la Mata Pages, R.; Montalvo Moreno, J.J. Tumors metastatic to the mandible: Analysis of nine cases and review of the literature. J. Oral Maxillofac. Surg. 1990, 48, 246–251. [Google Scholar] [CrossRef]
- Maestre-Rodríguez, O.; González-García, R.; Mateo-Arias, J.; Moreno-García, C.; Serrano-Gil, H.; Villanueva-Alcojol, L.; Campos-de-Orellana, A.M.; Monje-Gil, F. Metastasis of renal clear-cell carcinoma to the oral mucosa, an atypical location. Med. Oral Patol. Oral Cir. Bucal. 2009, 14, e601–e604. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Will, T.A.; Agarwal, N.; Petruzzelli, G.J. Oral cavity metastasis of renal cell carcinoma: A case report. J. Med. Case Rep. 2008, 2, 313. [Google Scholar] [CrossRef]
- Nesbitt, A.L.; Lim, Z.L.T.; Chan, K.J.; Zardawi, I.; Pridgeon, S.W. Metastatic renal cell carcinoma presenting with both acute stroke and an oral lesion. Urol. Case Rep. 2019, 23, 75–77. [Google Scholar] [CrossRef]
- Patel, S.; Barros, J.; Nwizu, N.N.; Ogbureke, K.U.E. Metastatic renal cell carcinoma to the oral cavity as first sign of disease: A case report. Clin. Case Rep. 2020, 8, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Narea-Matamala, G.; Fernández-Toro, M.d.l.A.; Villalabeitía-Ugarte, E.; Landaeta-Mendoza, M.; Rojas-Alcayaga, G. Oral metastasis of renal cell carcinoma, presentation of a case. Med. Oral Patol. Oral Cir. Bucal. 2008, 13, E742–E744. [Google Scholar] [PubMed]
- Massaccesi, M.; Morganti, A.G.; Serafini, G.; Di Lallo, A.; Deodato, F.; Picardi, V.; Scambia, G. Late tonsil metastases from renal cell cancer: A case report. Tumori J. 2009, 95, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Ito, H.; Nakayama, R.; Noguchi, T.; Jinbu, Y.; Kusama, M.; Ichimura, K. Metastatic Clear Cell Carcinoma of the Mandible in a Patient with Renal Cancer undergoing Haemodialysis. Asian J. Oral Maxillofac. Surg. 2009, 21, 43–47. [Google Scholar] [CrossRef]
- Ohmura, S.; Kitagawa, T.; Kida, Y.; Fujita, K.; Masuda, M.; Ohtani, T. Renal cell carcinoma metastatic to the mandibular angle. Jpn. J. Oral Maxillofac. Surg. 1981, 27, 662–667. [Google Scholar] [CrossRef]
- Nakano, H.; Naito, K.; Suzuki, S.; Naito, K.; Kubota, T.; Takizawa, S. Metastatic renal cell carcinoma in the cheek: Report of a case. J. Oral Maxillofac. Surg. Med. Pathol. 2013, 25, 291–293. [Google Scholar] [CrossRef]
- Ficarra, G.; Pierleoni, L.; Panzoni, E. Metastatic renal cell carcinoma involving Wharton’s duct. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 81, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Tunio, M.A.; Al Asiri, M.; Ahmad, S.; Fareed, M.; Bayoumi, Y. Tongue metastasis as an initial manifestation of metastasis in renal cell carcinoma: A case report. J. Solid Tumors 2012, 2, 39. [Google Scholar] [CrossRef][Green Version]
- Milner, P.; Janas, A.; Grzesiak-Janas, G. Clear cell renal carcinoma metastasis in the oral cavity—Case report. J. Pre-Clin. Clin. Res. 2015, 8, 127–129. [Google Scholar] [CrossRef]
- Santana, L.N.; Ribeiro, J.T.; Domingues, M.; De Oliveira, M.G.; Rivero, L.F.; Carrard, V.C.; Trevizani, M.A. A rare case of oral metastasis of renal clear cell carcinoma: Case report and review of literature. J. Oral Diagn. 2000, 5, e20200006. [Google Scholar]
- Kizaekka, A.; Chengot, P.; Mannion, C. Recurrent oral metastatic lesion of renal cell carcinoma—A case report. Int. J. Oral Craniofacial Sci. 2019, 5, 024–026. [Google Scholar] [CrossRef]
- Paraskevopoulos, K.; Vahtsevanos, K.; Ntomouchtsis, A.; Kalaitsidou, I.; Patrikidou, A.; Andreadis, C.; Antoniades, K. Metastatic tumors to the oral cavity—A retrospective analysis. Int. Res. J. Otolaryngol. 2021, 4, 10. [Google Scholar]
- Morita, Y.; Iwagami, T.; Kawakita, C.; Kusuyama, Y.; Niki-Yonekawa, A.; Morita, N. Oral metastasis of renal cell carcinoma mimicking recurrence of excised malignant myoepithelioma: A case report. Mol. Clin. Oncol. 2018, 9, 66–69. [Google Scholar] [CrossRef]
