Abstract
The purpose of this research is to explore the biomechanical consequences of maternal injuries on fetal movements. Additionally, the research aims to comprehend the relationship between these injuries and fetal movement within the amniotic sac and to understand the extent to which the amniotic fluid can provide protection during severe injuries. The focus is on the potential impact these injuries could have on surgical procedures and preventative strategies. Using advanced computational simulations, the study investigates how various maternal injuries can influence the behavior of amniotic fluid and the subsequent stress exerted on fetal development. The findings suggest that maternal injuries can induce stress, primarily affecting the posterior regions of the fetus and the umbilical cord, depending on the boundary and initial conditions. This stress is associated with fetal displacement within the amniotic sac. While the amniotic fluid provides a certain level of protection, its limitations become apparent during severe injuries. These insights have implications for the field of surgery, particularly fetal procedures. They underscore the need for improved protective measures and the development of personalized obstetric and neonatal care strategies. Moreover, the study highlights the potential of computational simulations in aiding surgeons. These simulations can provide a more accurate understanding of the critical areas to focus on during surgical procedures, thereby enhancing the precision and safety of these operations.
1. Introduction
Fetal trauma resulting from maternal injury requiring surgery is a complex and challenging situation. Trauma during pregnancy, including accidents and violence, is a common complication and can have serious consequences for both the mother and the fetus. Studies have shown that maternal death due to trauma is more common than any other medical complication during pregnancy [1,2]. In the field of computational medical simulations, the ability to observe and analyze the dynamics of a trauma event on a patient-specific basis is a valuable tool. This research focuses on the development of such simulations in the realm of obstetrics, specifically focusing on fetal trauma. The simulations provide a detailed, second-by-second observation of the stresses a fetus undergoes during a trauma event, akin to having a detailed view of the body during the trauma. The ability to pause, rotate, and inspect the fetus at any given time point offers invaluable insights for surgical planning. The simulations are primarily conducted with the fetus in the most common position—head down, facing the back. However, the flexibility of the model allows for the exploration of different scenarios, including varying external forces, angles of impact, and fetal positions.
Trauma, recognized as the primary cause of nonobstetric death among pregnant women [3], is estimated to affect approximately 1 in 12 pregnancies [4]. Over the past quarter-century, the occurrence of trauma during pregnancy has dramatically escalated, positioning it as the leading cause of nonobstetrical maternal death in the United States [5]. The risk associated with major trauma is substantial, with fetal death rates estimated between 40% and 50% [5]. The situation is further complicated by maternal shock, which is linked to an alarming 80% fetal mortality rate [5]. Pelvic fractures, the most prevalent maternal injury leading to fetal death, are a significant concern [5]. Furthermore, nearly 28% of pregnant trauma patients show evidence of fetal–maternal hemorrhage [5]. Understanding the normal physiology of maternal–fetal interactions is essential for the diagnosis and management of trauma during pregnancy [1,2]. Given the high fetal mortality rate associated with maternal shock, rapid evaluation and treatment of the mother should be prioritized in trauma scenarios [5]. The approach to managing surgical trauma during pregnancy is injury-specific [1,2]. Upon the detection of direct fetal injuries, the resolution to initiate the delivery process requires thorough deliberation, incorporating both the gestational age and the severity of the injuries into the decision-making process [6].
The effective management of trauma during pregnancy necessitates an interdisciplinary approach that integrates the expertise of obstetrics, surgery, anesthesiology, perinatology, and intensive care teams [7,8,9]. Coordinated care and collaboration between resuscitators, obstetricians, and vascular surgeons are crucial for optimal outcomes.
This research is centered around understanding the biomechanical impact of injuries to the mother on the dynamics of the fetus. We employed boundary conditions obtained from experiments in our use of the Smoothed Particle Hydrodynamics (SPH) method, which was combined with the high-order Finite Element Method (FEM). This combination was used to model the reaction of the amniotic fluid during traumatic situations. The SPH method allows for a thorough comprehension of the distribution and transmission of forces within the amniotic fluid to the fetus [10]. This enhances our comprehension of how changes in amniotic fluid dynamics can affect different parts of the fetus during maternal injury. There is a significant gap in the literature, with very few relevant studies, both computational and experimental, available on this topic [11,12]. Understandably, studying this topic can be challenging because it involves interactions between amniotic fluid and a multitude of complex geometries with varying properties. In contrast to previous studies in this field, we do not simplify the geometries or neglect to simulate the fluid as a fluid domain. Instead, we embrace the complexity of these interactions, providing a more accurate and comprehensive understanding of the effects of maternal trauma on fetal dynamics. This is a significant departure from existing methods, marking a first-of-its-kind application of these numerical methods in prenatal care.
2. Materials and Methods
Two levels of explanation are presented to accommodate readers with varying backgrounds. The methods used in the study are first introduced in non-technical terms, providing all readers with the ability to comprehend the foundational techniques used. This initial explanation offers enough insight for a clear understanding of the research processes and findings. Subsequent to this overview, more technical and detailed discussions regarding SPH and high-order FEM follow. Those subsections are designed for experts or readers with a specific interest in computational modeling and seek a deeper understanding of the intricacies involved in this approach. Readers not interested in these intricate details are encouraged to move to the Results section, where the core conclusions of the study and their significance to obstetric care can be fully appreciated in isolation from the more complex methodological specifics.
