Abstract
Background: Prehabilitation is gaining increasing interest and shows promising effects on short- and long-term outcomes among patients undergoing major surgery. The effect of multimodal, interdisciplinary prehabilitation has not yet been studied in patients with severe heart failure scheduled for the implantation of a left-ventricular assist device (LVAD). Methods: This randomized controlled multi-center study evaluates the effect of preoperative combined optimization of nutritional and functional status. Patients in the intervention group are prescribed daily in-bed cycling and oral nutrition supplements (ONS) from study inclusion until the day before LVAD-implantation. Patients in the control group receive standard of care treatment. The primary outcomes for the pilot study that involves 48 patients are safety (occurrence of adverse events), efficacy (group separation regarding the intake of macronutrients), feasibility of the trial protocol (compliance (percentage of received interventions) and confirmation of recruitment rates. Secondary outcomes include longitudinal measurements of muscle mass, muscle strength, physical function and quality of life, next to traditional clinical outcomes (30-day mortality, hospital and ICU length of stay, duration of mechanical ventilation and number of complications and infections). If the pilot study is successful, a larger confirmatory, international multicenter study is warranted.
1. Introduction
With more than 23 million diagnosed patients worldwide, heart failure remains a major public health problem [1,2]. With a life-time risk of 20–46% of being diagnosed with heart failure, the prevalence of heart failure ranges from about 1 to 12% worldwide [3] and about 10% in the elderly population [4,5]. Heart failure is a common cause of death and 50% of patients die within 5 years after the diagnosis [3,6].
When the symptoms of chronic heart failure are non-responsive to medical therapy, the implantation of a left-ventricular assist-device (LVAD) is one possible invasive treatment that is increasingly used as a definitive therapy for terminal heart failure or a bridge-to-transplant. Roughly 1000 LVAD systems are implanted in Germany every year [7]. From 2006 until 2016, 19,000 LVAD devices were implanted in the USA, with increasing numbers of new LVAD implants per year, as well as LVAD implants as destination therapy [1]. In-hospital mortality decreased significantly during the last few years and roughly 60–80% of the patients survived the first year after LVAD implantation [8,9] with a median survival time of 48 months [10].
However, all major surgical procedures are associated with systemic inflammatory response syndrome (SIRS). While SIRS is anticipated after cardiac surgery and LVAD implantation, this syndrome can cause multiple acute and persistent organ dysfunctions, perioperative complications, longer hospital stay and delayed rehabilitation [11,12,13,14,15]. Postoperative complications are a major determinant of morbidity, mortality, length of hospital stay, hospital costs and quality of life after heart surgery [16].
With an increasingly comorbid and elderly population with severe chronic heart failure (CHF) and reduced general conditions receiving more complex cardiac surgeries, new strategies are, therefore, urgently needed to improve the long-term outcome and quality of life of these patients [17,18,19]. The patient’s nutritional status and muscle mass, along with other preoperative factors, such as age and comorbidities, influence the patient’s postoperative clinical and functional outcome in the ICU and the years after discharge [20,21]. Prehabilitation involves optimizing the patient’s health status before elective surgery. Possible strategies include optimization of the patients’ nutritional and functional status via optimization of blood glucose and vital parameters to psychological interventions and beyond [15].
In patients with heart disease, preoperative ONS have been administered in the setting of elective cardiac surgery and patients with heart failure (Table 1), showing feasibility and safety, as well as good patient compliance. However, the benefits observed so far cannot be generalized and should lead to an increased interest in these subjects.

Table 1.
Studies addressing preoperative oral nutrition supplements in cardiac surgery and chronic heart failure. Abbreviations: CHF: chronic heart failure, EF: ejection fraction, LOS: length of stay, NYHA: heart failure classified by New York Heart Association, ONS: oral nutrition supplements, TNF: tumor necrosis factor; QOL: quality of life.
While several studies have proven the efficacy and safety of exercise in these patient groups, most studies focused on patients classified as moderate stage CHF and few studies investigated the effects of preoperative aerobic exercise training. To the authors’ knowledge, no exercise studies regarding patients with end-stage heart failure or patients planned for ventricular assist devices are currently being carried out or have been published. However, an exercise protocol for patients planned for LVAD implantation should regard the severely reduced functional capacity, as well as the compromised hemodynamics, of this patient group. Therefore, it is necessary to compare preoperative LVAD patients to both patients with CHF, as well as critically ill cardiac surgery patients, and apply similar monitoring and exercise protocols to ensure maximum patient safety.
