Ventricular Peritoneal Shunting Using Modified Keen’s Point Approach: Technical Report and Cases Series
Abstract
1. Introduction
2. Materials and Methods
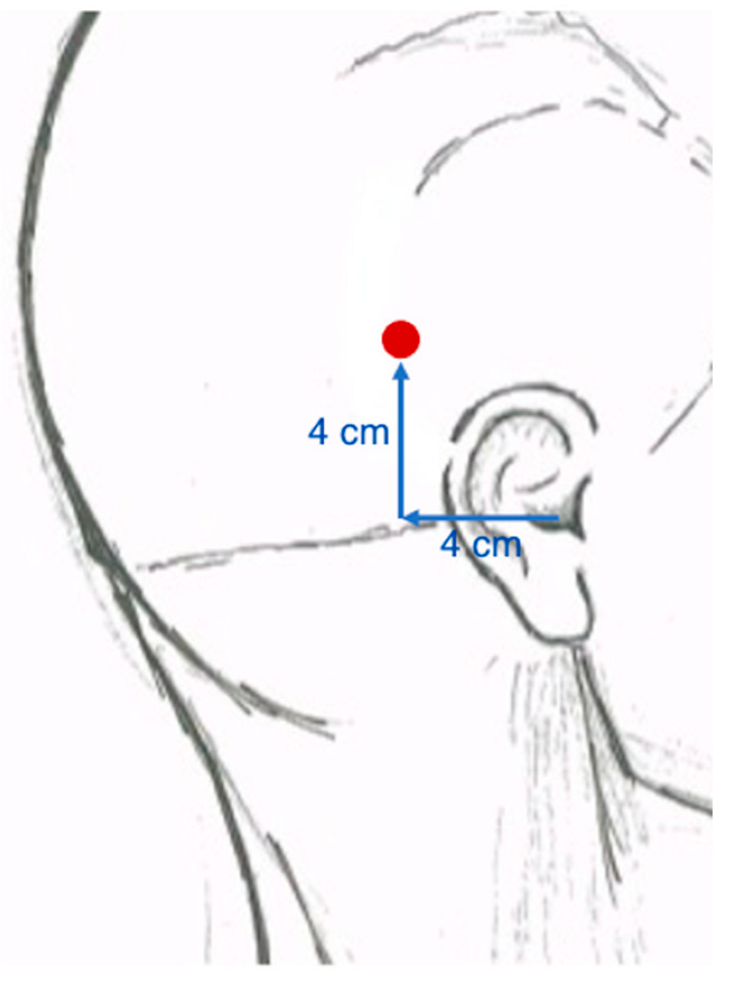
Surgical Technique
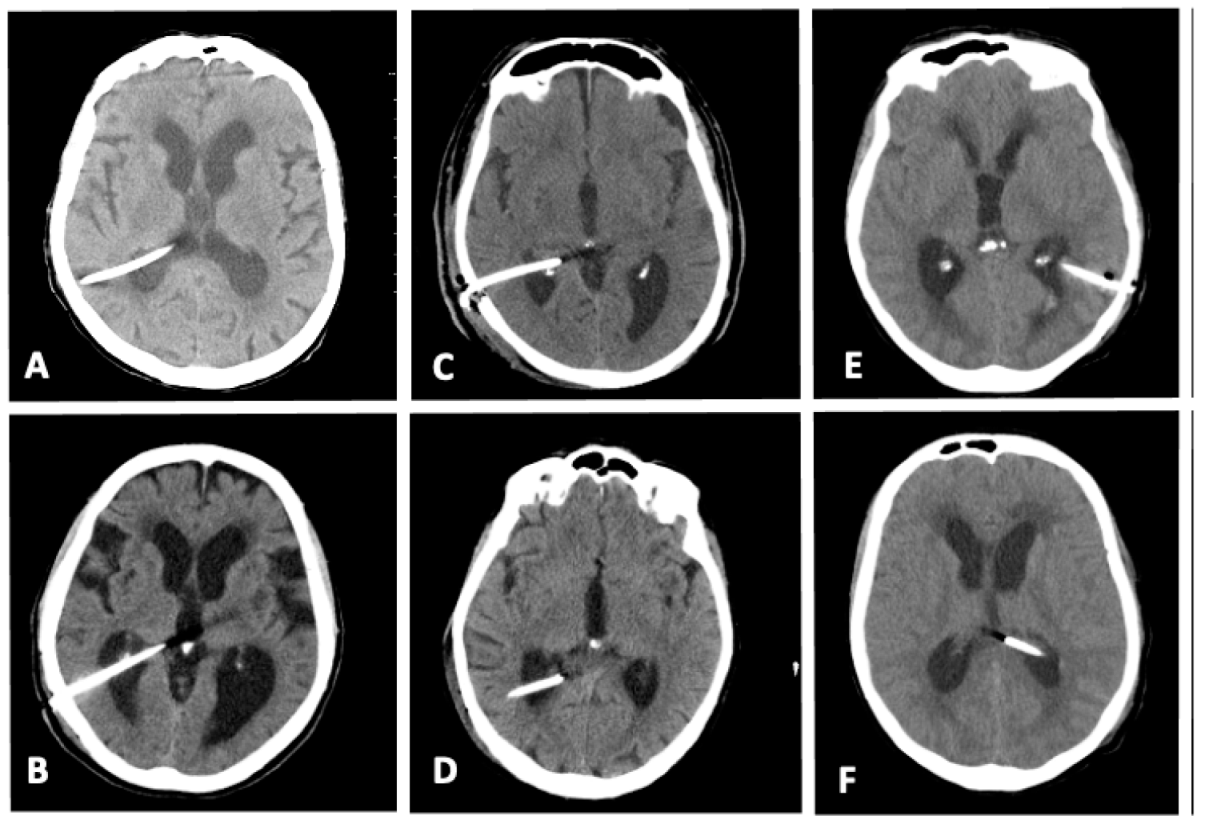
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bir, S.C.; Patra, D.P.; Maiti, T.K.; Sun, H.; Guthikonda, B.; Notarianni, C.; Nanda, A. Epidemiology of adult-onset hydrocephalus: Institutional experience with 2001 patients. Neurosurg. Focus 2014, 41, E5. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A.M.; Riva-Cambrin, J.; Yavin, D.; Hockley, A.; Pringsheim, T.M.; Jette, N.; Lethebe, B.C. Age-specific global epidemiology of hydrocephalus: Systematic review, metanalysis and global birth surveillance. PLoS ONE 2018, 13, e0204926. [Google Scholar] [CrossRef] [PubMed]
- Dobran, M.; Nasi, D.; Mancini, F.; Gladi, M.; Polonara, G.; Marini, A.; Lattanzi, S.; Scerrati, M. Relationship Between the Location of the Ventricular Catheter Tip and the Ventriculoperitoneal Shunt Malfunction. Clin. Neurol. Neurosurg. 2018, 175, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.; Gruen, J.P.; Luciano, M.G. Introduction: Adult hydrocephalus. Neurosurg. Focus 2016, 41, E1. [Google Scholar] [CrossRef]
- Anderson, I.A.; Saukila, L.M.F.; Robins, J.M.W.; Akhunbay-Fudge, C.Y.; Goodden, J.R.; Tyagi, A.K.; Chumas, P.D. Factors associated with 30-day ventriculoperitoneal shunt failure in pediatric and adult patients. J. Neurosurg. 2018, 130, 145–153. [Google Scholar] [CrossRef]
- Feletti, A.; d’Avella, D.; Wikkelsø, C.; Klinge, P.; Hellström, P. European iNPH Multicenter Study Group. Ventriculoperitoneal Shunt Complications in the European Idiopathic Normal Pressure Hydrocephalus Multicenter Study. Oper. Neurosurg. 2018, 17, 97–102. [Google Scholar] [CrossRef]
- Reddy, G.K.; Bollam, P.; Caldito, G. Long-Term Outcomes of Ventriculoperitoneal Shunt Surgery in Patients with Hydrocephalus. World Neurosurg. 2014, 81, 404–410. [Google Scholar] [CrossRef]
- Wells, D.L.; Allen, J.M. Ventriculoperitoneal shunt infections in adult patients. AACN Adv. Crit. Care 2013, 24, 6–12. [Google Scholar] [CrossRef]
- Ahmadvand, S.; Dayyani, M.; Etemadrezaie, H.; Ghorbanpour, A.; Zarei, R.; Shahriyari, A.; Emadzadeh, M.; Ganjeifar, B.; Zabihyan, S. Rate and Risk Factors of Early Ventriculoperitoneal Shunt Revision: A Five-Year Retrospective Analysis of a Referral Center. World Neurosurg. 2020, 134, e505–e511. [Google Scholar] [CrossRef]
