Abstract
Background: Inflammation has long been a key tenet in the diagnosis and management of malignancies, likely contributing to cancer incidence, staging, and progression. Systemic inflammation, in particular, is often elevated prior to and during cancer development. Systemic inflammation in the context of cancer diagnosis and monitoring is measured by various inflammatory indexes such as the systemic inflammatory response index (SIRI), plasma-to-lymphocyte ratio (PLR), systemic immune inflammation index (SII), and neutrophil-to-lymphocyte ratio (NLR). We set out to determine the relationship between pre- and post-treatment levels of these inflammatory indexes and the prognosis and outcomes of oropharyngeal cancer (OPC). Methods: A retrospective chart review was performed of 172 patients with OPC who underwent treatment for oropharyngeal cancer at University Medical Center in Lubbock, TX between May 2013 to May 2023. Sites of primary cancer were obtained through chart review. HPV infection status and differentiation of the tumor were noted for each patient. Treatment modalities were classified as surgery, radiation, chemotherapy, or concurrent chemotherapy and radiation. Treatment outcomes were classified based on recurrence and death secondary to disease. The relationships between treatment outcome and the described inflammatory indexes were evaluated. Appropriate parametric tests were selected based on the large number of variables. Results: Pre-treatment SIRI and Albumin levels were positively predictive in determining locoregional recurrence (p = 0.031 and p = 0.039). NLR, SII, and SIRI levels taken at three months post-treatment were also found to be positively predictive of locoregional recurrence (p = 0.005, p < 0.0005, and p = 0.007). SIRI taken at six months post-treatment was also found to be positively predictive of locoregional recurrence (p = 0.008). SII at six months post-treatment was found to be positively predictive of survival (p = 0.027). Conclusion: This study suggested that post-treatment levels of several inflammatory indexes, particularly SIRI, NLR, and SII, may be useful in determining the long-term outlook and recurrence of head and neck cancer following treatment.
1. Introduction
Inflammation has long been a key tenet in the diagnosis and management of malignancies, likely contributing to cancer incidence, staging, and progression [1,2]. Systemic inflammation, in particular, is often elevated prior to and during cancer development [1]. Systemic inflammation in the context of cancer diagnosis and monitoring is measured by various inflammatory indexes such as the systemic inflammatory response index (SIRI), plasma-to-lymphocyte ratio (PLR), systemic immune inflammation index (SII), and neutrophil-to-lymphocyte ratio (NLR). There is robust literature showing the elevation of various inflammatory indexes in different cancers.
There have been several studies in the literature examining the relationship between the aforementioned inflammatory indexes and various cancer-related outcomes. The neutrophil-to-lymphocyte ratio (NLR) has been shown in several studies to be a biomarker associated with decreased survival and prognosis in several solid tumor cancers [3]. Higher NLR has been associated with increased tumor growth and prognosis in various cancers of the gastrointestinal system [4,5,6]. Elevated NLR has also been shown to be an indicator of poor prognosis in squamous cell carcinoma of the head and neck [7]. Elevated platelet-to-lymphocyte ratios (PLR) have also been correlated with poor prognosis in a host of different cancers, including melanoma, rectal, and hepatocellular carcinoma [6,8,9].
SIRI is an inflammatory index that is based on the ratio of neutrophil, monocyte, and lymphocyte count [10]. Several studies have shown that SIRI is an important inflammatory index in the progression of many tumors [10]. It was shown to be an independent prognosis indicator in gallbladder cancer [11]. It was also shown to be a prognostic biomarker in head and neck squamous cell carcinoma [12].
An elevated systemic immune inflammation index (SII) indicates poor prognosis in patients treated with immune checkpoint inhibitor immunotherapy [13]. Elevated SII has been associated with poor prognosis in several malignancies, including bladder and non-small cell lung cancer [14,15]. Oropharyngeal cancer is one of the most common malignancies worldwide and the 7th leading cause of cancer deaths worldwide [16]. OPC is associated with tobacco use, alcohol consumption, and human papillomavirus (HPV) [16]. Additionally, OPC, particularly the HPV-associated variant, has increased in the United States [16]. The literature regarding the association of the various inflammatory indexes and prognosis of various malignancies is quite robust; however, oropharyngeal cancer (OPC) has been sparsely explored in this area. Furthermore, exploring and comparing which inflammatory indexes are the best determinates of outcome and prognosis in OPC is an emerging area in the literature we set out to explore.