Exercise programs have been used in patients with CHF and in preoperative cardiac surgery and intensive care unit (ICU) patients, as described in Table 2. In-bed cycling is a physiotherapeutic approach, focusing on the lower limb muscles, which are especially affected by immobilization and are critical to ambulation and functional independence [26,27]. The technique can be used actively or passively and several studies also demonstrated benefits from passive exercise [28,29]. In-bed cycling has proven to be safe, well-tolerated and feasible in patients with heart failure and in critically ill patients. Thus, the treatment has gained popularity within the last few years and several studies are currently being conducted.

Table 2.
Selected studies of exercise in patients with heart failure, preoperative cardiosurgical patients and ICU patients. Abbreviations: 6MWD: 6-min walk distance, BNP: brain natriuretic peptide, CABG: coronary artery bypass graft, CHF: chronic heart failure, ECMO: extracorporeal membrane oxygenation, EF: ejection fraction, HR: heart rate, ICU: intensive care unit, LOS: length-of stay, NYHA: heart failure classified by New York Heart Association, ONS: oral nutrition supplements, pVO2: peak oxygen consumption, SF-36: Short Form 36, TNF: tumor necrosis factor; QOL: quality of life.
As shown above, a multitude of studies over the last few years have shown that neither the sole adequacy of energy-protein intake nor exercise alone are sufficient to induce anabolism in past trials and to significantly improve the nutritional and functional status of the patients. In addition, neither pure weight gain nor gain of fat mass without the buildup of muscle mass should be the main target of therapy, especially against the background of sarcopenic obesity. In the same vein, the pure gain of muscle mass does not always lead to improved physical function, which should be the overall aim to improve the patient’s long-term outcome and quality of life. Therefore, the authors hypothesize that a combination of both exercise and nutrition may have a greater prehabilitation effect than one of the interventions given alone.
To our knowledge, there are no studies regarding the preoperative optimization of nutrition and physical exercise in patients scheduled for elective cardiac surgery or for LVAD implantation. Therefore, the PROPER-LVAD study aims to optimize the nutritional and functional status of patients scheduled for an elective LVAD implantation via a preoperative combined intervention that consists of oral nutritional supplements (ONS) and exercise (in-bed cycling).
2. Materials and Methods
2.1. Registration and Ethics
The study will be conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committees of all participating centers, including RWTH Aachen University (EK 313-19, initial date of approval 3 December 2019) and Bad Oeynhausen (AZ 2021-859_1). This study was registered at clinicaltrials.gov (NCT04205760).
2.2. Study Design and Sites
This is a randomized controlled multi-center clinical pilot trial. A total of 40 patients will be recruited in the Heart and Diabetes Center NRW, Bad Oeynhausen and at the University Hospital RWTH Aachen in Germany. The coordination and project management will be performed by the leading site RWTH Aachen University.
This pilot study consists of the following 2 phases:
- Phase 1 is a non-randomized run-in phase, where only ONS are administered to 8 patients. This phase evaluates the safety of the ONS therapy alone in this group of hemodynamically compromised patients.
- Phase 2 is a randomized controlled clinical trial, where the full study protocol is administered to 40 patients. In Phase 2, patients are either randomized to the full study protocol, consisting of ONS + in-bed cycling, or to the control group (regular hospital diet and physiotherapy as per standard protocol only).
2.3. Patients
A total of 48 adult patients who are scheduled for the elective implantation of an LVAD are screened and enrolled consecutively. Written informed consent is obtained from all patients, after receiving written information and having the study procedures explained and questions answered in a face-to face visit with a medical doctor with sufficient time to consider. Eligibility criteria are listed in Table 3.

Table 3.
In- and exclusion criteria of the PROPER-LVAD study. Abbreviations: ECMO: extracorporeal membrane oxygenation, GFR: glomerular filtration rate, INTERMACS: Interagency Registry for Mechanically Assisted Circulatory Support classification; LVAD: left ventricular assist device.
2.4. Study Intervention
2.4.1. Nutrition Intervention and Monitoring
All patients receive a regular hospital diet according to hospital standard targeting of 2000 kcal and 48–98 g protein per day. All patients are screened for nutritional risk using the NRS 2002.