- Bhargav, A.G.; Rinaldo, L.; Lanzino, G.; Elder, B.D. Comparison of Complication and Revision rates after frontal versus parietal approach for ventricular shunt placement in idiopathic normal pressure hydrocephalus. World Neurosurg. 2019, 126, e1017–e1022. [Google Scholar] [CrossRef]
- Duong, J.; Elia, C.J.; Miulli, D.; Dong, F.; Sumida, A. An approach using the occipital parietal point for placement of ventriculoperitoneal catheters in adults. Surg. Neurol. Int. 2019, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.V.; Gagliardi, F.; Bailo, M.; Spina, A.; Gragnagnello, C.; Caputy, A.J.; Mortini, P. Ventriculo-peritoneal shunt. In Operative Cranial Neurosurgical Anatomy, 1st ed.; Thieme Medical Publishers: New York, NY, USA, 2018; pp. 322–326. [Google Scholar]
- Guo, L.; Chen, X.; Yu, B.; Shen, L.; Zhang, X. Delayed Intracerebral Hemorrhage Secondary to Ventriculoperitoneal Shunt: A Retrospective Study. World Neurosurg. 2017, 107, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.L.; Vivas-Buitrago, T.; Adam, A.; Lu, J.; Robison, J.; Elder, B.D.; Goodwin, C.R.; Jusué-Torres, I.; Rigamonti, D. Ventriculoatrial versus ventriculoperitoneal shunt complications in idiopathic normal pressure hydrocephalus. Clin. Neurol. Neurosurg. 2017, 157, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jeremiah, K.J.; Cherry, C.L.; Wan, K.R.; Toy, J.A.; Wolfe, R.; Danks, R.A. Choice of valve type and poor ventricular catheter placement: Modifiable factors associated with ventriculoperitoneal shunt failure. J. Clin. Neurosci. 2016, 27, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Junaid, M.; Ahmed, M.; Rashid, M.U. An experience with ventriculoperitoneal shunting at keen’s point for hydrocephalus. Pak. J. Med. Sci. 2018, 34, 691–695. [Google Scholar] [CrossRef]
- McDowell, M.M.; Chiang, M.C.; Agarwal, N.; Friedlander, R.M.; Wecht, D.A. Exclusive use of fixed pressure valves for cerebrospinal fluid diversion in a modern adult cohort. Heliyon 2018, 4, e01099. [Google Scholar] [CrossRef]
- Merkler, A.E.; Ch’Ang, J.; Parker, W.E.; Murthy, S.B.; Kamel, H. The Rate of Complications after Ventriculoperitoneal Shunt Surgery. World Neurosurg. 2017, 98, 654–658. [Google Scholar] [CrossRef]
- Spirig, J.M.; Frank, M.N.; Regli, L.; Stieglitz, L.H. Shunt age-related complications in adult patients with suspected shunt dysfunction. A recommended diagnostic workup. Acta Neurochir. 2017, 159, 1421–1428. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Zhang, J.; Gu, Z.; Song, Y. The risk of intracranial infection in adults with hydrocephalus after ventriculoperitoneal shunt surgery: A retrospective study. Int. Wound J. 2020, 17, 722–728. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Mekary, R.; Glancz, L.J.; Yunusa, I.; Baticulon, R.E.; Fieggen, G.; Wellons, J.C.; Park, K.B.; Warf, B.C. Global hydrocephalus epidemiology and incidence: Systematic review and meta-analysis. J. Neurosurg. 2018, 130, 1065–1079. [Google Scholar] [CrossRef]