We set out to determine the relationship between the SIRI, PLR, SII, and NLR inflammatory indexes and the prognosis and outcomes of OPC. Additionally, we wanted to determine which indexes are the most significant prognosis indicators of OPC. We conducted a single institution retrospective chart review of 172 patients with OPC and evaluated their inflammatory index levels.
2. Methodology
With approval from the Texas Tech University Health Sciences Center Internal Review Board, a retrospective chart review identified 172 patients who underwent treatment for oropharyngeal cancer from 9 May 2013 to 9 May 2023. Patients who did not have any available pre-treatment and post-treatment blood work to analyze and those who did not have greater than three months of follow-up post-treatment were excluded.
Basic demographic information, such as gender, age, BMI, and ethnicity, as well as identified risk factors, such as smoking and alcohol use, were gathered (Table 1). Sites of primary cancer were obtained through chart review, which was classified as tonsil, base of tongue, soft palate, posterior, and overlapping. Furthermore, HPV infection status (positive, negative, n/a) and differentiation of the tumor (well, moderate, poor, undifferentiated) were noted for each patient. Tumor staging was recorded utilizing the 7th and 8th editions of oropharyngeal cancer staging, according to the American Cancer Society. Treatment intent was tabulated as either curative or palliative therapy, with the treatment modalities classified as surgery, radiation, chemotherapy, or concurrent chemotherapy and radiation. Patients who received adjuvant treatments were also noted. Post-treatment margin status was either negative, positive, or n/a, and treatment outcomes were classified based on recurrence and death secondary to disease. If recurrence was observed, the location was noted as either the primary site, neck, both, or not specified. The relationships between treatment outcome and pre- and post-treatment levels of inflammatory indexes such as systemic inflammatory response index (SIRI), plasma-to-lymphocyte ratio (PLR), systemic immune inflammation index (SII), and neutrophil-to-lymphocyte ratio (NLR) were evaluated.

Table 1.
Patient Demographics.
Statistical analysis was performed using SPSS (Version 23, IBM, Armonk, NY, USA). Multiple testing was used based on the large number of variables. Descriptive statistics were performed by independent t-tests and the Mann–Whiney U-test for mean comparisons of variables with two groupings. A one-way ANOVA (analysis of variance) test was utilized for variables with groupings of three or more. Chi-squared and Fisher’s exact tests were used to analyze categorical variables. Univariate survival estimates were generated by the Kaplan–Meier method and compared with the log-rank test. Multivariate survival analysis was performed using the Cox proportional hazard regression model, utilizing all variables approaching significance (p < 0.2) to control for confounding covariates. All tests were two-tailed, and results were considered significant for p = 0.05.
3. Results
The initial screening revealed 263 patients, and 91 were excluded from the study because of either lack of blood work or appropriate follow-up. As shown in Table 1, the demographics for the final cohort included 172 patients, 79.1% (n = 136) male and 20.9% (n = 36) female. Most patients identified as Caucasian (86%, n = 148), and 2.9% (n = 5) identified as black. A total of 46.5% (n = 80) of the patients had a smoking history of more than ten pack-years, 14% (n = 24) less than 10, and 39.5% (n = 68) had no previous smoking history. Regarding alcohol history, 48.3% (n = 83) denied any alcohol use, while 30.8% (n = 53) drank socially and 20.9% (n = 36) drank heavily. The most common site for oropharyngeal cancer was BOT, at 44.2% (n = 76), followed by the tonsil (27.9%, n = 48), overlapping (16.3%, n = 28), posterior (6.4%, n = 11), and soft palate (5.2%, n = 9). HPV status was divided into positive (37.7%, n = 66), negative (30.3%, n = 53), and n/a (30.3%, n = 53), and differentiation status into well (6.4%, n = 11), moderate (36.0%, n = 62), poor (27.3%, n = 47), and undifferentiated (30.2%, n = 52).
Tumor staging was divided according to the 7th and 8th editions of the American Cancer Society, as shown in Table 2. Table 3 describes the treatment intent and outcomes, which showed that for 94.2% (n = 162) of patients, the goal of treatment was curative, with surgery (57.6%, n = 99) employed as the most common primary treatment, followed by chemotherapy and radiation combined (39.0%, n = 67). Chemotherapy and radiation combined (50%, n = 86) was the most common adjuvant treatment. Following treatment, 23.8% (n = 41) of the total patients had recurrence of disease, with local recurrence at the neck being the most common (35%, n = 14), followed by the primary site (32.5%, n = 13), both (25%, n = 10), and unspecified (7.5%, n = 3). In total, 59 patients (34.3%, n = 59) died, and of those, 26 patients (44.1%, n = 26) died due to disease.