The following subsections provide a detailed explanation of the geometrical model and numerical methods utilized, presented in a more accessible, non-technical language.
2.1. Digital Twin of Maternal-Fetal Anatomy
The geometrical model employed in the study is a digital representation of the maternal–fetal anatomy (Figure 1), derived from clinical imaging data. It consists of three primary components: the uterus, the amniotic sac, and the fetus. The amniotic sac is modeled as a fluid-filled, compliant membrane encasing the fetus, allowing for both the movement of the fetus and the dynamic response of the amniotic fluid during a traumatic event. The fetus itself is constructed with a high degree of anatomical accuracy, reflecting the size, shape, and position within the amniotic sac according to gestational age. The fetus is in a head-down (cephalic) position. This is reflective of the typical fetal position during the later stages of pregnancy, specifically during the third trimester. This positioning is crucial as it significantly influences the dynamics of maternal traumas and their potential impact on the fetus. To accurately capture the interactions between the amniotic fluid and the fetus during traumas, the model integrates the physical properties of the amniotic fluid, fetal tissue, and the sac’s membrane. Advanced computational simulations are then conducted to analyze the forces and pressures acting on the fetus as a result of maternal traumas and how these stresses might contribute to potential developmental abnormalities.
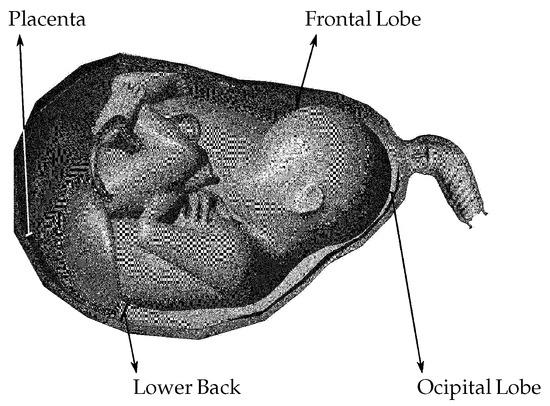
Figure 1.
An enhanced depiction of a fetus, encompassing the placenta and umbilical cord, situated within the uterus. Herein, designates the distance from the fetus’ frontal lobe (FL) to the closest point on the uterus (U), signifies the distance from the fetus’ occipital lobe (OL) to the uterus, represents the distance from the placenta (P) to the uterus, and indicates the distance from the fetus’ lower back (LB) to the uterus. These specified distances will be measured over time to enhance comprehension of the relative motion between the fetus/placenta and the uterus.
2.2. Numerical Methods
In this study, two main computational techniques, namely SPH and high-order FEM, were utilized to address the question of how a mother’s trauma could affect her developing baby. SPH is like a detailed and sophisticated simulation game for fluids. Imagine if we could follow each droplet of water in a pool to see how it moves and interacts with other droplets when someone dives in. SPH allows us to simulate this on a computer, but instead of a pool, we are studying the amniotic fluid inside the womb. Note that the SPH (fluid) particles are not physical entities like cells or atoms. Instead, they should be understood as massless points in a computational grid, each associated with an “energy” field. These particles “push” each other when their respective energy fields influence each other, thus simulating the behavior of a fluid. During a trauma, a mother’s movements can be quite severe, and we need to understand how this might create waves or ripples that could harm the fetus. High-order FEM is somewhat similar, but instead of droplets, think of it as a highly advanced version of a crash test analysis. Car manufacturers use it to study crash tests to understand how collisions affect the car and the people inside. FEM helps us simulate this for the fetus within the womb, allowing us to predict how different forces from a mother’s movements during a trauma can impact the baby’s body without having to actually expose the mother or child to such risks.
The study employs these two techniques together to create a validated virtual model that represents a mother and fetus during trauma. SPH provides detailed insights into the behavior of the protective amniotic fluid, whereas FEM offers a look at the potential physical responses of the fetus under such conditions. The utilization of these methods aims to enhance the understanding of fetal safety amidst maternal traumas, offering a realistic simulation of such an event while ensuring patient safety by avoiding the risks associated with real-world experimentation.
The following subsections offer comprehensive technical explanations of SPH and high-order FEM for those desiring an in-depth understanding of these methods’ implementation. While this detailed information enhances the discussion of the techniques employed, it is optional and intended for readers with a specific interest in the methodological nuances. Engaging with this level of detail is not a prerequisite for grasping the core results and implications of the research. Those who prefer to focus on the primary conclusions may proceed without reviewing the subsequent technical sections.
