Short-Term Postoperative Outcome of Baerveldt Glaucoma Implant with Two Tubes Inserted into the Vitreous Cavity
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Outcome Measures
2.2. Statistical Analyses
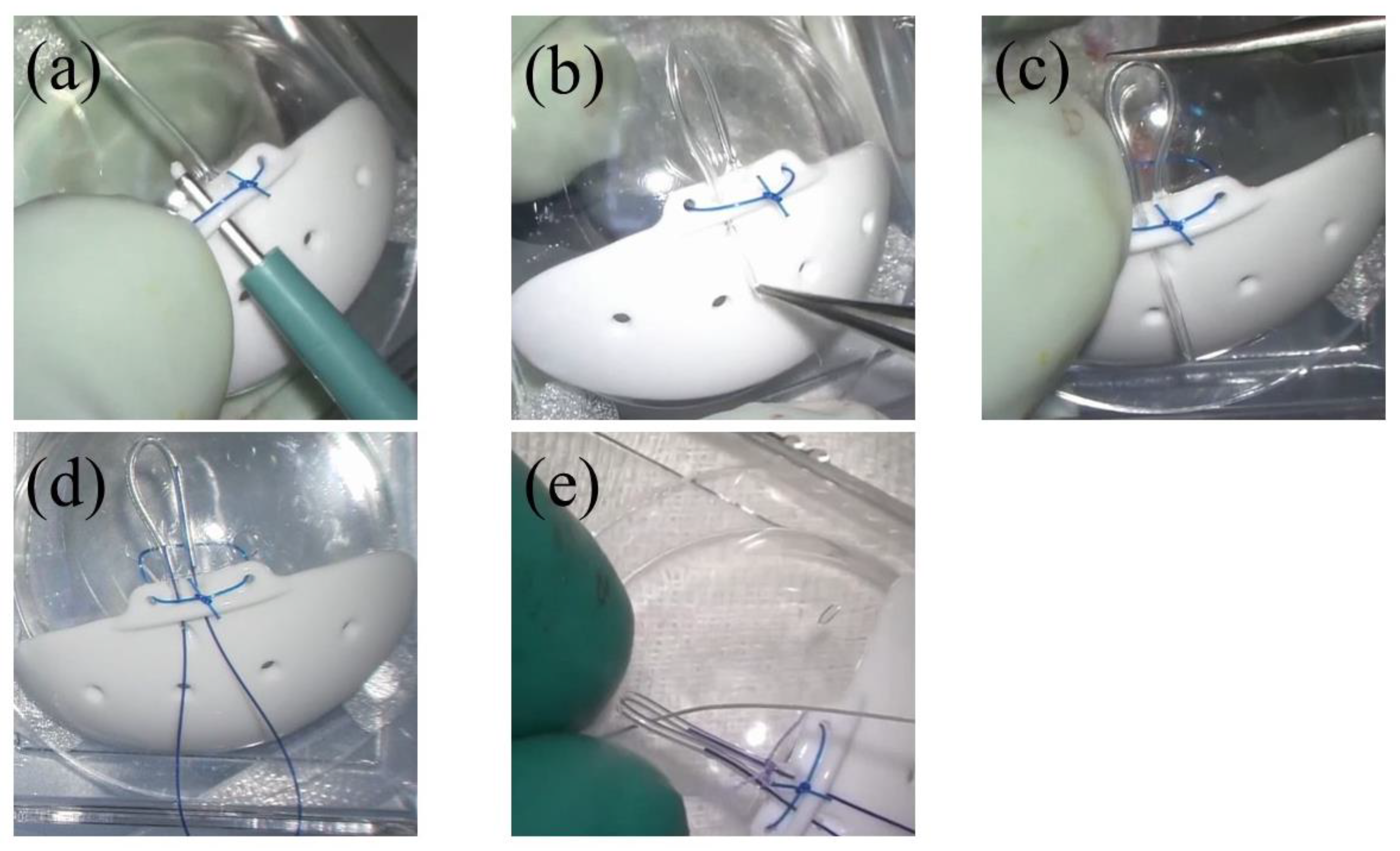
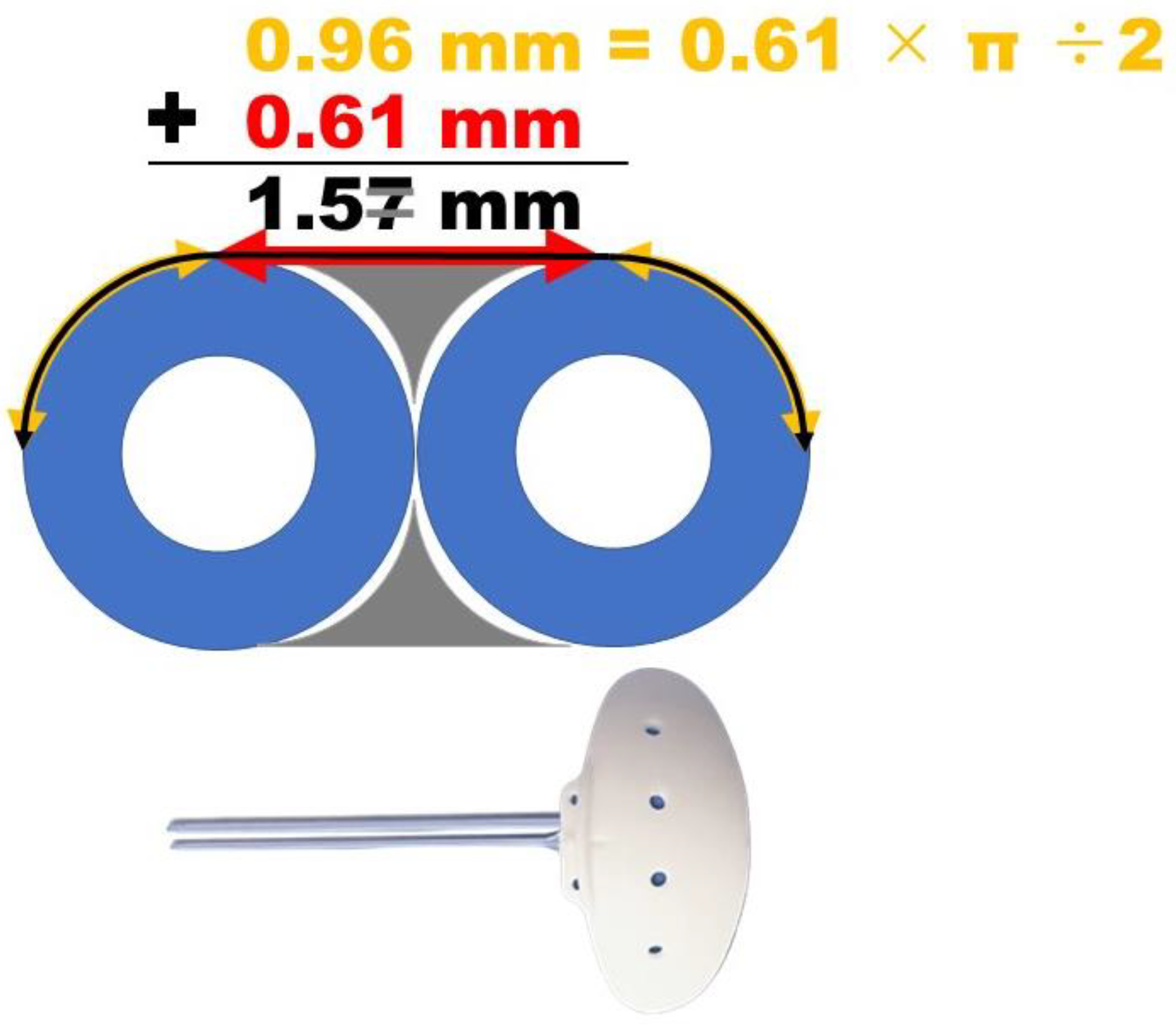
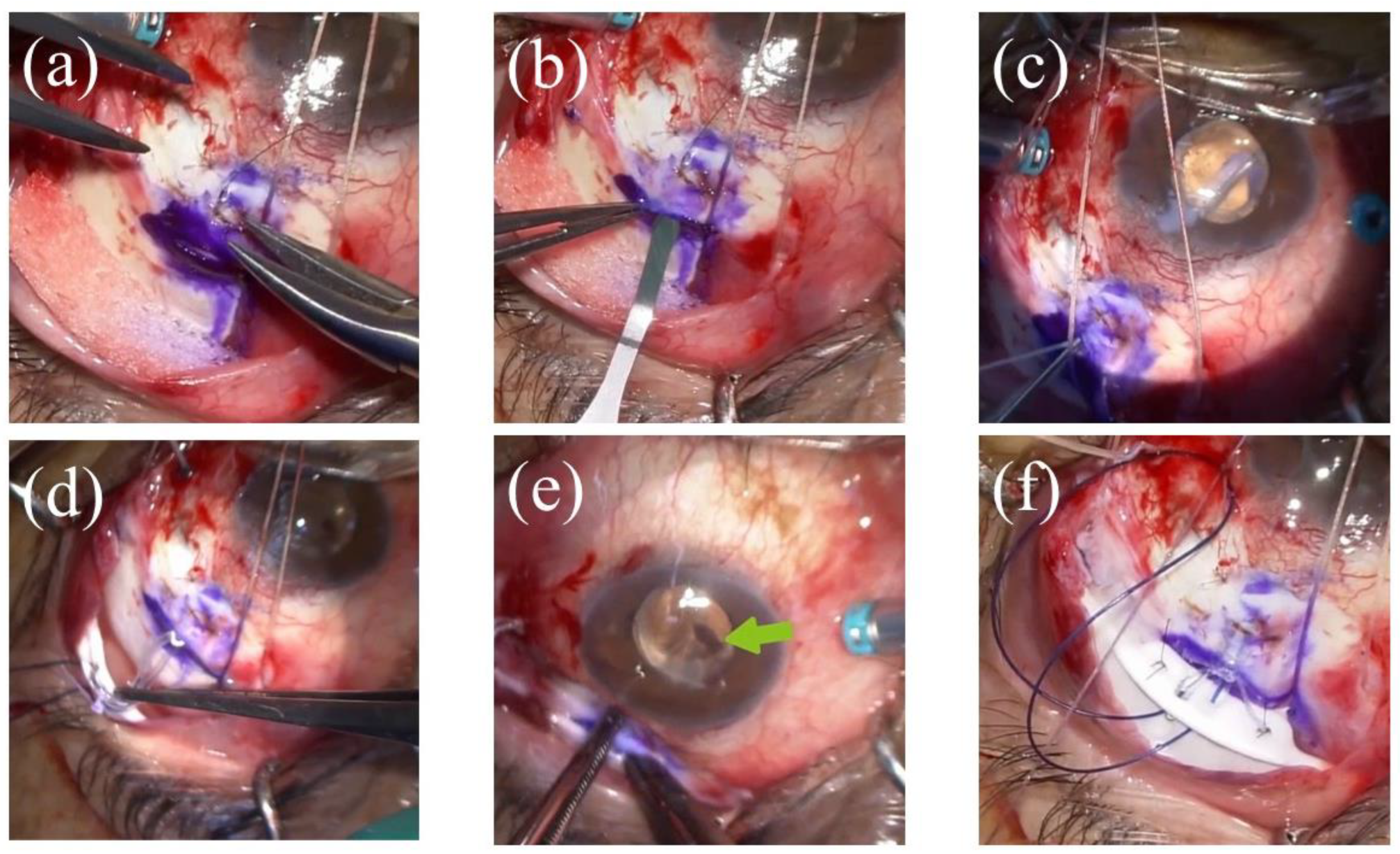
2.3. Surgical Technique
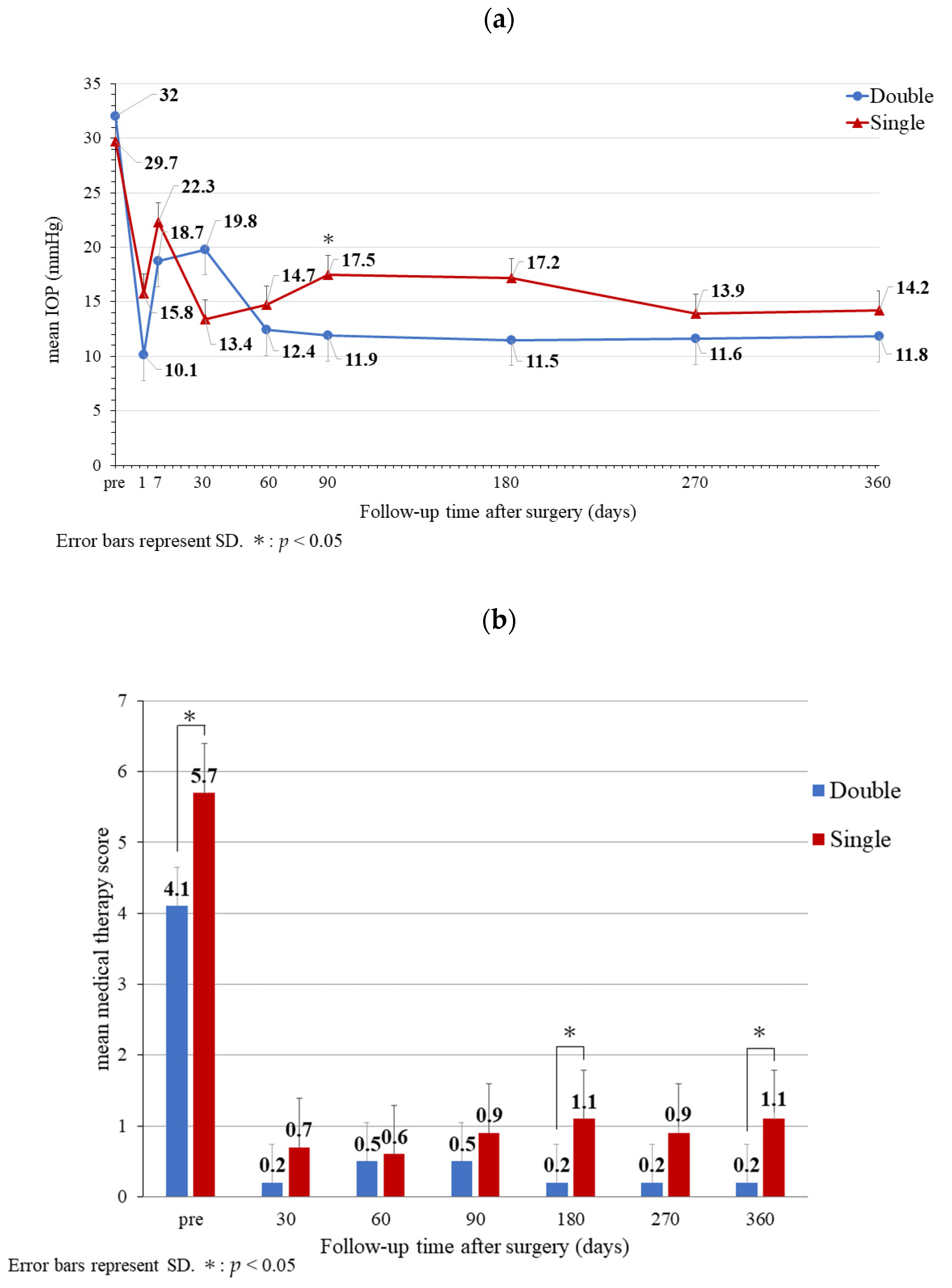
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sidoti, P.A.; Dunphy, T.R.; Baerveldt, G.; LaBree, L.; Minckler, D.S.; Lee, P.P.; Heuer, D.K. Experience with the Baerveldt Glaucoma Implant in Treating Neovascular Glaucoma. Ophthalmology 1995, 102, 1107–1118. [Google Scholar] [CrossRef]
- Gedde, S.J.; Schiffman, J.C.; Feuer, W.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L. Tube versus Trabeculectomy Study Group. Treatment Outcomes in the Tube Versus Trabeculectomy (TVT) Study After Five Years of Follow-Up. Am. J. Ophthalmol. 2012, 153, 789–803.e2. [Google Scholar] [CrossRef] [PubMed]
- Gedde, S.J.; Feuer, W.J.; Lim, K.S.; Barton, K.; Goyal, S.; Ahmed, I.I.; Brandt, J.D. Treatment Outcomes in the Primary Tube Versus Trabeculectomy Study After 3 Years of Follow-Up. Ophthalmology 2020, 127, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.K.; Lee, J.H.; Warren, J.L.; Teng, C.C. GlaucoMap—Distribution of Glaucoma Surgical Procedures in the United States. Clin Ophthalmol. 2020, 14, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.T.; Madike, R.; Huang, S.; Cameron, C.; Selva, D.; Casson, R.J.; Wong, C.X. Changing Trends in Glaucoma Surgery within Australia. Br. J. Ophthalmol. 