Primary Human Ligament Fibroblast Adhesion and Growth on 3D-Printed Scaffolds for Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Scaffold Fabrication
2.2. Surface Coating of the Scaffolds
2.3. Isolation and Culture of Primary Human Ligament Fibroblasts
2.4. Cell Seeding and Culture on the Scaffolds
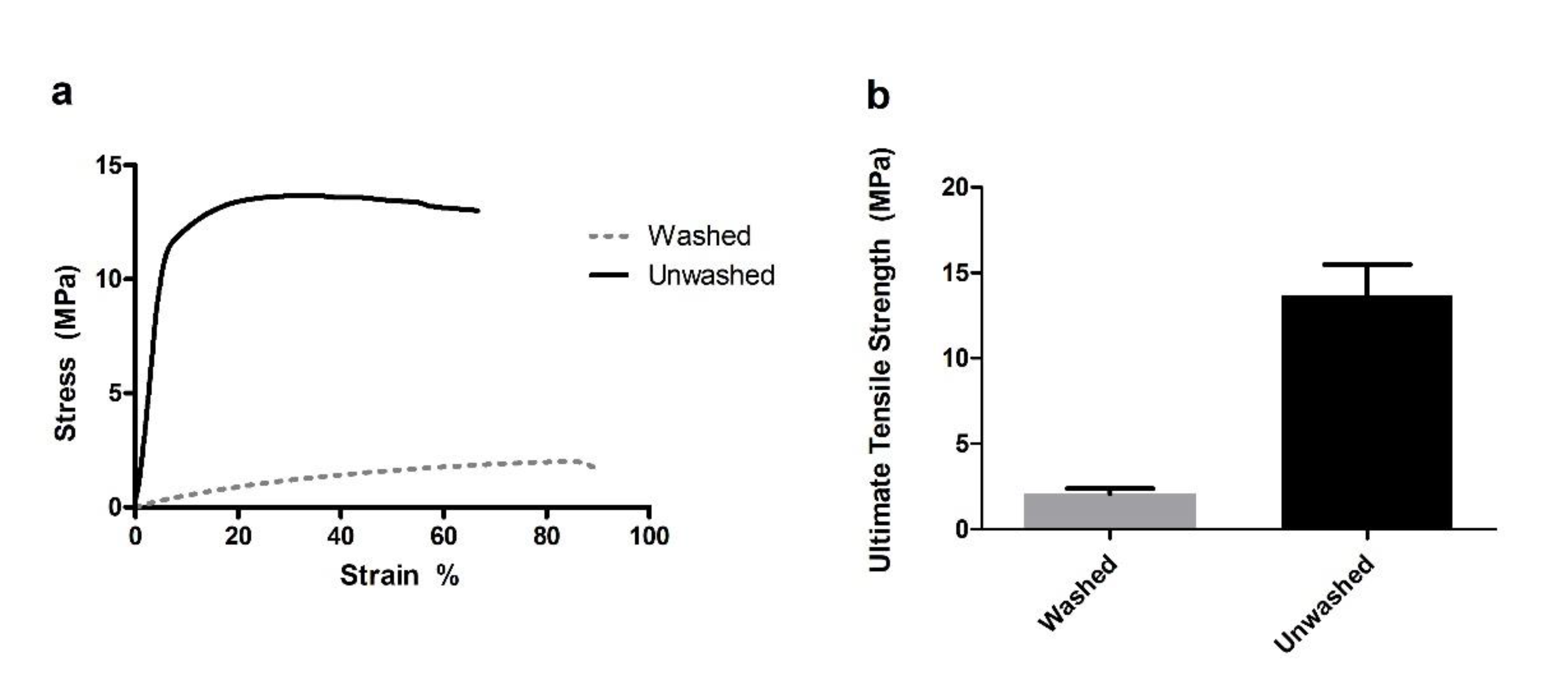
2.5. Mechanical Testing
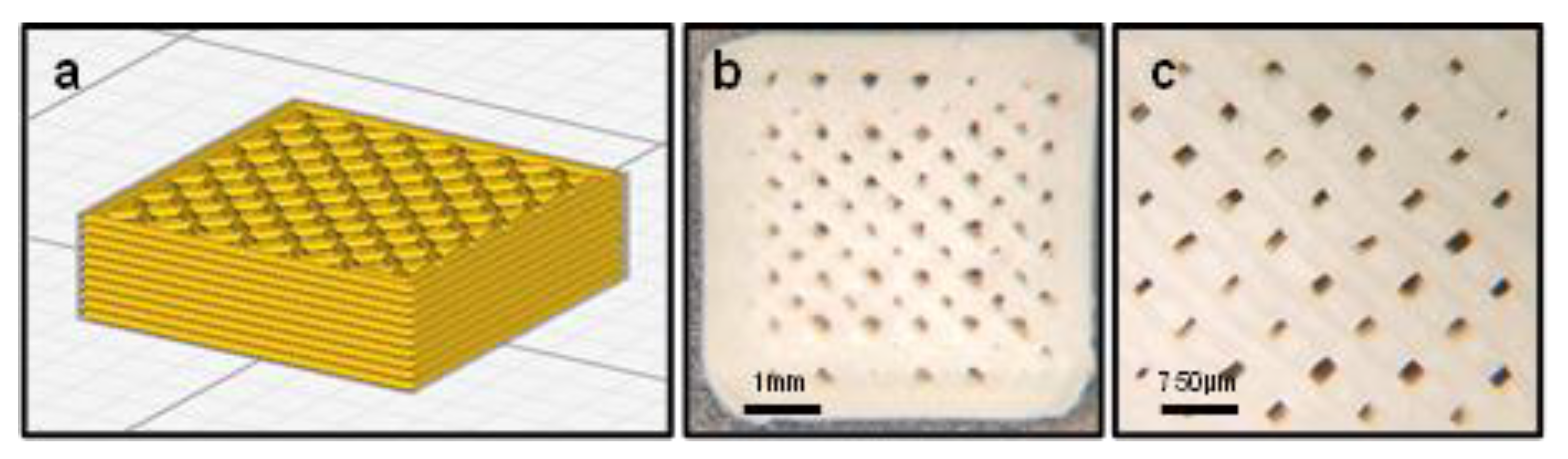
2.6. Scaffold Geometry
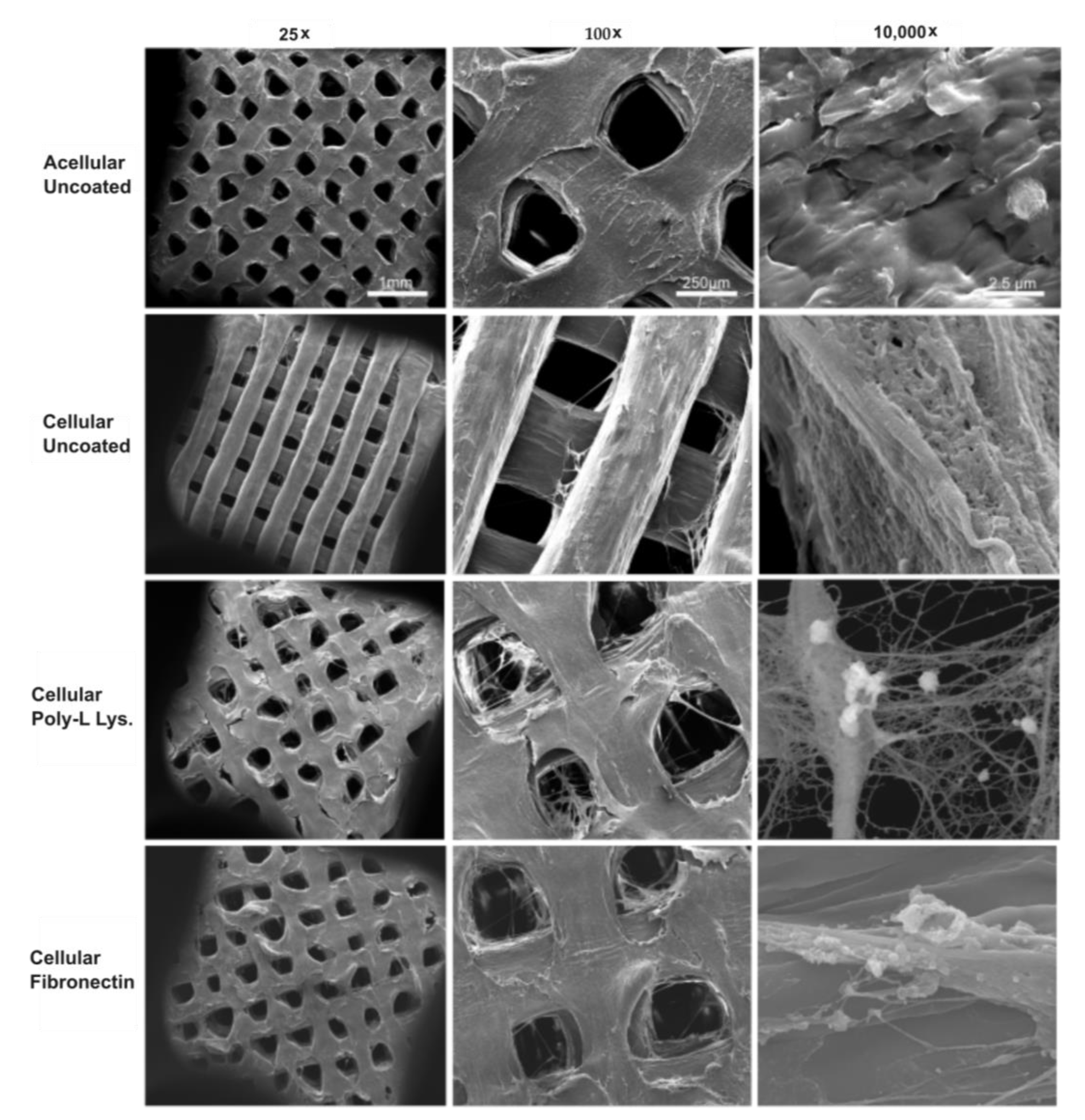
2.7. Surface Morphology
2.8. Gene Expression
2.9. Cell Viability and Proliferation
2.10. Immunofluorescence and Protein Analyses
2.11. Statistical Analysis
3. Results
3.1. Mechanical Testing and Scaffold Characterization
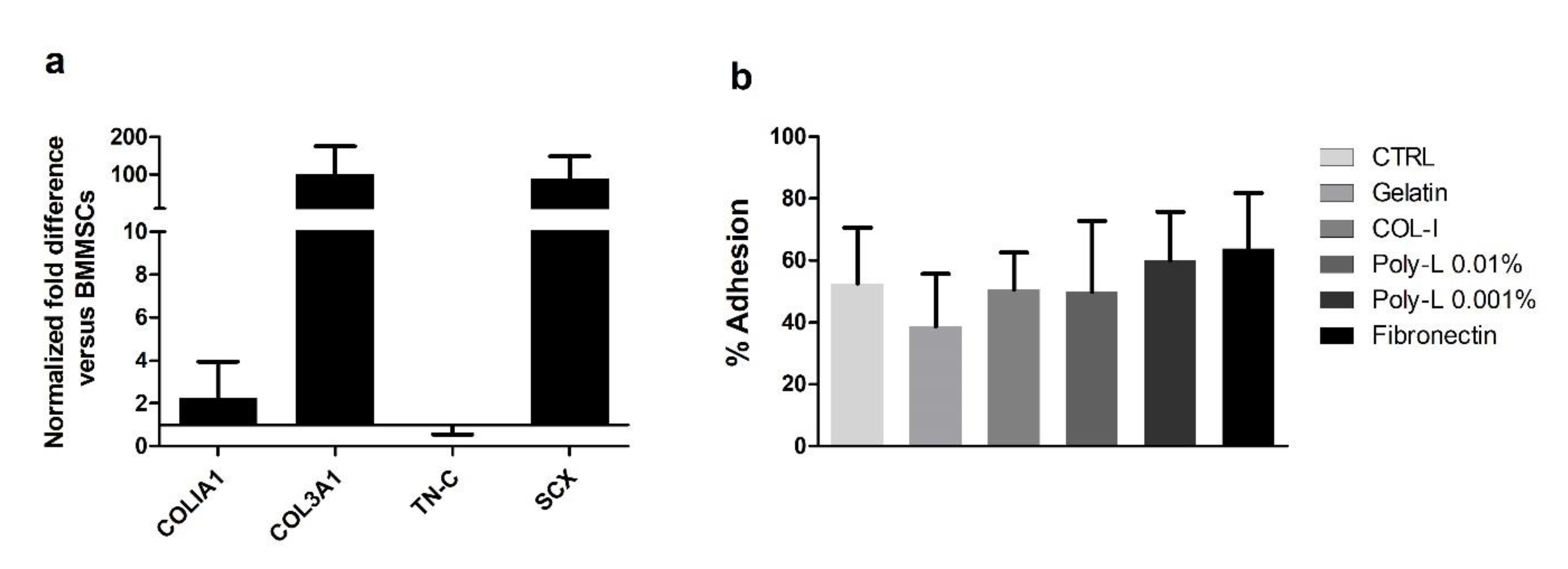
3.2. Cell Isolation, Characterization, and Scaffold Adhesion
3.3. Cell Viability, Proliferation, and DNA Quantification
3.4. Ligamentogenic Matrix Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Majewski, M.; Susanne, H.; Klaus, S. Epidemiology of athletic knee injuries: A 10-year study. Knee 2006, 13, 184–188. [Google Scholar] [CrossRef]
- Kiapour, A.M.; Murray, M.M. Basic science of anterior cruciate ligament injury and repair. Bone Jt. Res. 2014, 3, 20–31. [Google Scholar] [CrossRef]
- Levine, J.W.; Kiapour, A.M.; Quatman, C.E.; Wordeman, S.C.; Goel, V.K.; Hewett, T.E.; Demetropoulos, C.K. Clinically Relevant Injury Patterns After an Anterior Cruciate Ligament Injury Provide Insight Into Injury Mechanisms. Am. J. Sports Med. 2012, 41, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.L.; Noyes, F.R.; Grood, E.S. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J. Bone Jt. Surg. Am. 1980, 62, 259–270. [Google Scholar] [CrossRef]
- Campbell, C.J.; Carson, J.D.; Diaconescu, E.D.; Celebrini, R.; Rizzardo, M.R.; Godbout, V.; Fletcher, J.A.; McCormack, R.; Outerbridge, R.; Taylor, T.; et al. Canadian Academy of Sport and Exercise Medicine Position Statement: Neuromuscular Training Programs Can Decrease Anterior Cruciate Ligament Injuries in Youth Soccer Players. Clin. J. Sport Med. 2014, 24, 263–267. [Google Scholar] [CrossRef]
- Gopinathan, P. Fate of the untreated anterior cruciate ligament-injured knee. J. Orthop. 2017, 14, A1–A3. [Google Scholar] [CrossRef]
- Friel, N.A.; Chu, C.R. The Role of ACL Injury in the Development of Posttraumatic Knee Osteoarthritis. Clin. Sports Med. 2013, 32, 1–12. [Google Scholar] [CrossRef]
- Calejo, I.; Costa-Almeida, R.; Reis, R.L.; Gomes, M.E. Enthesis Tissue Engineering: Biological Requirements Meet at the Interface. Tissue Eng. Part B Rev. 2019, 25, 330–356. [Google Scholar] [CrossRef] [PubMed]
- Costa-Paz, M.; Ayerza, M.A.; Tanoira, I.; Astoul, J.; Muscolo, D.L. Spontaneous Healing in Complete ACL Ruptures: A Clinical and MRI Study. Clin. Orthop. Relat. Res. 2012, 470, 979–985. [Google Scholar] [CrossRef]
- Daniel, D.M.; Stone, M.L.; Dobson, B.E.; Fithian, D.C.; Rossman, D.J.; Kaufman, K. Fate of the ACL-injured Patient. Am. J. Sports Med. 1994, 22, 632–644. [Google Scholar] [CrossRef]