- Morone, P.J.; Dewan, M.C.; Zuckerman, S.L.; Tubbs, R.S.; Singer, R.J. Craniometrics and Ventricular Access: A Review of Kocher’s, Kaufman’s, Paine’s, Menovksy’s, Tubbs’, Keen’s, Frazier’s, Dandy’s and Sanchez’s Points. Oper. Neurosurg. 2019, 18, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, M.M.; Adeeb, N.; Griessenauer, C.J.; Sheikh, H.; Shahidi, S.; Tubbs, R.I.; Tubbs, R.S. The ventricular system of the brain: A comprehensive review of its history, anatomy, histology, embryology and surgical considerations. Child’s Nerv. Syst. 2014, 30, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Hayhurst, C.; Beems, T.; Jenkinson, M.D.; Byrne, P.; Clark, S.; Kandasamy, J.; Goodden, J.; Nandoe Tewarie, R.D.; Mallucci, C.L. Effect of electromagnetic-navigated shunt placement on failure rates: A prospective multicenter study. J. Neurosurg. 2010, 113, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Choux, M.; Genitori, L.; Lang, D.; Lena, G. Shunt implantation: Reducing the incidence of shunt infection. J. Neurosurg. 1992, 77, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Raffa, G.; Marseglia, L.; Gitto, E.; Germanò, A. Antibiotic-impregnated catheters reduce ventriculoperitoneal shunt infection rate in high-risk newborns and infants. Child’s Nerv. Syst. 2015, 31, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Raffa, G.; LATorre, D.; Conti, A.; Cardali, S.M.; Angileri, F.F.; Germanò, A. The efficacy of 90 cm-long peritoneal shunt catheters in newborns and infants. J. Neurosurg. Sci. 2017, 61, 33–38. [Google Scholar] [CrossRef]
- Kemp, J.; Flannery, A.M.; Tamber, M.S.; Duhaime, A.C. Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Task Force. Pediatric hydrocephalus: Systematic literature review and evidence-based guidelines—Part 9: Effect of ventricular catheter entry point and position. J. Neurosurg. Pediatr. 2014, 14, 72–76. [Google Scholar] [CrossRef]
- Dickerman, R.D.; McConathy, W.J.; Morgan, J.; Stevens, Q.E.; Jolley, J.T.; Schneider, S.; Mittler, M.A. Failure rate of frontal versus parietal approaches for proximal catheter placement in ventriculoperitoneal shunts: Revisited. J. Clin. Neurosci. 2005, 12, 781–783. [Google Scholar] [CrossRef]