2021, 106, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M.; Early Manifest Glaucoma Trial Group. Reduction of Intraocular Pressure and Glaucoma Progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, A.B. An Assessment of Flow and Pressure Control in Experimental Models of Glaucoma Drainage Surgery. Ph.D. Thesis, UCL (University College London), London, UK, 2014. [Google Scholar]
- Kusaka, M.; Kujime, Y.; Yamakawa, M.; Akimoto, M. Baerveldt Tube Shunt Implantation Through a Long Scleral Tunnel. Eur. J. Ophthalmol. 2019, 29, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Akamine, R.; Miyamoto, N.; Kusaka, M.; Akimoto, M. Tube Placement into the Long Scleral Tunnel with a Catheter Needle without a Scleral Valve and/or Graft Patch: 1-Year Outcome. EC Ophthalmol. 2022, 13, 1–9. [Google Scholar]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software ‘EZR’for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Christakis, P.G.; Kalenak, J.W.; Tsai, J.C.; Zurakowski, D.; Kammer, J.A.; Harasymowycz, P.J.; Mura, J.J.; Cantor, L.B.; Ahmed, I.I. The Ahmed Versus Baerveldt Study: Five-Year Treatment Outcomes. Ophthalmology 2016, 123, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Lavin, M.J.; Franks, W.A.; Wormald, R.P.; Hitchings, R.A. Clinical Risk Factors for Failure in Glaucoma Tube Surgery: A Comparison of Three Tube Designs. Arch. Ophthalmol. 