2.3. Advanced Methodological Insights
The fetus is constantly subjected to external loads, including the forces exerted by the uterine environment and maternal movements. The fetus has the ability to adapt and respond to these loads. Various simulation methods could be employed to investigate the dynamics of the amniotic fluid surrounding the developing fetus. Two predominant methodologies in this domain are SPH and the FEM. SPH, a computational technique frequently employed in the realm of fluid dynamics, enables intricate simulations of complex free surface flows. This approach yields substantial insights into the interplay between the fetus and the amniotic fluid under diverse conditions, making it particularly germane to biomedical research that necessitates an understanding of complex fluid–structure interactions within the maternal body. The integration of SPH and FEM provides versatility in addressing complex geometries and boundary conditions. This combination can effectively emulate the motion of the amniotic fluid within the actual maternal womb.
The methodology is articulated through three interrelated subsections below. The employed numerical approach is predicated on the utilization of governing equations. These equations are designed to address the deformations present in both the solid and fluid phases, as well as their mutual interactions. A geometrical model is developed to preserve the small-scale characteristics of a realistic fetus, which is encapsulated within a fluid domain inside the uterus. To simulate realistic conditions, boundary and initial conditions are prescribed, thereby subjecting the uterus to external loading.
2.3.1. Smoothed Particle Hydrodynamics
Smoothed Particle Hydrodynamics represents a computational methodology that is extensively employed within the discipline of fluid dynamics (Figure 2). This mesh-free approach is recognized for its proficiency in simulating intricate free surface flows and has been widely applied across numerous biomedical domains [13]. Its application is particularly prominent in the exploration of health conditions pertaining to the brain [14] and blood flow interacting with heart valves [15]. A significant advantage of utilizing SPH within biomedical research lies in its capacity to accurately model complex fluid–structure interactions occurring within the human body. Hence, similarly, in the context of maternal traumas and their impact on the fetus, SPH is a pivotal tool in advancing our understanding of the interaction between the fetus and the amniotic fluid under various loading conditions.
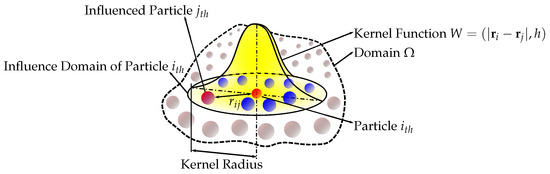
Figure 2.
Smoothed-particle hydrodynamics kernel approximation. Adapted from [16], MDPI, 2021.
In the case of traumatic fetal injury, for instance, SPH simulations can assist in exploring the dispersion of forces within the amniotic fluid and their influence on the fetus. This process involves considerable deformation and displacement of the fetal structures within the uterus, which can result in fetal injuries. This, in turn, has implications for various fetal regions, including the head, limbs, and essential organs. By offering a more nuanced representation of these intricate fluid–structure interactions, computational simulations yield significant insights into the fundamental mechanisms underlying fetal injuries. Such understanding is important in the development of advanced therapeutic strategies or preventive measures aimed at mitigating the effects of maternal traumas on fetal health.
2.3.2. Geometrical Model
The model comprises several distinct parts, as illustrated in Figure 1. Each part is assigned a unique set of material properties, derived from data available in existing literature [17,18,19]. The gap between the fetus and the uterus is populated with 16,731 fluid particles. The IMPETUS Afea SPH Solver (IMPETUS Afea AS, Flekkefjord, Norway) is utilized to compute fluid motion and boundary interactions, while the IMPETUS Afea Solver is employed to calculate the deformations in the solid elements.
The number of fluid particles, specifically 16,731, was determined based on a prescribed inter-particle distance. The smaller the inter-particle distance, the higher the number of fluid particles, and consequently, the higher the resolution of the simulation. The specific value of the inter-particle distance was chosen to ensure that the fluid domain was adequately resolved while keeping the computational cost manageable. To confirm the adequacy of the chosen inter-particle distance and the resulting number of fluid particles, a sensitivity study was conducted. In this study, the number of fluid particles was increased (i.e., the inter-particle distance was decreased), and the results of the simulation were compared. The aim was to ensure that the results of the simulation were not significantly affected by the number of fluid particles, indicating that the results had converged.
2.3.3. Boundary Conditions
The model in this study is designed to simulate the specific scenarios where a pregnant woman might be subjected to an external force during a traumatic event. In this particular case, the external force is an explosion, specifically mimicking a situation where a vehicle drives over a mine. This scenario was chosen as it represents a highly dangerous situation that could lead to severe trauma. The manikin used in the experiments was placed in a structure that closely resembles a vehicle. This setup was chosen to replicate as closely as possible the conditions inside a vehicle that is driving over a mine. The manikin’s placement and the structure’s design were both considered to ensure the accuracy of the simulation. These measurements are experimental and were collected by a private equity firm. Due to the proprietary nature of these data, the raw measurements are not available for sharing in this article. However, to provide a comprehensive understanding of the impact of these conditions, we have provided the normalized acceleration values. These normalized values, derived from the raw experimental data, provide a clear representation of the biomechanical effects under the given conditions. These values are presented in the Results section of this article.