Table 2.
Tumor Stages.

Table 3.
Treatments and oncologic outcomes.
Table 4 shows the pre-treatment, three-month, and six-month mean values of the inflammatory markers SIRI, PLR, SII, NLR, and PNI values divided according to sex, ethnicity, smoking, and alcohol history, location of the tumor, HPV status, tumor differentiation, AJCC staging (7th and 8th edition), treatment intent, primary treatment, adjuvant treatment, margin status, recurrence, location of recurrence, and death. Of these values, we found that pre-treatment SIRI and Albumin levels were positively predictive in determining locoregional recurrence (p = 0.031 and p = 0.039). NLR, SII, and SIRI levels taken at three months post-treatment were also found to be positively predictive of locoregional recurrence (p = 0.005, p < 0.0005, and p = 0.007), as well as SIRI taken at six months post-treatment (p = 0.008). Lastly, SII at six months post-treatment was positively predictive of survival (p = 0.027). Figure 1, Figure 2, Figure 3 and Figure 4 summarize survival outcomes on Kaplan–Meier Curves. Decreased inflammatory markers are associated with improved survival outcomes on Kaplan–Meier Curves. However, only SII at six months post-treatment value was statistically significant on the Cox proportional hazard test (p = 0.027).

Table 4.
Pre-treatment and post-treatment values of SIRI, PLR, SII, NLR, and PNI.
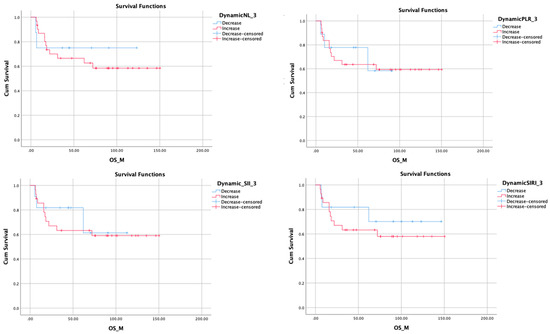
Figure 1.
Kaplan–Meier plots showing overall survival outcomes related to 3 months changes of related inflammatory markers. Survival favors a decrease in inflammatory markers.
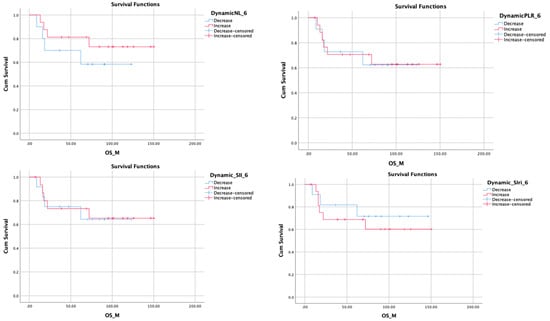
Figure 2.
Kaplan–Meier plots showing Overall Survival Outcomes related to 6 months Inflammatory marker changes.
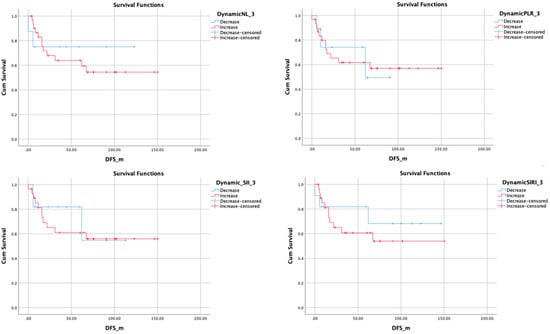
Figure 3.
Kaplan–Meier plots showing Disease Free Survival related to 3 months changes of related inflammatory markers.
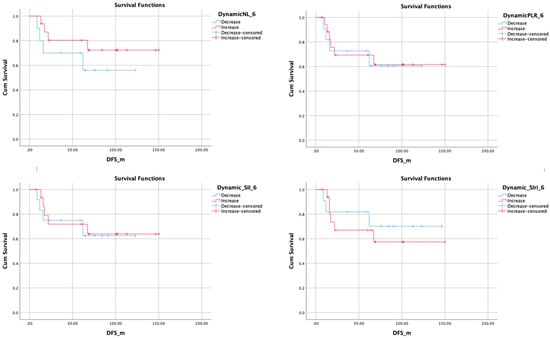
Figure 4.