- Whitehead, W.E.; Riva-Cambrin, J.; Wellons, J.C.; Kulkarni, A.V.; Limbrick, D.D.; Wall, V.L.; Rozzelle, C.J.; Hankinson, T.C.; McDonald, P.J.; Krieger, M.D. Anterior versus posterior entry site for ventriculoperitoneal shunt insertion: A randomized controlled trial by the Hydrocephalus Clinical Research Network. J. Neurosurg. Pediatr. 2021, 29, 257–267. [Google Scholar] [CrossRef]
- Yamada, S.M.; Kitagawa, R.; Teramoto, A. Relationship of the location of the ventricular catheter tip and function of the ventriculoperitoneal shunt. J. Clin. Neurosci. 2013, 20, 99–101. [Google Scholar] [CrossRef]
- Jorgensen, J.; Williams, C.; Sarang-Sieminski, A. Hydrocephalus and Ventriculoperitoneal Shunts: Modes of Failure and Opportunities for Improvement. Crit. Rev. Biomed. Eng. 2016, 44, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Little, A.S.; Zabramski, J.M.; Peterson, M.; Goslar, P.W.; Wait, S.D.; Albuquerque, F.C.; McDougall, C.G.; Spetzler, R.F. Ventriculoperitoneal shunting after aneurysmal subarachnoid hemorrhage: Analysis of the indications, complications and outcome with a focus on patients with borderline ventriculomegaly. Neurosurgery 2008, 62, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Orrego-González, E.; Enriquez-Marulanda, A.; Ascanio, L.C.; Jordan, N.; Hanafy, A.K.; Moore, J.M.; Ogilvy, C.S.; Thomas, A.J. A Cohort Comparison Analysis of Fixed Pressure Ventriculoperitoneal Shunt Valves with Programmable Valves for Hydrocephalus Following Nontraumatic Subarachnoid Hemorrhage. Oper. Neurosurg. 2020, 18, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.K.; Bollam, P.; Caldito, G. Ventriculoperitoneal shunt surgery and the risk of shunt infection in patients with hydrocephalus: Long-term single institution experience. World Neurosurg. 2012, 78, 155–163. [Google Scholar] [CrossRef] [PubMed]
- McGirt, M.J.; Zaas, A.; Fuchs, H.E.; George, T.M.; Kaye, K.; Sexton, D.J. Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin. Infect. Dis. 2003, 36, 858–862. [Google Scholar] [CrossRef]
- Al-Schameri, A.R.; Hamed, J.; Baltsavias, G.; Winkler, P.; Machegger, L.; Richling, B.; Emich, S. Ventriculoatrial Shunts in Adults, Incidence of Infection and Significant Risk Factors: A Single-Center Experience. World Neurosurg. 2016, 94, 345–351. [Google Scholar] [CrossRef]
- Birjandi, A.; Zare, E.; Hushmandi, F. Ventriculoperitoneal shunt infection: A review of treatment. Neurosurg. Q. 2012, 22, 145–148. [Google Scholar] [CrossRef]
- Korinek, A.M.; Fulla-Oller, L.; Boch, A.L.; Golmard, J.L.; Hadiji, B.; Puybasset, L. Morbidity of ventricular cerebrospinal fluid shunt surgery in adults: An 8-year study. Neurosurgery 2011, 68, 985–994. [Google Scholar] [CrossRef]