2.4. Validation
In computational simulations, it is a common practice to validate the results on simpler models and experiments before applying them to more complex models. This approach is often used to gain confidence in the accuracy and reliability of the simulations and to ensure that the obtained results are consistent with known experimental data [20]. Simpler models and experiments are typically used as a starting point because they allow for easier analysis and understanding of the underlying phenomena. These simplified models often involve fewer variables, assumptions, and computational complexity, which makes them more manageable and computationally efficient.
During the validation process, the results of the simplified models are compared with experimental data or analytical solutions to assess their agreement. If the simplified models are able to accurately reproduce the observed behavior or trends, it provides confidence that the underlying principles and assumptions of the simulation approach are valid. Once the simpler models have been validated, the results can then be extrapolated or assumed to hold true for more complex models. This assumption is based on the understanding that the simplified models capture the essential aspects of the phenomenon being studied, and that the more complex models can be seen as extensions or refinements of the simpler models. This kind of validation has been conducted by the developers of IMPETUS Afea and can be found in their technical reports.
However, it is important to note that this assumption is not always justified, as more complex models may introduce additional complexities, interactions, or phenomena that were not present in the simplified models. Therefore, it is crucial to exercise caution and carefully consider the limitations and assumptions made when translating the results from simpler models to more complex ones. Nevertheless, in a previous study, we employed a complex geometric model of the skull and brain with the subarachnoid space filled with SPH particles. Hence, the same computational methodology was previously employed to simulate coup and contrecoup brain injuries [21]. In order to validate the accuracy of this approach and the associated geometrical model, our simulation results were juxtaposed with the experimental cadaver tests conducted by Nahum et al. [22]. These cadaveric tests involved the application of an impact impulse to the frontal lobe of human specimens, followed by the measurement of the ensuing pressure responses in the cerebrospinal fluid. These measurements were then compared with our computational simulations. A significant correlation was observed between our simulation results and the cadaver data pertaining to the coup response. Furthermore, we used it to model other interactions between biological fluids and structures within the human body, such as the dynamics of blood flow and its interaction with heart valves [15,23].
3. Results
In Figure 3, the colored lines represent the acceleration measurements obtained experimentally. The acquired experimental values are applied to the uterine model depicted in Figure 1. Subsequently, calculations are conducted using the IMPETUS Afea software (Engine v8.1.603) described above. In addition, Figure 3 provides a detailed representation of the relative displacement of the fetus/placenta in relation to the uterus during the type of movement typically observed in traumatic events. The graph displays normalized distances between the fetus/placenta and the uterus at four distinct points over a period of time. These measurements have been standardized based on their initial values prior to the onset of trauma (T = 0 ms). When the values surpass one, it indicates an expansion in the separation between the fetus/placenta and the uterus, while a value below one signifies a contraction in distance. Despite the manifestation of oscillatory acceleration patterns (also represented in the graphs), the variations in the distances between the fetus/placenta and the uterus remain relatively minor, a phenomenon attributed to the cushioning effect of the amniotic fluid.
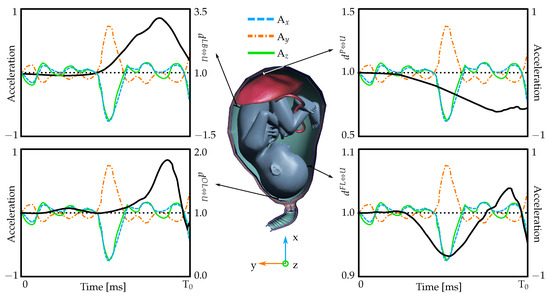
Figure 3.
Scaled distance values (scaled by the respective distances in the initial state, i.e., before the trauma) between the uterus and fetus/placenta in four different locations. When the solid black line exceeds the value of one, it represents an increase in the spatial separation between the uterus and the fetus or placenta. Conversely, a value less than one signifies a decrease in this distance in comparison to the initial measurement.
Succinctly speaking, the strain experienced by the fetus in response to external loads can be considered a form of deformation. Deformation refers to the change in the shape of an object in response to applied forces. In the case of the fetus, the strain distribution and behavior can be influenced by various factors, including the position of the fetus, the elasticity of the fetal tissues, the mechanical properties of the uterine environment, and the presence of amniotic fluid. Maternal exposure to external loading can induce sudden and intense movements in the uterus, leading to changes in the strain distribution on the surface of the fetus. These changes in strain can affect the fetal tissues and organs, potentially leading to adverse effects on fetal development. Figure 4a shows strain values on the fetus at three different time points. It can be seen that the maximum values are located on the frontal lobe of the fetus.
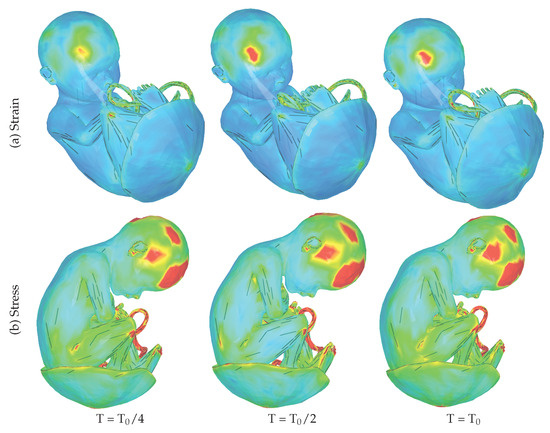
Figure 4.