Kaplan-Meier plots showing disease-free survival related to 6 months changes of related inflammatory markers.
4. Discussion
This retrospective project aimed to evaluate the role the inflammatory indexes, SIRI, PLR, SII, NLR, and PNI have on head and neck cancer prognosis. These inflammatory indexes have been well described in the literature in the context of cancer incidence and prognosis [1,2,8]. However, there has not been a definitive consensus regarding their role in head and neck cancer [17]. Other effective prognosis indicators in head and neck cancer include TNM (Tumor, Node, Metastasis) staging and HPV-positive status of head and neck cancer [18].
SIRI is calculated by multiplying neutrophils by the ratio of monocytes/lymphocytes [12]. SIRI has been shown to be an independent predictor of disease-specific survival in head and neck squamous cell carcinoma [12]. SIRI was also shown to be a predictor of local, regional, and distant recurrence-free survival [12]. It is seen as an effective prognosis indicator in head and neck cancers [18]. On that note, our findings on SIRI were particularly robust regarding locoregional recurrence. We found that pre-treatment SIRI was positively predictive in determining locoregional recurrence (p = 0.031). SIRI taken at three months post-treatment was also found to be positively predictive of locoregional recurrence (p = 0.007). Finally, SIRI taken at six months post-treatment was also found to be positively predictive of locoregional recurrence (p = 0.008). This is consistent with the previous literature stating that pre-treatment SIRI levels are a predictor of local and regional recurrence-free survival [12]. This study builds on the existing literature by showing that SIRI taken at three-month and six-month intervals after treatment was significantly predictive of locoregional recurrence.
SII is calculated by multiplying platelet count by neutrophil count/lymphocyte count [19]. SII has been used as a prognosis predictor in several cancers, including cervical, colorectal, bladder, lung, and liver cancer [19]. A high SII in head and neck cancer patients prior to receiving treatment has been shown to be predictive of decreased overall survival and more advanced tumor status [12]. Our results did not show the significance of the prognosis effectiveness in pre-treatment levels of SII. However, there was significance using post-treatment SII levels in both recurrences, which was taken at three months post-treatment (p < 0.0005), and, survival which was taken at six months post-treatment (p = 0.027).
NLR, which is the neutrophil-to-lymphocyte ratio, and PLR, which is the platelet-to-lymphocyte ratio, are inflammatory markers that have been shown to have prognostic value in several cancers [20]. Higher pre-treatment PLR and NLR values are associated with poor survivability in pancreatic, gastrointestinal, bladder, and ovarian cancers [20]. Elevated pre-treatment PLR and NLR have been associated with poor survivability in head and neck cancer patients, regardless of treatment modality (surgery or chemotherapy) [20]. NLR levels taken at three months post-treatment were also found to be positively predictive of locoregional recurrence (p = 0.005). Again, we did not find significance with the pre-treatment levels of NLR, which most of the existing literature focuses on. We did, however, find significance in the three-month post-treatment NLR level. It should be noted that we did not find significance in both pre-treatment and post-treatment levels of NLR.
Finally, PNI, the prognostic nutritional index, is calculated from the serum albumin concentration and the total lymphocyte count in the peripheral blood [21]. A low PNI has been shown to be associated with worse overall survival in patients with esophageal cancer [21]. Furthermore, a low PNI has been shown to be significantly associated with poor overall survival in patients with head and neck cancer, although it was not a significant indicator of disease-specific survival [22].
The limitations of this study are that it is a single-center retrospective chart review with only 172 patients. Our study was underpowered to find significance for pre-treatment levels of several of the inflammatory indexes in locoregional recurrence and overall survival. Despite these limitations, this study signifies a notable addition to the literature, displaying the value of post-treatment levels of inflammatory indexes as a prognosis and recurrence indicator in head and neck cancer.