- Vavken, P.; Murray, M.M. The Potential for Primary Repair of the ACL. Sports Med. Arthrosc. Rev. 2011, 19, 44–49. [Google Scholar] [CrossRef]
- Murray, M.M. Current Status and Potential of Primary ACL Repair. Clin. Sports Med. 2009, 28, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Odensten, M.; Good, L.; Gillquist, J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J. Bone Jt. Surg. Am. 1989, 71, 965–974. [Google Scholar] [CrossRef]
- Woo, S.L.-Y.; Chan, S.S.; Yamaji, T. Biomechanics of knee ligament healing, repair and reconstruction. J. Biomech. 1997, 30, 431–439. [Google Scholar] [CrossRef]
- Murray, M.M. History of ACL Treatment and Current Gold Standard of Care. In The ACL Handbook: Knee Biology, Mechanics, and Treatment; Murray, M.M., Vavken, P., Fleming, B., Murray, M.M., Vavken, P., Fleming, B., Eds.; Springer: New York, NY, USA, 2013; pp. 19–28. [Google Scholar] [CrossRef]
- Björnsson, H.; Samuelsson, K.; Sundemo, D.; Desai, N.; Sernert, N.; Rostgård-Christensen, L.; Karlsson, J.; Kartus, J. A Randomized Controlled Trial With Mean 16-Year Follow-up Comparing Hamstring and Patellar Tendon Autografts in Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2016, 44, 2304–2313. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, J.G.; Wang, J.H.; Jung, C.H.; Lim, H.C. Long-Term Results of Anterior Cruciate Ligament Reconstruction Using Bone–Patellar Tendon–Bone: An Analysis of the Factors Affecting the Development of Osteoarthritis. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Selmi, T.A.S.; Fithian, D.; Neyret, P. The evolution of osteoarthritis in 103 patients with ACL reconstruction at 17 years follow-up. Knee 2006, 13, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Barenius, B.; Ponzer, S.; Shalabi, A.; Bujak, R.; Norlén, L.; Eriksson, K. Increased Risk of Osteoarthritis After Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2014, 42, 1049–1057. [Google Scholar] [CrossRef]
- Woo, S.L.-Y.; Wu, C.; DeDe, O.; Vercillo, F.; Noorani, S. Biomechanics and anterior cruciate ligament reconstruction. J. Orthop. Surg. Res. 2006, 1, 2. [Google Scholar] [CrossRef]
- Herrington, L.; Wrapson, C.; Matthews, M.; Matthews, H. Anterior Cruciate Ligament reconstruction, hamstring versus bone–patella tendon–bone grafts: A systematic literature review of outcome from surgery. Knee 2005, 12, 41–50. [Google Scholar] [CrossRef]
- Oluwadamilola, A.; Yousaf, S.; Zare, M.; Mozafari, M.; Youseffi, M.; Twigg, P.; Sefate, F. 14—Scaffolds for ligament tissue engineering. In Handbook of Tissue Engineering Scaffolds: Volume One; Mozafari, M., Sefat, F., Atala, A., Mozafari, M., Sefat, F., Atala, A., Eds.; 2019; pp. 299–327. Available online: http://www.sciencedirect.com/science/article/pii/B9780081025635000149 (accessed on 4 August 2022).