(a) Strain: The series of images represent the fetus, overlaid with a color map that demonstrates the strain (deformation) experienced due to external loads at three distinct time points. These time points correspond to sudden and intense uterine movements triggered by maternal movement after exposure to external forces. The strain is dimensionless. (b) Stress: The series of images represent the fetus, overlaid with a color map that illustrates the 1st deviatoric principal stress experienced due to external loads at three distinct time points. The stress ranges from 0 MPa (blue) to 9 MPa (red).
It can be observed in Figure 4b that during maternal exposure to external forces, the stress distribution within the fetal tissues exhibits a rapid increase immediately following the initial peak in velocity and remains consistently elevated during subsequent velocity peaks. In this context, the values signify the magnitude and direction of stress acting within the fetal tissues during maternal exposure to external loading. It provides valuable insight into the distribution and intensity of mechanical forces experienced by the fetus under these conditions. Notably, the highest stress values are observed in specific regions, including the frontal lobe, occipital lobe, temporal and parietal lobes, as well as the umbilical cord, while the placenta is relatively unaffected. The observed localization of high stress values in critical regions of the fetal brain, as well as the umbilical cord, suggests that these structures are particularly vulnerable to the mechanical strain induced by maternal movement during external trauma.
Clinically, these findings have certain implications for fetal well-being. The high stress levels experienced by the frontal, occipital, temporal, and parietal lobes raise concerns regarding potential neurological consequences for the developing brain. The vulnerability of the umbilical cord to elevated stress levels further emphasizes the importance of careful monitoring and timely intervention to safeguard fetal circulation and oxygenation. However, the absence of higher stress on the placenta suggests that it may possess adaptive mechanisms or structural characteristics that confer resilience against the mechanical forces exerted during maternal traumas. This finding underscores the placenta’s vital role in protecting the fetus from potential harm. Nevertheless, the placenta, being the interface between the mother and fetus, is always susceptible to trauma. However, our model specifically simulates a scenario where the fetus is in the cephalic position (head down) and the trauma originates from an explosion under the car. In this particular situation, the initial impact is directed toward the fetus’ head.
4. Discussion
Amniotic fluid plays a vital role in fetal growth and development. Broek et al. (2013) investigated the relationship between amniotic fluid deficiency and congenital abnormalities, focusing on fluctuating asymmetry in developing limbs [24]. The study found that fetuses with severe amniotic fluid deficiencies had higher asymmetry in limb development compared to fetuses with sufficient amniotic fluid volumes. The researchers suggested that low amniotic fluid levels increase mechanical pressure, leading to greater asymmetry in limb development. This highlights the importance of amniotic fluid as a protective buffer for the fetus, reducing the risk of compression between the fetus and the uterine wall. The study also suggests that amniotic fluid disorders, such as oligohydramnios and anhydramnios, can influence fluctuating asymmetry in fetuses. Amniotic fluid volume serves as an important diagnostic tool for assessing fetal well-being. Dubil and Magann (2013) reviewed the diagnostic use of amniotic fluid volume in indicating fetal well-being [25]. The paper discussed amniotic fluid dynamics, methods for measuring and quantifying amniotic fluid, and various amniotic fluid disorders, including oligohydramnios and polyhydramnios. Understanding amniotic fluid volume and its significance in fetal well-being is crucial for monitoring and managing pregnancies effectively [26]. In 2005, Ott published a reassessment of the association between the volume of amniotic fluid and perinatal outcomes [27]. The primary objective of his research was to intensify the scrutiny of the connection between the Amniotic Fluid Index (AFI) and complications in neonates. However, the research team did not discover any substantial correlation between AFI and neonatal complications, nor between AFI and the duration of stays at the neonatal intensive care unit. The conclusion drawn from the study was that the AFI did not have a significant correlation with perinatal outcomes. This suggests that disorders related to amniotic fluid do not have a direct bearing on the health of a neonate.
Despite its importance, the topic of amniotic fluid and its disorders remains relatively poorly understood. Problems related to the amniotic fluid during pregnancy are still being studied. Just recently, researchers looked at cases where there was either too much or too little amniotic fluid [28,29]. They wanted to identify factors that could increase the risk of these issues and see how they affected both the mother and the baby [30]. They found that a significant number of women had either too much or too little amniotic fluid. Factors such as the mother’s weight and diabetes were linked to these problems, while age, education, and the number of previous pregnancies were not. They also found that the amount of amniotic fluid affected when and how the baby was born, but it did not directly impact the baby’s health. The study highlights the need for more research with larger groups of women to better understand amniotic fluid problems and their effects on pregnancy.