- Working Group on Neurosurgical Outcomes Monitoring; Woo, P.Y.; Wong, H.T.; Pu, J.K.; Wong, W.K.; Wong, L.Y.; Lee, M.W.; Yam, K.Y.; Lui, W.M.; Poon, W.S. Primary ventriculoperitoneal shunting outcomes: A multicentre clinical audit for shunt infection and its risk factors. Hong Kong Med. J. 2016, 22, 410–419. [Google Scholar] [CrossRef]
- Wong, J.M.; Ziewacz, J.E.; Ho, A.L.; Panchmatia, J.R.; Bader, A.M.; Garton, H.J.; Laws, E.R.; Gawande, A.A. Patterns in neurosurgical adverse events: Cerebrospinal fluid shunt surgery. Neurosurg. Focus 2012, 33, E13. [Google Scholar] [CrossRef]
- Choksey, M.S.; Malik, I.A. Zero tolerance to shunt infections: Can it be achieved? J. Neurol. Neurosurg. Psychiatry 2004, 75, 87–91. [Google Scholar] [PubMed]
- Faillace, W.J. A no-touch technique protocol to diminish cerebrospinal fluid shunt infection. Surg. Neurol. 1995, 43, 344–350. [Google Scholar] [CrossRef]
- Kalangu, K.K.N.; Esene, I.N.; Dzowa, M.; Musara, A.; Ntalaja, J.; Badra, A.K. Towards zero infection for ventriculoperitoneal shunt insertion in resource-limited settings: A multicenter prospective cohort study. Child’s Nerv. Syst. 2020, 36, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Kestle, J.R.; Riva-Cambrin, J.; Wellons, J.C., 3rd; Kulkarni, A.V.; Whitehead, W.E.; Walker, M.L.; Oakes, W.J.; Drake, J.M.; Luerssen, T.G.; Simon, T.D.; et al. Hydrocephalus Clinical Research Network. A standardized protocol to reduce cerebrospinal fluid shunt infection: The Hydrocephalus Clinical Research Network Quality Improvement Initiative. J. Neurosurg. Pediatr. 2011, 8, 22–29. [Google Scholar] [CrossRef]
- Pirotte, B.J.; Lubansu, A.; Bruneau, M.; Loqa, C.; Van Cutsem, N.; Brotchi, J. Sterile surgical technique for shunt placement reduces the shunt infection rate in children: Preliminary analysis of a prospective protocol in 115 consecutive procedures. Child’s Nerv. Syst. 2007, 23, 1251–1261. [Google Scholar] [CrossRef]
- Sweeney, J.; Zyck, S.; Tovar-Spinoza, Z.; Krishnamurthy, S.; Chin, L.; Bodman, A. Evidence-Based Perioperative Protocol for Ventriculoperitoneal Shunt Infection Reduction at a Single Institution. World Neurosurg. 2019, 128, e814–e822. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Q.; Jiang, X.; Zhang, T. Prevention options for ventriculoperitoneal shunt infections: A retrospective analysis during a five-year period. Int. J. Clin. Exp. Med. 2015, 8, 19775–19780. [Google Scholar]