This study built on the existing literature showing the value of inflammatory indexes as a prognosis indicator in head and neck cancer. Much of the literature has focused on utilizing pre-treatment levels of inflammatory indexes; this study uniquely examined both pre-treatment and post-treatment values (measured at three- and six-month intervals). This study also demonstrates the value of using inflammatory indexes to evaluate locoregional tumor recurrence in head and neck cancer. Our study indicates that SIRI taken at both three and six months post-treatment and NLR and SII at three months post-treatment were found to be predictive of locoregional recurrence. Other findings include that SII was the only inflammatory index to show a significant correlation with post-treatment levels and overall survival. Overall, we have uniquely highlighted the value post-treatment inflammatory indexes may have as prognosis and locoregional indicators for head and neck cancer.
5. Conclusions
This study suggested that post-treatment levels of several inflammatory indexes, particularly SIRI, NLR, and SII, may be useful in determining the long-term outlook and recurrence of head and neck cancer following treatment. Specifically, SIRI taken at both three and six months post-treatment and NLR and SII at three months post-treatment were found to be predictive of locoregional recurrence. Furthermore, SII was the only inflammatory index to show a significant correlation with post-treatment levels and overall survival. This builds on the existing literature that primarily focuses on pre-treatment levels of inflammatory indexes and their prognosis value for overall survival. Additionally, it highlights the need for future studies to evaluate further the role post-treatment inflammatory index levels have on future recurrence and overall prognosis of head and neck cancer.
Author Contributions
Conceptualization, Y.D.; Methodology, Y.D.; Validation, Y.D.; Formal analysis, Y.D.; Investigation, W.J. and J.F.Z.; Data Curation, D.K.N., I.S.M., A.S. and H.W.; Original draft preparation, W.J. and J.F.Z.; Review and editing, W.J., J.F.Z. and Y.D.; Supervision, Y.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was exempt from formal IRB review by the Institutional Review Board for the protection of human subjects by Texas Tech Health Science Center Lubbock/Odessa IRB. IRB number: L20-102. This research meets the criteria for exemption from formal review by the IRB, in accordance with 45 CFR 46.104(d)(4)(iii). Information required to make this determination has been provided by the investigator. A waiver of individual HIPAA authorization has been requested and found to be appropriate. This application was screened for exempt status according to TTUHSC policies and the provisions of applicable federal regulations. The study was found not to require formal IRB review because the research falls into one of the categories specifically designated as exempt per 45 CFR 46.104(d).
Informed Consent Statement
No Informed Consent was required due to the retrospective nature of the study.
Data Availability Statement
Dataset is available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nøst, T.H.; Alcala, K.; Urbarova, I.; Byrne, K.S.; Guida, F.; Sandanger, T.M.; Johansson, M. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur. J. Epidemiol. 2021, 36, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Li, J.; Yao, X.; Zhang, X.; Liu, G.; Zhang, Z.; Weng, S. Prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer liver metastasis: A meta-analysis of results from multivariate analysis. Int. J. Surg. 2022, 107, 106959. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, R.; Inagawa, S.; Sano, N.; Tadano, S.; Adachi, S.; Yamamoto, M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur. J. Surg. Oncol. 2018, 44, 607–612. [Google Scholar] [CrossRef]
- Schobert, I.T.; Savic, L.J.; Chapiro, J.; Bousabarah, K.; Chen, E.; Laage-Gaupp, F.; Tefera, J.; Nezami, N.; Lin, M.; Pollak, J.; et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of tumor response in hepatocellular carcinoma after DEB-TACE. Eur. Radiol. 2020, 30, 5663–5673. [Google Scholar] [CrossRef] [PubMed]