In the intervention group only, the ONS are started as soon as feasible after study inclusion for Phase 1 and after randomization for Phase 2, but no more than 24 h afterwards. The ONS are administered to the patient until the day before surgery, with the goal to administer the ONS for at least 5 consecutive days.
The ONS used in this study is the Fresubin® PRO kcal DRINK (Fresenius Kabi® Deutschland GmbH, Bad Homburg/Germany), which is a food for special medical purposes for use under medical supervision. Fresubin® PRO kcal DRINK is a nutritionally complete, high energy (2.4 kcal/mL), high protein (0.144 g/mL) milk shake-style nutritional supplement, enriched with vitamins, minerals and trace elements.
The ONS are provided in accordance with the patient’s nutritional status. Since actual food intake is expected to be reduced up to 50% in this patient group, patients with high nutritional risk defined as NRS 2002 ≥ 3 receive 3 ONS/day (equaling 1440 kcal and 86.4 g protein), in addition to the regular hospital diet. Patients with low nutritional risk defined as NRS < 3 are provided 2 ONS/day (equaling 960 kcal and 57.6 g protein), in addition to the regular hospital diet. The nutritional intake of all patients is assessed during the entire study intervention period using the quarter-plate-method (www.fightmalnutrition.eu (accessed on 1 December 2017)).
Regular monitoring of blood sugars, electrolytes, phosphate, and triglycerides occur as per standard clinical protocol, but at least twice weekly. Possible adverse effects of ONS, such as nausea, vomiting, diarrhea and obstipation, are be monitored daily, and the amount of nutrition and medication will be adjusted.
Special cases include the following:
- In the unlikely event that the patient is discharged from the hospital prior to LVAD implantation, the study ONS will be stopped at the day of discharge and further nutrition therapy will be left to the discretion of the following facility.
- If a single dose of ONS was failed to be administered to the patient, it can be made up for the next day, since it is the average protein and energy intake that will allow the patient to improve physical function and adverse effects, due to the fact that increasing the amount of ONS by a single unit on a day is not likely.
- If more than a single dose of ONS was failed to be administered to the patient, it shall not be made up for on the next day to avoid adverse effects for the patient.
- In case of gastrointestinal or metabolic intolerance, patients with fluid restriction and patients experiencing discomfort during the nutrition intervention, the dosage of ONS will be adjusted according to the clinical symptoms and blood work. This will allow the patients to consume one drink less per day than initially planned (e.g., 1 ONS/d in patients without nutritional risk or 2 ONS/d in patients with nutritional risk).
2.4.2. Exercise Intervention and Monitoring
In Phase 2 only, in-bed cycling is started as soon as possible after randomization, but no more than 24 h thereafter. The in-bed cycling is delivered once daily for 50 min for at least 5 days a week, with vigorous verbal encouragement from the trained research staff who are delivering the intervention to promote active cycling. The cycling is be continued until evening before LVAD implantation.
Standard safety criteria will be assessed prior to initiating cycling treatments. The cycling is supervised by medically trained research staff. The patients are placed in a semi-recumbent position. The patient’s heart rate and transcutaneous oxygen saturation of blood (SpO2) is monitored continuously; the systolic and diastolic blood pressure is be measured non-invasively in 2.5-min intervals. The in-bed cycling is performed actively with graded increasing resistance or passively, according to our protocol (Figure 1). There are 3 cycling periods for 12 min each with a 2-min assisted interval in-between the cycling periods, allowing the patient to rest. Before and after the in-bed cycling, there is a 5-min warm-up, as well as a 5-min cool-down. In between the cycling periods, the resistance of the ergometer will be adjusted according to the physiologic response of the patient, as shown in Figure 1.
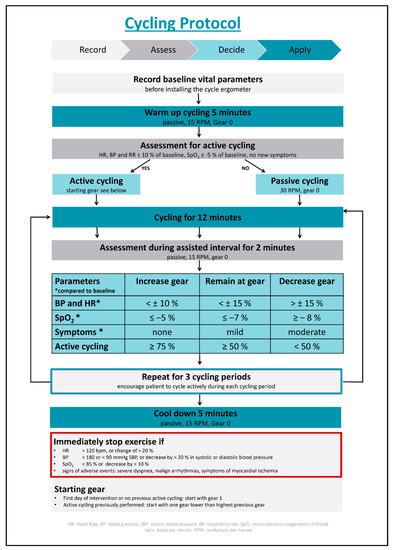
Figure 1.