Despite the protective role of the amniotic fluid, various external events can induce physiological changes and affect the fetus in different ways. The specific impacts may vary based on factors such as the type and intensity of external trauma, and the fetus’ position within the womb, aspects that are not explored in this study. In essence, this research underscores the mitigating role of the amniotic fluid in shielding the fetus during certain external boundary conditions and showcases the potential of computational simulations in shedding light on amniotic fluid dynamics and its contribution to fetal protection.
While the amniotic fluid plays a crucial role in protecting the fetus by acting as a cushion and mitigating the effects of sudden movements, it has limitations in shielding the fetus when faced with violent and rapid traumatic events that can occur during traumas. These biomechanical stresses and the potential displacement resulting from trauma can influence the overall well-being of the fetus, thus emphasizing the importance of managing traumas effectively during pregnancy and the need for further research on protective mechanisms for high-impact situations.
Our findings indicate that maternal traumas can exert significant biomechanical stress on the developing fetus, with particular emphasis on contact pressure, shear stress, and physical displacement within the amniotic fluid environment. Despite the protective role of amniotic fluid, the intensity and rapid succession of traumatic forces during traumas suggest a potential for adverse fetal outcomes. This underscores an urgent need for improved therapeutic interventions aimed at mitigating these risks.
Furthermore, our study highlights the intricate interplay between the protective properties of the amniotic fluid and the dynamics of maternal traumas, pointing to a paucity of research in this domain. New strategies are required to enhance fetal safety in expectant mothers, which may include advancements in prenatal care. The need for an interdisciplinary approach combining obstetrics, neurology, and pediatric care is evident, and our work contributes to the body of knowledge that will inform future research directions and clinical practices.
The limitations of the approach taken in this study using simulations to investigate the effects of maternal traumas on fetal dynamics could include the following. The computational models, even though advanced, may not fully capture the complex physical interactions between the amniotic fluid and the fetus during actual trauma events. Simulations are based on experimentally acquired boundary conditions, which might have limitations in their accuracy or may not cover all possible scenarios experienced in clinical settings. The study is constrained by the paucity of similar published research, which limits the ability to validate findings against a broad spectrum of existing studies. While the models provide insight into stress distribution and fetal displacement, they cannot fully predict clinical outcomes or account for biological variability among individual pregnancies. This study points to the need for further research to assess the efficacy of protective mechanisms during traumas in pregnancy, indicating an awareness that the current understanding and available solutions are limited. Hence, despite the limitations, the study represents a step forward in understanding the biomechanical effects of maternal traumas on fetal dynamics and sets the stage for future research and potential clinical applications.
Given the established significance of amniotic fluid volume as both a vital sign of fetal health and a determinant of perinatal outcomes, it is imperative to further explore the repercussions of maternal traumas and the resulting biomechanical stresses on the developing fetus. The application of advanced computational methods, such as those employed in this study, offers a promising avenue for such research. These methods could serve as sophisticated tools to simulate and analyze the complex fluid–structure interactions that occur during maternal traumas, thereby illuminating potential risks and informing targeted interventions that may mitigate adverse effects on fetal development. The advancement of this research is critical for improving outcomes in pregnancies complicated by maternal neurological conditions and amniotic fluid disorders. For example, the substantial role of amniotic fluid in fetal development and the intricate relationship between its volume and congenital abnormalities highlight an area of prenatal care that needs further investigation. By leveraging the computational techniques demonstrated in this study, future research could focus on the biomechanical impact of amniotic fluid deficiency on fetal morphology. These methods, capable of replicating the nuanced fluid–structure interactions within the uterine environment during maternal traumas, could illuminate the nuances of fetal development compromised by fluid imbalances. The continued development and application of such computational models could provide invaluable insights into the prevention and management of congenital abnormalities resulting from amniotic fluid disorders, thus refining the standards of obstetric care.
5. Conclusions
In summing up this study, the following points encapsulate our key findings, their implications, and the path forward.
- Biomechanical risk and vulnerability: Our study underscores the significant biomechanical risk that traumas pose to the fetus, particularly in specific regions such as posterior areas and the umbilical cord. Despite the substantial protective capacity of amniotic fluid, intense trauma events can still cause stress and displacement within the uterine environment.
- Implications for research and clinical practice: The insights gained from our simulation techniques, including SPH and FEM, emphasize the need for further research to enhance protective measures for the fetus during trauma events. This research has clinical implications, suggesting a need for personalized care strategies and potential revisions in the management of traumas in pregnant women, thereby advocating for increased awareness and education among healthcare providers.
The posterior region of the fetus, specifically the frontal lobe, is found to be particularly susceptible, likely due to its proximity to the hard surface of the mother’s spine. Conversely, areas of the fetus that come into contact with softer surfaces, such as the anterior or superior regions, are cushioned by both the amniotic fluid and the mother’s internal organs. The model, while advanced, does not currently include spinal bones for the fetus, indicating an area for future improvement. The findings underscore the importance of considering the fetal position at different stages of pregnancy, as it can significantly impact the outcomes of a trauma event. These insights pave the way for more patient-specific and effective surgical planning in obstetrics.