- Prol, C.; Ruiz-Oslé, S.; Malaxetxebarría, S.; Dolado, A.; Del Hoyo, O.M.; Barbier, L. Oral and Maxillary Metastases: Retrospective Clinical Analysis of 21 Cases. Rev. Española Cirugía Oral Maxilofac. Publicación Soc. Española Cirugía Oral Maxilofac. 2019, 41, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Shimono, H.; Hirai, H.; Oikawa, Y.; Mochizuki, Y.; Kuroshima, T.; Tomioka, H.; Kayamori, K.; Ikeda, T.; Harada, H. Metastatic tumors in the oral region: A retrospective chart review of clinical characteristics and prognosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 648–652. [Google Scholar] [CrossRef]
- Ali, R.A.E.; Mohamed, K.E.H. Metastatic Clear Cell Renal Cell Carcinoma Presenting with a Gingival Metastasis. Clin. Pract. 2016, 6, 847. [Google Scholar] [CrossRef][Green Version]
- Selvi, F.; Faquin, W.C.; Michaelson, M.D.; August, M. Three Synchronous Atypical Metastases of Clear Cell Renal Carcinoma to the Maxillary Gingiva, Scalp and the Distal Phalanx of the Fifth Digit: A Case Report. J. Oral Maxillofac. Surg. 2016, 74, 1286.e1–1286.e9. [Google Scholar] [CrossRef]
- Jatti, D.; Puri, G.; Aravinda, K.; Dheer, D.S. An atypical metastasis of renal clear cell carcinoma to the upper lip: A case report. J. Oral Maxillofac. Surg. 2015, 73, 371.e1–371.e6. [Google Scholar] [CrossRef]
- Sikka, S.; Sikka, P.; Kaur, G.; Shetty, D.C. A review of histopathological and immunohistochemical parameters in diagnosis of metastatic renal cell carcinoma with a case of gingival metastasis. J. Cancer Res. Ther. 2013, 9, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Ganini, C.; Lasagna, A.; Ferraris, E.; Gatti, P.; Paglino, C.; Imarisio, I.; Morbini, P.; Benazzo, M.; Porta, C. Lingual metastasis from renal cell carcinoma: A case report and literature review. Rare Tumors 2012, 4, e41. [Google Scholar] [CrossRef]
- Lutcavage, G.J.; Branham, G.B.; Winterholler, B.W.; Wood, D.A. Renal cell carcinoma metastasis to the hard palate. J. Oral Maxillofac. Surg. 1984, 42, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Abubakerr, M.; Gollins, S. Tongue metastasis as an initial presentation of renal cell carcinoma: A case report and literature review. J. Med. Case Rep. 2008, 2, 249. [Google Scholar] [CrossRef]
- Basely, M.; Bonnel, S.; Maszelin, P.; Verdalle, P.; Bussy, E.; de Jaureguiberry, J.P. A rare presentation of metastatic renal clear cell carcinoma to the tongue seen on FDG PET. Clin. Nucl. Med. 2009, 34, 566–569. [Google Scholar] [CrossRef]
- Mansourian, E.; Ahmadnia, H.; Amirmajdi, N. Renal cell carcinoma presenting as mandibular metastasis. Saudi J. Kidney Dis. Transplant. 2013, 24, 789. [Google Scholar] [CrossRef]
- Ord, R.A.; Malins, T.; Ward-Booth, P.R. Vascular metastatic renal carcinoma of the maxilla. Report of two cases. Int. J. Oral Maxillofac. Surg. 1990, 19, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Capodiferro, S.; Limongelli, L.; Mastropasqua, M.G.; Favia, G.; Lajolo, C.; Colella, G.; Tempesta, A.; Maiorano, E. Metastatic Tumors of the Oro-Facial Tissues: Clear Cell Renal Cell Carcinoma: A Clinico-Pathological and Immunohistochemical Study of Seven Cases. J. Clin. Med. 2020, 9, 1151. [Google Scholar] [CrossRef]
- Andabak Rogulj, A.; Tomasovic Loncaric, C.; Muller, D.; Blivajs, I.; Andabak, M.; Vucicevic Boras, V.; Sekerija, M. Solid malignant metastases in the jaw bones. Br. J. Oral Maxillofac. Surg. 2018, 56, 705–708. [Google Scholar] [CrossRef]
- Derakhshan, S.; Rahrotaban, S.; Mahdavi, N.; Mirjalili, F. Metastatic renal cell carcinoma presenting as maxillary lesion: Report of two rare cases. J. Oral Maxillofac. Pathol. 2018, 22 (Suppl. S1), S39–S43. [Google Scholar] [PubMed]