- Gentleman, E.; Lay, A.N.; Dickerson, D.A.; Nauman, E.A.; Livesay, G.A.; Dee, K.C. Mechanical characterization of collagen fibers and scaffolds for tissue engineering. Biomaterials 2003, 24, 3805–3813. [Google Scholar] [CrossRef]
- Laurent, C.; Liu, X.; De Isla, N.; Wang, X.; Rahouadj, R. Defining a scaffold for ligament tissue engineering: What has been done, and what still needs to be done. J. Cell. Immunother. 2018, 4, 4–9. [Google Scholar] [CrossRef]
- Woo, S.L.-Y.; Hollis, J.M.; Adams, D.J.; Lyon, R.M.; Takai, S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex: The effects of specimen age and orientation. Am. J. Sports Med. 1991, 19, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, P.; Cooke, M.E.; Weber, M.H.; Rosenzweig, D.H. Current Biomedical Applications of 3D Printing and Additive Manufacturing. Appl. Sci. 2019, 9, 1713. [Google Scholar] [CrossRef]
- Calore, A.R.; Sinha, R.; Harings, J.; Bernaerts, K.V.; Mota, C.; Moroni, L. Additive Manufacturing Using Melt Extruded Thermoplastics for Tissue Engineering. Methods Mol. Biol. Clifton NJ 2021, 2147, 75–99. [Google Scholar] [CrossRef]
- Diegel, O. 10.02—Additive Manufacturing: An Overview. In Comprehensive Materials Processing; Hashmi, S., Batalha, G.F., Van Tyne, C.J., Yilbas, B., Hashmi, S., Batalha, G.F., Van Tyne, C.J., Yilbas, B., Eds.; Elsevier B.V.: Oxford, UK, 2014; pp. 3–18. Available online: http://www.sciencedirect.com/science/article/pii/B9780080965321010001 (accessed on 2 September 2020).