Our aim is to integrate this model seamlessly into existing clinical workflows, serving as a predictive tool for evaluating the impact of maternal trauma on fetal dynamics. It also serves as a valuable training resource for medical professionals. A key feature of our approach is its emphasis on data privacy and ethical considerations. All computations can be performed in-house, eliminating the need for data to be transferred outside of the hospital premises or for online access. This ensures the highest level of protection for patient information. The goal is to refine these techniques for routine use, fostering a learning environment for healthcare professionals, and maintaining high ethical standards in prenatal care.
Author Contributions
Conceptualization, A.D.A., M.B., J.M., T.D., K.N., R.C.-A. and M.T.; methodology, A.D.A., M.B., J.M., T.D., K.N., R.C.-A. and M.T.; software, A.D.A., M.B., J.M., T.D., K.N., R.C.-A. and M.T.; validation, A.D.A., M.B., J.M., T.D., K.N., R.C.-A. and M.T.; formal analysis, A.D.A., M.B., J.M., T.D., K.N., R.C.-A. and M.T.; investigation, A.D.A., M.B., J.M., T.D., K.N., R.C.-A. and M.T.; resources, A.D.A., M.B., J.M., T.D., K.N., R.C.-A. and M.T.; data curation, A.D.A., M.B., J.M., T.D., K.N., R.C.-A. and M.T.; writing—original draft preparation, A.D.A., M.B., J.M., T.D., K.N., R.C.-A. and M.T.; writing—review and editing, A.D.A., M.B., J.M., T.D., K.N., R.C.-A. and M.T.; visualization, M.T.; supervision, M.T.; project administration, M.T.; funding acquisition, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest. Author R.C.A. is currently employed by Pfizer, but at the time of her involvement with this study, she was employed by the New York Presbyterian Queens Hospital. No funders, companies, or institutions had any role in the design of the study, the collection, analyses, or interpretation of data, the writing of the manuscript, or the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| SPH | Smoothed-Particle Hydrodynamics |
| FEM | Finite Element Method |
| BLE | Bluetooth Low Energy |
| SDK | Software Development Kit |
| IMU | Imperial Measurement Unit |
| AFI | Amniotic Fluid Index |
References
- Sakamoto, J.; Michels, C.; Eisfelder, B.; Joshi, N. Trauma in Pregnancy. Emerg. Med. Clin. N. Am. 2019, 37, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Petrone, P.; Jiménez-Morillas, P.; Axelrad, A.; Marini, C.P. Traumatic injuries to the pregnant patient: A critical literature review. Eur. J. Trauma Emerg. Surg. 2017, 45, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Lucia, A.; Dantoni, S.E. Trauma Management of the Pregnant Patient. Crit. Care Clin. 2016, 32, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Al-Thani, H.; El-Menyar, A.; Sathian, B.; Mekkodathil, A.; Thomas, S.; Mollazehi, M.; Al-Sulaiti, M.; Abdelrahman, H. Blunt traumatic injury during pregnancy: A descriptive analysis from a level 1 trauma center. Eur. J. Trauma Emerg. Surg. 2018, 45, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Krywko, D.M.; Toy, F.K.; Mahan, M.E.; Kiel, J. Pregnancy Trauma. [Updated 12 September 2022]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430926/ (accessed on 14 August 2024).
- Stokes, S.C.; Rubalcava, N.S.; Theodorou, C.M.; Bhatia, M.B.; Gray, B.W.; Saadai, P.; Russo, R.M.; McLennan, A.; Bichianu, D.C.; Austin, M.T.; et al. Recognition and management of traumatic fetal injuries. Injury 2022, 53, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Genc, S. Trauma in pregnancy: An analysis of the adverse perinatal outcomes and the injury severity score. Turk. J. Trauma Emerg. Surg. 2023, 29, 1039–1050. [Google Scholar] [CrossRef]
- Santoso, G.; Ammarullah, M.I.; Sugiharto, S.; Rachayu, R.M.; Mughni, A.; Bayuseno, A.P.; Jamari, J. Von Mises stress analysis of surgery chair designed for laparoscopic surgeon with lifting mechanism. AIP Adv. 2024, 14, 045104. [Google Scholar] [CrossRef]
- Santoso, G.; Ammarullah, M.I.; Sugiharto, S.; Hidayat, T.; Khoeron, S.; Bayuseno, A.P.; Jamari, J. TRIZ-based method for developing a conceptual laparoscopic surgeon’s chair. Cogent Eng. 2024, 11, 1. [Google Scholar] [CrossRef]
- Arias, J.; Kurgansky, G.; Wei, O.C.; Chan-Akeley, R.; Toma, M. fluid–structure interaction analysis of amniotic fluid with fetus and placenta inside uterus exposed to military blasts. Injury 2023, 54, 110843. [Google Scholar] [CrossRef]