1992, 110, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Heuer, D.K.; Lloyd, M.A.; Abrams, D.A.; Baerveldt, G.; Minckler, D.S.; Lee, M.B.; Martone, J.F. Which Is Better? One or Two? A Randomized Clinical Trial of Single-Plate Versus Double-Plate Molteno Implantation for Glaucomas in Aphakia and Pseudophakia. Ophthalmology 1992, 99, 1512–1519. [Google Scholar] [CrossRef]
- Souza, C.; Tran, D.H.; Loman, J.; Law, S.K.; Coleman, A.L.; Caprioli, J. Long-Term Outcomes of Ahmed Glaucoma Valve Implantation in Refractory Glaucomas. Am. J. Ophthalmol. 2007, 144, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Britt, M.T.; LaBree, L.D.; Lloyd, M.A.; Minckler, D.S.; Heuer, D.K.; Baerveldt, G.; Varma, R. Randomized Clinical Trial of the 350-mm2 Versus the 500-mm2 Baerveldt Implant: Longer Term Results: Is Bigger Better? Ophthalmology 1999, 106, 2312–2318. [Google Scholar] [CrossRef]
- Lloyd, M.A.; Baerveldt, G.; Fellenbaum, P.S.; Sidoti, P.A.; Minckler, D.S.; Martone, J.F.; LaBree, L.; Heuer, D.K. Intermediate-Term Results of a Randomized Clinical Trial of the 350-Versus-the 500-mm2 Baerveldt Implant. Ophthalmology 1994, 101, 1456–1463; discussion 1463. [Google Scholar] [CrossRef]
- Sheybani, A.; Reitsamer, H.; Ahmed, I.I. Fluid Dynamics of a Novel Micro-Fistula Implant for the Surgical Treatment of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4789–4795. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, R.F. Flow of Aqueous Humor in Humans [The Friedenwald Lecture]. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3145–3166. [Google Scholar] [PubMed]