- Chalouhi, N.; Whiting, A.; Anderson, E.C.; Witte, S.; Zanaty, M.; Tjoumakaris, S.; Gonzalez, L.M.F.; Hasan, D.; Starke, R.M.; Hann, S.; et al. Comparison of techniques for ventriculoperitoneal shunting in 523 patients with subarachnoid hemorrhage. J. Neurosurg. 2014, 121, 904–907. [Google Scholar] [CrossRef]
- Kamenova, M.; Croci, D.; Guzman, R.; Mariani, L.; Soleman, J. Low-dose acetylsalicylic acid and bleeding risks with ventriculoperitoneal shunt placement. Neurosurg. Focus 2016, 41, E4. [Google Scholar] [CrossRef]
- Savitz, M.H.; Bobroff, L.M. Low incidence of delayed intracerebral hemorrhage secondary to ventriculoperitoneal shunt insertion. J. Neurosurg. 1999, 91, 32–34. [Google Scholar] [CrossRef]
- Mahaney, K.B.; Chalouhi, N.; Viljoen, S.; Smietana, J.; Kung, D.K.; Jabbour, P.; Bulsara, K.R.; Howard, M.; Hasan, D.M. Risk of hemorrhagic complication associated with ventriculoperitoneal shunt placement in aneurysmal subarachnoid hemorrhage patients on dual antiplatelet therapy. J. Neurosurg. 2013, 119, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Tervonen, J.; Leinonen, V.; Jaaskelainen, J.E.; Koponen, S.; Huttunen, T.J. Rate and Risk Factors for Shunt Revision in Pediatric Patients with Hydrocephalus-A Population-Based Study. World Neurosurg. 2017, 101, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.S.; Nagahama, Y.; Nakagawa, D.; Starke, R.M.; Dlouhy, B.J.; Torner, J.C.; Jabbour, P.; Allan, L.; Derdeyn, C.P.; Greenlee, J.D.W.; et al. Hemorrhage associated with ventriculoperitoneal shunt placement in aneurysmal subarachnoid hemorrhage patients on a regimen of dual antiplatelet therapy: A retrospective analysis. J. Neurosurg. 2018, 129, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Kung, D.K.; Policeni, B.A.; Capuano, A.W.; Rossen, J.D.; Jabbour, P.M.; Torner, J.C.; Howard, M.A.; Hasan, D. Risk of ventriculostomy-related hemorrhage in patients with acutely ruptured aneurysms treated using stent-assisted coiling. J. Neurosurg. 2011, 114, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, M.; Sainte-Rose, C.; Cinalli, G.; Maixner, W.; Malucci, C.; Zerah, M.; Pierre-Kahn, A.; Renier, D.; Hoppe-Hirsch, E.; Aicardi, J. Epilepsy in children with shunted hydrocephalus. J. Neurosurg. 1999, 90, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Dan, N.G.; Wade, M.J. The incidence of epilepsy after ventricular shunting procedures. J. Neurosurg. 1986, 65, 19–21. [Google Scholar] [CrossRef]
- Klepper, J.; Büsse, M.; Strassburg, H.M.; Sörensen, N. Epilepsy in shunt-treated hydrocephalus. Dev. Med. Child Neurol. 1998, 40, 731–736. [Google Scholar] [CrossRef]