- Haglund, L.; Ahangar, P.; Rosenzweig, D.H. Advancements in 3D printed scaffolds to mimic matrix complexities for musculoskeletal repair. Curr. Opin. Biomed. Eng. 2019, 10, 142–148. [Google Scholar] [CrossRef]
- Cooke, M.E.; Ramirez-Garcialuna, J.L.; Rangel-Berridi, K.; Park, H.; Nazhat, S.N.; Weber, M.H.; Henderson, J.E.; Rosenzweig, D.H. 3D Printed Polyurethane Scaffolds for the Repair of Bone Defects. Front. Bioeng. Biotechnol. 2020, 8, 557215. [Google Scholar] [CrossRef] [PubMed]
- Leong, N.L.; Kabir, N.; Arshi, A.; Nazemi, A.; Wu, B.; Petrigliano, F.A.; McAllister, D.R. Evaluation of Polycaprolactone Scaffold with Basic Fibroblast Growth Factor and Fibroblasts in an Athymic Rat Model for Anterior Cruciate Ligament Reconstruction. Tissue Eng. Part A 2015, 21, 1859–1868. [Google Scholar] [CrossRef]
- Podporska-Carroll, J.; Ip, W.Y.; Gogolewski, S. Biodegradable poly(ester urethane) urea scaffolds for tissue engineering: Interaction with osteoblast-like MG-63 cells. Acta Biomater. 2014, 10, 2781–2791. [Google Scholar] [CrossRef]
- Vaquette, C.; Kahn, C.; Frochot, C.; Nouvel, C.; Six, J.-L.; De Isla, N.; Luo, L.-H.; Cooper-White, J.; Rahouadj, R.; Wang, X. Aligned poly(L-lactic-co-e-caprolactone) electrospun microfibers and knitted structure: A novel composite scaffold for ligament tissue engineering. J. Biomed. Mater. Res. Part A 2010, 94, 1270–1282. [Google Scholar] [CrossRef]
- Kimura, Y.; Hokugo, A.; Takamoto, T.; Tabata, Y.; Kurosawa, H. Regeneration of Anterior Cruciate Ligament by Biodegradable Scaffold Combined with Local Controlled Release of Basic Fibroblast Growth Factor and Collagen Wrapping. Tissue Eng. Part C Methods 2008, 14, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.; Hollister, S.J.; Dalton, P.D. Additive manufacturing of polymer melts for implantable medical devices and scaffolds. Biofabrication 2017, 9, 012002. [Google Scholar] [CrossRef]
- Sahoo, S.; Ouyang, H.; Goh, J.C.-H.; Tay, T.-E.; Toh, S. Characterization of a Novel Polymeric Scaffold for Potential Application in Tendon/Ligament Tissue Engineering. Tissue Eng. 2006, 12, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, S.; Sundaram, M.N.; Kadavan, J.D.; Jayakumar, R. Layered chitosan-collagen hydrogel/aligned PLLA nanofiber construct for flexor tendon regeneration. Carbohydr. Polym. 2016, 153, 492–500. [Google Scholar] [CrossRef]
- Domingos, M.; Intranuovo, F.; Gloria, A.; Gristina, R.; Ambrosio, L.; Bártolo, P.; Favia, P. Improved osteoblast cell affinity on plasma-modified 3-D extruded PCL scaffolds. Acta Biomater. 2013, 9, 5997–6005. [Google Scholar] [CrossRef]
- Poh, P.S.; Hutmacher, D.W.; Holzapfel, B.M.; Solanki, A.K.; Stevens, M.M.; Woodruff, M.A. In vitro and in vivo bone formation potential of surface calcium phosphate-coated polycaprolactone and polycaprolactone/bioactive glass composite scaffolds. Acta Biomater. 2016, 30, 319–333. [Google Scholar] [CrossRef]
- Sousa, I.; Mendes, A.; Pereira, R.F.; Bártolo, P.J. Collagen surface modified poly(ε-caprolactone) scaffolds with improved hydrophilicity and cell adhesion properties. Mater. Lett. 2014, 134, 263–267. [Google Scholar] [CrossRef]
- Wang, W.; Caetano, G.; Ambler, W.S.; Blaker, J.J.; Frade, M.A.; Mandal, P.; Diver, C.; Bártolo, P. Enhancing the Hydrophilicity and Cell Attachment of 3D Printed PCL/Graphene Scaffolds for Bone Tissue Engineering. Materials 2016, 9, 992. [Google Scholar] [CrossRef]
- You, J.; Preen, R.J.; Bull, L.; Greenman, J.; Ieropoulos, I. 3D printed components of microbial fuel cells: Towards monolithic microbial fuel cell fabrication using additive layer manufacturing. Sustain. Energy Technol. Assess. 2017, 19, 94–101. [Google Scholar] [CrossRef]