- Kudsieh, B.; Fernández-Vigo, J.I.; Agujetas, R.; Montanero, J.M.; Ruiz-Moreno, J.M.; Fernández-Vigo, J.Á.; García-Feijóo, J. Numerical Model to Predict and Compare the Hypotensive Efficacy and Safety of Minimally Invasive Glaucoma Surgery Devices. PLoS ONE 2020, 15, e0239324. [Google Scholar] [CrossRef] [PubMed]
- Fea, A.M.; Menchini, M.; Rossi, A.; Posarelli, C.; Malinverni, L.; Figus, M. Early Experience with the New XEN63 Implant in Primary Open-Angle Glaucoma Patients: Clinical Outcomes. J. Clin. Med. 2021, 10, 1628. [Google Scholar] [CrossRef] [PubMed]
- Breckenridge, R.R.; Bartholomew, L.R.; Crosson, C.E.; Kent, A.R. Outflow resistance of the Baerveldt glaucoma drainage implant and modifications for early postoperative intraocular pressure control. J. Glaucoma 2004, 13, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.L.; Groman-Lupa, S. Design and Performance of a Large Lumen Glaucoma Drainage Device. Eye 2017, 31, 152–156. [Google Scholar] [CrossRef][Green Version]
- Salazar, D.; Shah, M. Double XEN implant in Childhood glaucoma. In Proceedings of the 9th World Glaucoma E-Congress, Kyoto, Japan, 30 June–3 July 2021. [Google Scholar]
- Kolomeyer, A.M.; Seery, C.W.; Emami-Naeimi, P.; Zarbin, M.A.; Fechtner, R.D.; Bhagat, N. Combined Pars Plana Vitrectomy and Pars Plana Baerveldt Tube Placement in Eyes with Neovascular Glaucoma. Retina 2015, 35, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Nishitsuka, K.; Sugano, A.; Matsushita, T.; Nishi, K.; Yamashita, H. Surgical Outcomes After Primary Baerveldt Glaucoma Implant Surgery with Vitrectomy for Neovascular Glaucoma. PLoS ONE 2021, 16, e0249898. [Google Scholar] [CrossRef] [PubMed]