- Altuntaş, O.; Petekkaya, İ.; Süslü, N.; Güllü, İ. Renal cell carcinoma metastatic to the tongue: A case report and review of the literature. J. Oral Maxillofac. Surg. 2015, 73, 1227–1230. [Google Scholar] [CrossRef]
- Amiruddin, S.; Yunus, M.R.M. Tongue mass in post nephrectomy patient. Egypt. J. Ear Nose Throat Allied Sci. 2013, 14, 147–149. [Google Scholar] [CrossRef][Green Version]
- Lieder, A.; Guenzel, T.; Lebentrau, S.; Schneider, C.; Franzen, A. Diagnostic relevance of metastatic renal cell carcinoma in the head and neck: An evaluation of 22 cases in 671 patients. Int. Braz J. Urol. 2017, 43, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Kase, A.M.; George, D.J.; Ramalingam, S. Clear Cell Renal Cell Carcinoma: From Biology to Treatment. Cancers 2023, 15, 665. [Google Scholar] [CrossRef] [PubMed]
- Schütz, V.; Lin, H.; Kaczorowski, A.; Zschäbitz, S.; Jäger, D.; Stenzinger, A.; Duensing, A.; Debus, J.; Hohenfellner, M.; Duensing, S. Long-Term Survival of Patients with Stage T1N0M1 Renal Cell Carcinoma. Cancers 2023, 15, 5715. [Google Scholar] [CrossRef] [PubMed]
- Semenescu, L.E.; Kamel, A.; Ciubotaru, V.; Baez-Rodriguez, S.M.; Furtos, M.; Costachi, A.; Dricu, A.; Tătăranu, L.G. An Overview of Systemic Targeted Therapy in Renal Cell Carcinoma, with a Focus on Metastatic Renal Cell Carcinoma and Brain Metastases. Curr. Issues Mol. Biol. 2023, 45, 7680–7704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albiges, L.; McGregor, B.A.; Heng, D.Y.C.; Procopio, G.; de Velasco, G.; Taguieva-Pioger, N.; Martín-Couce, L.; Tannir, N.M.; Powles, T. Vascular endothelial growth factor-targeted therapy in patients with renal cell carcinoma pretreated with immune checkpoint inhibitors: A systematic literature review. Cancer Treat. Rev. 2024, 122, 102652. [Google Scholar] [CrossRef] [PubMed]
- Kaddissi, A.E.; Ducleon, G.G.; Lefort, F.; Mezepo, G.; Frontczak, A.; Goujon, M.; Mouillet, G.; Almotlak, H.; Gross-Goupil, M.; Thiery-Vuillemin, A. Metastatic renal cell cancer and first-line combinations: For which patients? (focus on tolerance and health-related quality of life). Bull. Cancer 2022, 109 (Suppl. S2), 2S19–2S30. [Google Scholar] [CrossRef] [PubMed]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; Mackenzie, M.; Wood, L.; Donskov, F.; Tan, M.H.; et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013, 14, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Brierley, D.J.; Crane, H.; Hunter, K.D. Lumps and Bumps of the Gingiva: A Pathological Miscellany. Head Neck Pathol. 2019, 13, 103–113. [Google Scholar] [CrossRef]
- Ide, F.; Obara, K.; Mishima, K.; Saito, I.; Horie, N.; Shimoyama, T.; Kusama, K. Peripheral odontogenic tumor: A clinicopathologic study of 30 cases: General features and hamartomatous lesions. J. Oral Pathol. Med. 2005, 34, 552–557. [Google Scholar] [CrossRef]
- Wulfrank, D.; Speelman, T.; Pauwels, C.; Roels, H.; De Schryver, A. Extranodal non-Hodgkin’s lymphoma of the head and neck. Radiother. Oncol. 1987, 8, 199–207. [Google Scholar] [CrossRef]
- Epstein, J.B.; Epstein, J.D.; Le, N.D.; Gorsky, M. Characteristics of oral and paraoral malignant lymphoma: A population-based review of 361 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 96, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fantasia, J.E.; Kaplan, R. Oral manifestations of acute myelomonocytic leukemia: A case report and review of the classification of leukemias. J. Periodontol. 2002, 73, 664–668. [Google Scholar] [CrossRef]
- Irani, S. Metastasis to the Jawbones: A review of 453 cases. J. Int. Soc. Prev. Community Dent. 2017, 7, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.F.; Valente, C.; Bekdache, O.; Maxwell, F.; Balasa, C.; Savignac, A.; Meyrignac, O. Update on Renal Cell Carcinoma Diagnosis with Novel Imaging Approaches. Cancers 2024, 16, 1926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).