- Sato, O.; Yamguchi, T.; Kittaka, M.; Toyama, H. Hydrocephalus and epilepsy. Child’s Nerv. Syst. 2001, 17, 76–86. [Google Scholar] [CrossRef]
- Piatt, J.H., Jr.; Carlson, C.V. Hydrocephalus and epilepsy: An actuarial analysis. Neurosurgery 1996, 39, 722–777. [Google Scholar] [CrossRef]
- Venes, J.L.; Dauser, R.C. Epilepsy following ventricular shunt placement. J. Neurosurg. 1987, 66, 154–155. [Google Scholar] [CrossRef]
| Epidemiologic Data | Number of Patients (Percentage on the Total) |
|---|---|
| Age | 63.97 +/− 16.4 years (range 17–84 years) |
| Gender | M: 96 (51.1%) F: 92 (48.9%) |
| Side of shunt | Right: 178 (94.7%) Left: 10 (5.3%) |
| Etiology of Hydrocephalus | Posthemorrhagic: 88 (46.8%) NPH: 65 (34.6%) Malformative: 13 (6.9%) Posttraumatic: 11 (5.6%) CNS tumors: 7 (3.7%) Post infections: 4 (2.1%) |
| Complications needing revision surgery | 21 (11.2%) |
| Causes of revision surgery | Ventricular catheter issues: 8 (4.3%) Abdominal issues: 8 (4.3%) Hardware failure: 3 (1.6%) Infection: 2 (1.1%) |
| Ventricular catheter positioning according to Hayhurst grading | Grade 1: 171 (90.1%) Grade 2: 9 (4.8%) Grade 3: 8 (4.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, E.V.; Bongetta, D.; Cofano, F.; Versace, A.; Garbossa, D.; Bertuccio, A.; Armocida, D.; d’Auria, P.; Farina, L.M.; Assietti, R.; et al. Ventricular Peritoneal Shunting Using Modified Keen’s Point Approach: Technical Report and Cases Series. Surgeries 2022, 3, 314-322. https://doi.org/10.3390/surgeries3040034
Colombo EV, Bongetta D, Cofano F, Versace A, Garbossa D, Bertuccio A, Armocida D, d’Auria P, Farina LM, Assietti R, et al. Ventricular Peritoneal Shunting Using Modified Keen’s Point Approach: Technical Report and Cases Series. Surgeries. 2022; 3(4):314-322. https://doi.org/10.3390/surgeries3040034
Chicago/Turabian StyleColombo, Elena Virginia, Daniele Bongetta, Fabio Cofano, Alessandro Versace, Diego Garbossa, Alessandro Bertuccio, Daniele Armocida, Patrizia d’Auria, Lisa Maria Farina, Roberto Assietti, and et al. 2022. "Ventricular Peritoneal Shunting Using Modified Keen’s Point Approach: Technical Report and Cases Series" Surgeries 3, no. 4: 314-322. https://doi.org/10.3390/surgeries3040034
APA StyleColombo, E. V., Bongetta, D., Cofano, F., Versace, A., Garbossa, D., Bertuccio, A., Armocida, D., d’Auria, P., Farina, L. M., Assietti, R., & Tartara, F. (2022). Ventricular Peritoneal Shunting Using Modified Keen’s Point Approach: Technical Report and Cases Series. Surgeries, 3(4), 314-322. https://doi.org/10.3390/surgeries3040034