- Go, M.; Ulrich, J.N.; Fleischman, D. Intraocular and Extraocular Hemorrhage Associated with Ligature Release of Non-Valved Glaucoma Drainage Implant. Am. J. Ophthalmol. Case Rep. 2017, 5, 114–116. [Google Scholar] [CrossRef]
- Takayama, K.; Someya, H.; Yokoyama, H.; Takamura, Y.; Morioka, M.; Sameshima, S.; Ueda, T.; Kitano, S.; Tashiro, M.; Sugimoto, M.; et al. Risk Factors of Neovascular Glaucoma After 25-Gauge Vitrectomy for Proliferative Diabetic Retinopathy with Vitreous Hemorrhage: A Retrospective Multicenter Study. Sci. Rep. 2019, 9, 14858. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.B.; Han, J.W.; Yim, H.B.; Lee, N.Y. Beneficial Effects of Adjuvant Intravitreal Bevacizumab Injection on Outcomes of Ahmed Glaucoma Valve Implantation in Patients with Neovascular Glaucoma: Systematic Literature Review. J. Ocul. Pharmacol. Ther. 2015, 31, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xu, X.; Zhang, X.; Sun, X. Clinical Outcomes of Ahmed Glaucoma Valve Implantation with or without Intravitreal Bevacizumab Pretreatment for Neovascular Glaucoma: A Systematic Review and Meta-Analysis. J. Glaucoma 2016, 25, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, M.B.; Smith, M.F. Prevention of Early Hypotony Associated with Molteno Implants by a New Occluding Stent Technique. Ophthalmology 1993, 100, 85–90. [Google Scholar] [CrossRef]