- Shi, L.; Myers, K. A finite porous-viscoelastic model capturing mechanical behavior of human cervix under multi-step spherical indentation. J. Mech. Behav. Biomed. Mater. 2023, 143, 105875. [Google Scholar] [CrossRef]
- Veljovic, F.; Straus, S.; Karabdic, I.; Masic, I. Spinal Column and Abdominal Muscles Loading in Pregnant Women Dependent on Working Postures. Acta Inform. Medica 2019, 27, 54–57. [Google Scholar] [CrossRef]
- Toma, M. The Emerging Use of SPH in Biomedical Applications. Significances Bioeng. Biosci. 2017, 1, 1. [Google Scholar] [CrossRef]
- Toma, M.; Dehesa-Baeza, A.; Chan-Akaley, R.; Nguyen, P.D.H.; Zwibel, H. Cerebrospinal Fluid Interaction with Cerebral Cortex during Pediatric Abusive Head Trauma. J. Pediatr. Neurol. 2020, 18, 223–230. [Google Scholar] [CrossRef]
- Toma, M.; Singh-Gryzbon, S.; Frankini, E.; Wei, Z.A.; Yoganathan, A.P. Clinical Impact of Computational Heart Valve Models. Materials 2022, 15, 3302. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.; Chan-Akeley, R.; Arias, J.; Kurgansky, G.D.; Mao, W. Fluid–Structure Interaction Analyses of Biological Systems Using Smoothed-Particle Hydrodynamics. Biology 2021, 10, 185. [Google Scholar] [CrossRef]
- Koh, C.T.; Tonsomboon, K.; Oyen, M.L. Fracture toughness of human amniotic membranes. Interface Focus 2019, 9, 20190012. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Liu, S.; Jin, X.; Mao, H.; Zhu, F.; Saif, T.; Zhou, R.; Fan, H.; Begeman, P.C.; Chou, C.C.; et al. Porcine growth plate experimental study and estimation of human pediatric growth plate properties. J. Mech. Behav. Biomed. Mater. 2020, 101, 103446. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Liu, X.; Wu, Y.; Qian, L.; Huang, W.; Li, Y. Comparison of the Biomechanical Properties between Healthy and Whole Human and Porcine Stomachs. Bioengineering 2024, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Abd Aziz, A.U.; Ammarullah, M.I.; Ng, B.W.; Gan, H.S.; Abdul Kadir, M.R.; Ramlee, M.H. Unilateral external fixator and its biomechanical effects in treating different types of femoral fracture: A finite element study with experimental validated model. Heliyon 2024, 10, e26660. [Google Scholar] [CrossRef]
- Toma, M.; Nguyen, P.D.H. Coup-contrecoup brain injury: Fluid–structure interaction simulations. Int. J. Crashworthiness 2019, 25, 175–182. [Google Scholar] [CrossRef]
- Nahum, A.M.; Gatts, J.D.; Gadd, C.W.; Danforth, J. Impact Tolerance of the Skull and Face. In SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1968. [Google Scholar] [CrossRef]
- Toma, M.; Einstein, D.R.; Kohli, K.; Caroll, S.L.; Bloodworth, C.H.; Cochran, R.P.; Kunzelman, K.S.; Yoganathan, A.P. Effect of Edge-to-Edge Mitral Valve Repair on Chordal Strain: Fluid–structure Interaction Simulations. Biology 2020, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- ten Broek, C.M.A.; Bots, J.; Varela-Lasheras, I.; Bugiani, M.; Galis, F.; Van Dongen, S. Amniotic Fluid Deficiency and Congenital Abnormalities both Influence Fluctuating Asymmetry in Developing Limbs of Human Deceased Fetuses. PLoS ONE 2013, 8, e81824. [Google Scholar] [CrossRef]
- Dubil, E.A.; Magann, E.F. Amniotic fluid as a vital sign for fetal wellbeing. Australas. J. Ultrasound Med. 2013, 16, 62–70. [Google Scholar] [CrossRef]
- Huri, M.; Di Tommaso, M.; Seravalli, V. Amniotic Fluid Disorders: From Prenatal Management to Neonatal Outcomes. Children 2023, 10, 561. [Google Scholar] [CrossRef]
- Ott, W.J. Reevaluation of the relationship between amniotic fluid volume and perinatal outcome. Am. J. Obstet. Gynecol. 2005, 192, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Raghu, P.; Kennedy, A.M.; Sugi, M.; Morgan, T.A.; Feldstein, V.; Pōder, L.; Penna, R. Assessment of Amniotic Fluid Volume in Pregnancy. RadioGraphics 2023, 43, 1. [Google Scholar] [CrossRef]
- Simmons, P.M.; Whittington, J.R.; Estrada, S.M.; Ounpraseuth, S.T.; Shnaekel, K.B.; Slaton, K.B.; Magann, E.F. What is the Impact of Abnormal Amniotic Fluid Volumes on Perinatal Outcomes in Normal Compared with At-Risk Pregnancies? Int. J. Women’s Health 2020, 12, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Palermo, M.S.F.; Espinosa, A.; Trasmonte, M. Disorders of Amniotic Fluid Volume: Oligoamnios and Polyhydramnios. In Perinatology; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 687–705. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).