- Tsai, K.J.; Dixon, S.; Hale, L.R.; Darbyshire, A.; Martin, D.; de Mel, A. Biomimetic heterogenous elastic tissue development. NPJ Regen. Med. 2017, 2, 16. [Google Scholar] [CrossRef]
- Ahangar, P.; Akoury, E.; Luna, A.S.R.G.; Nour, A.; Weber, M.H.; Rosenzweig, D.H. Nanoporous 3D-Printed Scaffolds for Local Doxorubicin Delivery in Bone Metastases Secondary to Prostate Cancer. Materials 2018, 11, 1485. [Google Scholar] [CrossRef]
- Fairag, R.; Rosenzweig, D.H.; Garcialuna, J.L.R.; Weber, M.H.; Haglund, L. Three-Dimensional Printed Polylactic Acid Scaffolds Promote Bone-like Matrix Deposition in Vitro. ACS Appl. Mater. Interfaces 2019, 11, 15306–15315. [Google Scholar] [CrossRef]
- Pitaru, A.A.; Lacombe, J.-G.; Cooke, M.E.; Beckman, L.; Steffen, T.; Weber, M.H.; Martineau, P.A.; Rosenzweig, D.H. 3D Printing to Microfabricate Stiff and Elastic Scaffolds that Mimic Ligament Tissue. Micromachines 2020, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Garcia-Luna, J.L.; Wong, T.H.; Chan, D.; Al-Saran, Y.; Awlia, A.; Abou-Rjeili, M.; Ouellet, S.; Akoury, E.; Lemarié, C.A.; Henderson, J.E.; et al. Defective bone repair in diclofenac treated C57Bl6 mice with and without lipopolysaccharide induced systemic inflammation. J. Cell. Physiol. 2018, 234, 3078–3087. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/jcp.27128 (accessed on 2 September 2020). [CrossRef]
- Park, H.; Cooke, M.E.; Lacombe, J.-G.; Weber, M.H.; Martineau, P.A.; Nazhat, S.N.; Rosenzweig, D.H. Continuous Two-Zone In Vitro Co-culture Model of the Enthesis. Biomed. Mater. Devices 2022, 1–10. Available online: https://link.springer.com/article/10.1007/s44174-022-00015-2 (accessed on 7 April 2023). [CrossRef]
- Park, H.; Nazhat, S.N.; Rosenzweig, D.H. Mechanical activation drives tenogenic differentiation of human mesenchymal stem cells in aligned dense collagen hydrogels. Biomaterials 2022, 286, 121606. [Google Scholar] [CrossRef]
- Ciucci, A.; Zannoni, G.F.; Buttarelli, M.; Lisi, L.; Travaglia, D.; Martinelli, E.; Scambia, G.; Gallo, D. Multiple direct and indirect mechanisms drive estrogen-induced tumor growth in high grade serous ovarian cancers. Oncotarget 2016, 7, 8155–8171. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; He, S.-T.; Yan, F.-H.; Zhou, P.-F.; Luo, K.; Zhang, Y.-D.; Xiao, Y.; Lin, M.-K. Dental pulp stem cells express tendon markers under mechanical loading and are a potential cell source for tissue engineering of tendon-like tissue. Int. J. Oral Sci. 2016, 8, 213–222. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Jiang, N.; Qin, T.; Zhang, W.; Liu, Z.; Liu, Y.; Li, D. Microfiber-reinforced nanofibrous scaffolds with structural and material gradients to mimic ligament-to-bone interface. J. Mater. Chem. B 2017, 5, 8579–8590. [Google Scholar] [CrossRef]
- Chen, X.; Qi, Y.-Y.; Wang, L.-L.; Yin, Z.; Yin, G.-L.; Zou, X.-H.; Ouyang, H.-W. Ligament regeneration using a knitted silk scaffold combined with collagen matrix. Biomaterials 2008, 29, 3683–3692. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Y.; Streubel, P.N.; Duan, B. Living nanofiber yarn-based woven biotextiles for tendon tissue engineering using cell tri-culture and mechanical stimulation. Acta Biomater. 2017, 62, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Xerogeanes, J.W.; Livesay, G.A.; Fu, F.H.; Woo, S.L.-Y. Biomechanical function of the human anterior cruciate ligament. Arthrosc. J. Arthrosc. Relat. Surg. 1994, 10, 140–147. [Google Scholar] [CrossRef]