- Trible, J.R.; Brown, D.B. Occlusive Ligature and Standardized Fenestration of a Baerveldt Tube with and without Antimetabolites for Early Postoperative Intraocular Pressure Control. Ophthalmology 1998, 105, 2243–2250. [Google Scholar] [CrossRef]
- Ferreira, J.; Fernandes, F.; Patricio, M.; Brás, A.; Rios, C.; Stalmans, I.; Pinto, L.A. Magnetic Resonance Imaging Study on Blebs Morphology of Ahmed Valves. J. Curr. Glaucoma Pract. 2015, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sano, I.; Tanito, M.; Uchida, K.; Katsube, T.; Kitagaki, H.; Ohira, A. Assessment of Filtration Bleb and Endplate Positioning Using Magnetic Resonance Imaging in Eyes Implanted with Long-Tube Glaucoma Drainage Devices. PLoS ONE 2015, 10, e0144595. [Google Scholar] [CrossRef] [PubMed][Green Version]
| (a) | ||||||||||||||||
| Preoperative | Postoperative (12 Months) | Surgical Complications | ||||||||||||||
| Case | Age (Years) | Sex | Eye | Type | Previous Surgeries | Antiplatelet Drug | SMTS | IOP (mmHg) | VA | CECD (Cells/mm2) | SMTS | IOP (mmHg) | VA | CECD (Cells/mm2) | Intraoperative Complications | Postoperative Complications |
| 1 | 49 | M | L | OIS | PEA + IOL, PPV + SFIOL | 5 | 31 | 10/200 | 2299 | 0 | 16 | 20/200 | 1252 | VH | ||
| 2 | 88 | M | R | CRVO | PEA + IOL, PPV | 2 | 32 | HM | 1887 | 0 | 10 | NLP | 1761 | VH, ME | ||
| 3 | 60 | M | R | PDR | PEA + IOL + LET, PPV | 6 | 25 | 30/200 | 2257 | 2 | 13 | 30/200 | 2304 | VH | ||
| 4 | 84 | M | L | PDR | PEA + IOL, PPV | Clopidogrel, Aspirin | 2 | 22 | 20/200 | 2208 | 0 | 11 | 60/200 | 2273 | Iridodialysis | VH |