- Ferrandino, R.M.; Roof, S.; Garneau, J.; Haidar, Y.; Bates, S.E.; Park, Y.H.A.; Bauml, J.M.; Genden, E.M.; Miles, B.; Sigel, K. Neutrophil-to-lymphocyte ratio as a prognostic indicator for overall and cancer-specific survival in squamous cell carcinoma of the head and neck. Head. Neck. 2020, 42, 2830–2840. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, P.; Liu, Y.; Wei, H.; Yang, X.; Chen, T.; Xiao, J. Platelet-to-lymphocyte ratio in advanced Cancer: Review and meta-analysis. Clin. Chim. Acta 2018, 483, 48–56. [Google Scholar] [CrossRef]
- Zhang, F.; Gong, W. Prognostic Value of the Platelet-to-Lymphocyte Ratio in Patients with Melanoma: A Meta-Analysis. Front. Oncol. 2020, 10, 1116. [Google Scholar] [CrossRef]
- Sun, L.; Hu, W.; Liu, M.; Chen, Y.; Jin, B.; Xu, H.; Du, S.; Xu, Y.; Zhao, H.; Lu, X.; et al. High Systemic Inflammation Response Index (SIRI) Indicates Poor Outcome in Gallbladder Cancer Patients with Surgical Resection: A Single Institution Experience in China. Cancer Res. Treat. 2020, 52, 1199–1210. [Google Scholar] [CrossRef]
- Xu, Y.; He, H.; Zang, Y.; Yu, Z.; Hu, H.; Cui, J.; Wang, W.; Gao, Y.; Wei, H.; Wang, Z. Systemic inflammation response index (SIRI) as a novel biomarker in patients with rheumatoid arthritis: A multi-center retrospective study. Clin. Rheumatol. 2022, 41, 1989–2000. [Google Scholar] [CrossRef]
- Valero, C.; Pardo, L.; Sansa, A.; Lorenzo, J.G.; López, M.; Quer, M.; León, X. Prognostic capacity of Systemic Inflammation Response Index (SIRI) in patients with head and neck squamous cell carcinoma. Head. Neck. 2020, 42, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.W.; Yang, Y.F.; Yang, C.C.; Yan, L.-J.; Ding, Z.-N.; Liu, H.; Xue, J.-S.; Dong, Z.-R.; Chen, Z.-Q.; Hong, J.-G.; et al. Systemic immune-inflammation index predicts prognosis of cancer immunotherapy: Systemic review and meta-analysis. Immunotherapy. 2022, 14, 1481–1496. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, D.; Huang, Y.; Xiong, Q.; Tan, D.; Liu, L.; Lin, T.; Wei, Q. The Prognostic and Clinicopathological Significance of Systemic Immune-Inflammation Index in Bladder Cancer. Front. Immunol. 2022, 13, 865643. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, S.; Liu, Y.; Ma, L.; Zhu, J.; Xin, Y.; Wang, Y.; Yang, C.; Cheng, Y. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J. Clin. Lab. Anal. 2019, 33, e22964. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Carioli, G.; Santucci, C.; Bertuccio, P.; Gallus, S.; Garavello, W.; Negri, E.; La Vecchia, C. Global trends in oral and pharyngeal cancer incidence and mortality. Int. J. Cancer. 2020, 147, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Yamahara, K.; Mizukoshi, A.; Lee, K.; Ikegami, S. Pretherapeutic nutritional/inflammatory factors as predictors for survival of both early and advanced staged head and neck cancer patients. Auris Nasus Larynx. 2021, 48, 731–737. [Google Scholar] [CrossRef]
- Bossi, P. Prognostic Nutritional Index: An easy nutritional screening for patients with head and neck cancer? ESMO Open 2018, 3, e000449. [Google Scholar] [CrossRef]
- Wang, Y.T.; Kuo, L.T.; Weng, H.H.; Hsu, C.-M.; Tsai, M.-S.; Chang, G.-H.; Lee, Y.-C.; Huang, E.I.; Tsai, Y.-T. Systemic Immun e-Inflammation Index as a Predictor for Head and Neck Cancer Prognosis: A Meta-Analysis. Front. Oncol. 2022, 12, 899518. [Google Scholar] [CrossRef]
- Abelardo, E.; Davies, G.; Kamhieh, Y.; Prabhu, V. Are Inflammatory Markers Significant Prognostic Factors for Head and Neck Cancer Patients? ORL J. Otorhinolaryngol. Relat. Spec. 2020, 82, 235–244. [Google Scholar] [CrossRef]
- Okadome, K.; Baba, Y.; Yagi, T.; Kiyozumi, Y.; Ishimoto, T.M.; Iwatsuki, M.M.; Miyamoto, Y.M.; Yoshida, N.M.; Watanabe, M.M.; Baba, H.M. Prognostic Nutritional Index, Tumor-infiltrating Lymphocytes, and Prognosis in Patients with Esophageal Cancer. Ann. Surg. 2020, 271, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Atasever Akkas, E.; Erdis, E.; Yucel, B. Prognostic value of the systemic immune-inflammation index, systemic inflammation response index, and prognostic nutritional index in head and neck cancer. Eur Arch Otorhinolaryngol. 2023, 280, 3821–3830, Erratum in Eur. Arch. Otorhinolaryngol. 2023, 280, 3831–3833. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).