- Rao, R.; Libring, S.; Fleisher, E.; Yankannah, Y.; Freeman, J.W. Design and characterization of three-dimensional twist-braid scaffolds for anterior cruciate ligament regeneration. Technology 2017, 5, 98–106. [Google Scholar] [CrossRef]
- Cooper, J.A.; Lu, H.H.; Ko, F.K.; Freeman, J.W.; Laurencin, C.T. Fiber-based tissue-engineered scaffold for ligament replacement: Design considerations and in vitro evaluation. Biomaterials 2005, 26, 1523–1532. [Google Scholar] [CrossRef]
- Duthon, V.L.A.; Barea, C.; Abrassart, S.; Fasel, J.H.; Fritschy, D.; Menetrey, J. Anatomy of the anterior cruciate ligament. Knee Surg. Sports Traumatol. Arthrosc. 2005, 14, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hao, R.; Qin, J.; Song, J.; Chen, X.; Rao, F.; Zhai, J.; Zhao, Y.; Zhang, L.; Xue, J. Electrospun Fibers Control Drug Delivery for Tissue Regeneration and Cancer Therapy. Adv. Fiber Mater. 2022, 4, 1375–1413. [Google Scholar] [CrossRef]
- Peixoto, T.; Carneiro, S.; Fangueiro, R.; Guedes, R.M.; Paiva, M.C.; Lopes, M.A. Engineering hybrid textile braids for tendon and ligament repair application. J. Appl. Polym. Sci. 2021, 139, 52013. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/app.52013 (accessed on 7 April 2023). [CrossRef]
- Chen, C.; Shi, Q.; Li, M.; Chen, Y.; Zhang, T.; Xu, Y.; Liao, Y.; Ding, S.; Wang, Z.; Li, X.; et al. Engineering an enthesis-like graft for rotator cuff repair: An approach to fabricate highly biomimetic scaffold capable of zone-specifically releasing stem cell differentiation inducers. Bioact. Mater. 2022, 16, 451–471. [Google Scholar] [CrossRef]




| Target Gene | Product Length (bp) | Genbank’s Gene ID |
|---|---|---|
| GAPDH | 1978 | NM_001170721 |
| COLIA1 | 462 | NM_001102599 |
| COL3A1 | ||
| TN-C | 1065 | AL445645.10 |
| SCX | 1930 | NG_053063.1 |
| Target Gene | Forward Primer (5′—3′) | Reverse Primer (3′—5′) |
|---|---|---|
| GAPDH | TCCCTGAGCTGAACGGGAAG | GGAGGAGTGGGTGTCGCTGT |
| COLIA1 | GGTGATGCTGGTCCTGTTG | CATCGTGAGCCTTCTCTTGAG |
| COL3A1 | CCCAGAACATCACATATCAC | CAAGAGGAACACATATGGAG |
| TN-C | ACCGTCTCTTCCGTCACTTCT | AACAACTTAGGACAATGCGTCT |
| SCX | AGAACACCCAGCCCAAACA | TCCTTGCTCAACTTTCTCTGGT |
| Property | Value |
|---|---|
| Dry mass (mg) | 88.3 ± 0.7 |
| SV/RV (%) | 60.7 ± 1.0 |
| SS (mm2) | 159.5 ± 2.2 |
| SF.Th (μm) | 479.9 ± 10.7 |
| SF. Sp (μm) | 271.7 ± 4.6 |
| Po. Tot (%) | 39.3 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacombe, J.-G.; Cooke, M.E.; Park, H.; Alshammari, S.M.; Gawri, R.; Nazhat, S.N.; Martineau, P.A.; Rosenzweig, D.H. Primary Human Ligament Fibroblast Adhesion and Growth on 3D-Printed Scaffolds for Tissue Engineering Applications. Surgeries 2023, 4, 196-211. https://doi.org/10.3390/surgeries4020021
Lacombe J-G, Cooke ME, Park H, Alshammari SM, Gawri R, Nazhat SN, Martineau PA, Rosenzweig DH. Primary Human Ligament Fibroblast Adhesion and Growth on 3D-Printed Scaffolds for Tissue Engineering Applications. Surgeries. 2023; 4(2):196-211. https://doi.org/10.3390/surgeries4020021
Chicago/Turabian StyleLacombe, Jean-Gabriel, Megan E. Cooke, Hyeree Park, Suliman Mohammed Alshammari, Rahul Gawri, Showan N. Nazhat, Paul A. Martineau, and Derek H. Rosenzweig. 2023. "Primary Human Ligament Fibroblast Adhesion and Growth on 3D-Printed Scaffolds for Tissue Engineering Applications" Surgeries 4, no. 2: 196-211. https://doi.org/10.3390/surgeries4020021
APA StyleLacombe, J.-G., Cooke, M. E., Park, H., Alshammari, S. M., Gawri, R., Nazhat, S. N., Martineau, P. A., & Rosenzweig, D. H. (2023). Primary Human Ligament Fibroblast Adhesion and Growth on 3D-Printed Scaffolds for Tissue Engineering Applications. Surgeries, 4(2), 196-211. https://doi.org/10.3390/surgeries4020021