| 5 | 57 | M | L | PDR | PEA + IOL, PPV, LET | 4 | 35 | CF | 2481 | 0 | 7 | 40/200 | 1497 | VH, CD | ||
| 6 | 66 | M | R | PDR | PEA + IOL, PPV, LET | 4 | 34 | CF | 874 | 0 | 11 | NLP | NR | VH, RRD, PB | ||
| 7 | 72 | M | L | PDR | PEA + IOL, PPV, LET | Aspirin | 3 | 30 | HM | 2092 | 0 | 14 | 8/200 | 2088 | VH | |
| 8 | 65 | M | L | PDR | PEA + IOL | 5 | 13 | 10/200 | 2381 | 0 | 15 | 160/200 | 2179 | VH | ||
| 9 | 66 | M | R | CRVO | PEA + IOL, PPV | 5 | 44 | 2/200 | 1414 | 0 | 10 | 8/200 | 2309 | ME, ERM | ||
| 10 | 41 | M | L | PDR | PEA + IOL, PPV | 5 | 54 | 100/200 | 1330 | 0 | 11 | 10/200 | 1117 | VH | ||
| (b) | ||||||||||||||||
| Preoperative | Postoperative (12 Months) | Surgical Complications | ||||||||||||||
| Case | Age (Years) | Sex | Eye | Type | Previous Surgeries | Antiplatelet Drug | SMTS | IOP (mmHg) | VA | CECD (cells/mm2) | SMTS | IOP (mmHg) | VA | CECD (cells/mm2) | Intraoperative Complications | Postoperative Complications |
| 1 | 49 | W | L | PDR | PEA + IOL + PPV, LET, BN | 7 | 29 | 2/200 | 2387 | 1 | 16 | 30/200 | 2028 | ME | ||
| 2 | 49 | W | R | PDR | PEA + IOL + PPV | 6 | 23 | 120/200 | 1818 | 1 | 18 | 200/200 | 2358 | |||
| 3 | 46 | M | R | PDR | PEA + IOL + PPV + LET | 4 | 22 | 8/200 | 2137 | 2 | 7 | 30/200 | 2049 | |||
| 4 | 67 | M | R | PDR | PEA + IOL, PPV | 6 | 32 | 20/200 | NR | 1 | 11 | HM | 2053 | RRD | ||
| 5 | 51 | M | L | PDR | PEA + IOL + PPV, PI | 7 | 40 | CF | 1859 | 0 | 11 | 60/200 | 2632 | IB | ||
| 6 | 75 | M | L | OIS | PEA + SFIOL + PPV | 5 | 34 | LP | NM | 2 | 18 | NLP | 1222 | VH | ||
| 7 | 51 | M | R | PDR | PEA + IOL + PPV | 5 | 30 | CF | 2770 | 0 | 18 | 60/200 | 2198 | VH, ME | ||
| 8 | 54 | M | R | PDR | PEA + IOL + PPV | 6 | 38 | 20/200 | NR | 4 | 16 | 10/200 | 2257 | |||
| 9 | 57 | M | L | PDR | PEA + IOL + PPV, LET | 5 | 24 | 120/200 | 1684 | 0 | 10 | 60/200 | 1529 | VH | ||
| 10 | 53 | M | L | PDR | PEA + IOL + PPV | 6 | 25 | 60/200 | 2639 | 0 | 17 | 60/200 | 2653 | ME | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomita, K.; Akamine, R.; Morino, K.; Kusaka, M.; Akimoto, M. Short-Term Postoperative Outcome of Baerveldt Glaucoma Implant with Two Tubes Inserted into the Vitreous Cavity. Surgeries 2022, 3, 323-333. https://doi.org/10.3390/surgeries3040035
Tomita K, Akamine R, Morino K, Kusaka M, Akimoto M. Short-Term Postoperative Outcome of Baerveldt Glaucoma Implant with Two Tubes Inserted into the Vitreous Cavity. Surgeries. 2022; 3(4):323-333. https://doi.org/10.3390/surgeries3040035
Chicago/Turabian StyleTomita, Kosei, Rinko Akamine, Kazuya Morino, Mami Kusaka, and Masayuki Akimoto. 2022. "Short-Term Postoperative Outcome of Baerveldt Glaucoma Implant with Two Tubes Inserted into the Vitreous Cavity" Surgeries 3, no. 4: 323-333. https://doi.org/10.3390/surgeries3040035
APA StyleTomita, K., Akamine, R., Morino, K., Kusaka, M., & Akimoto, M. (2022). Short-Term Postoperative Outcome of Baerveldt Glaucoma Implant with Two Tubes Inserted into the Vitreous Cavity. Surgeries, 3(4), 323-333. https://doi.org/10.3390/surgeries3040035