Cycling protocol. Abbreviations: BP: blood pressure, HR: heart rate, RPM: rotations per minute, RR: blood pressure; SPO2: transcutaneous oxygen saturation.
The ergometer used in this study is the MotoMed Letto II cycle ergometer (RECK-Technik GmbH & Co. KG Medizintechnik, Betzenweiler/Germany). It is a bed cycling device approved for intensive care and rehabilitation. This device has one passive gear, a motor assisted gear and several active gears, ranging from a very light to very heavy resistance.
Monitoring of adverse effects related to bedside cycling will occur continuously during all exercise sessions. The exercise will immediately be terminated if one of the following adverse effects occurs:
- Severe dyspnea, dizziness, or syncope;
- New malign arrhythmias, new signs of myocardial ischemia;
- Non-physiological reactions of vital parameters, including the following:
- ○
- Heart rate > 120 bpm, or change of >20%;
- ○
- Blood pressure (BP) > 180 mmHg systolic BP or decrease by 20% in systolic or diastolic BP;
- ○
- Transcutaneous oxygen saturation < 85% or decrease by >10%.
Terminated cycling sessions will be attempted to be completed on the same day, after allowing the patient to rest for a while.
Special cases include the following:
- In the unlikely event that the patient is discharged from the hospital prior to LVAD implantation, the exercise will be stopped at day of discharge.
- If an exercise session was missed by the patient, it should not be made up for the next day.
- If an exercise intervention needs to be terminated early due to interruptions, it will be attempted to be completed during the same day, adding warm-up and cool-down phases to the remaining minutes of exercise.
2.5. Control Group
Patients randomized to the control group will receive a standard hospital diet. Patients in the control group will not be offered ONS as standard of care. If the treating medical team decides that ONS are detected in a patient and there is no clinical equipoise regarding the administration of ONS, the patient should not be included in this study.
Patients randomized to the control group will receive standard physiotherapy, not involving cycle-ergometry.
2.6. Study Duration
The total study duration will be 48 months (including set-up, final evaluation and statistics). The recruitment phase started in January 2022 and will end in June 2023 (15 months). The planned duration of study inclusion per patient will be ~2 weeks for Phase 1 and 7 months for Phase 2, the latter including the perioperative hospital stay (~1 month) and a follow-up period of 6 months.
2.7. Outcomes
The primary goal of this pilot study will be to prove the safety and feasibility of the study intervention. The data collected will provide a basic concept for a following larger-scale clinical trial that aims to evaluate the clinical significance of oral nutrition support and exercise in the population of high-risk cardiac surgery patients with LVAD implantation. For Phase one, only safety and compliance will be determined. In Phase 2, the safety and feasibility will be determined, as summarized in Table 4.

Table 4.
Primary outcome parameters. Abbreviations: ONS: oral nutrition supplements.
The secondary outcomes include longitudinal measurements of the following parameters at study inclusion, day before surgery, ICU and hospital discharge and scheduled at routine follow ups at 3 and 6 months after surgery:
- Muscle mass (quadriceps muscle layer thickness);
- Muscle strength (handgrip strength, quadriceps strength);
- Function (6-min walk distance, Short Physical Performance Battery, Functional Status Score for the ICU, Manual Muscle Testing, Clinical Frailty Scale, Barthel Index, Lawton Instrumental and Katz Activities of Daily Living, and Mini Mental State Exam);
- Quality of life (Short Form 36).
In addition, classical clinical outcomes (30-day mortality, hospital and ICU-length of stay, duration of mechanical ventilation, number of complications and infections) will be evaluated.
3. Discussion and Outlook
To the best of authors knowledge, this is the first study to evaluate the functional and morphological efficacy of a combined nutrition and exercise intervention as prehabilitation for patients scheduled for LVAD implantation. If this intervention protocol proves to be feasible in the pilot study, it could be considered and used as a preparation program for patients scheduled for LVAD implantation in clinical practice. If this intervention is proven to be effective in a following confirmatory clinical trial in LVAD patients, the combined intervention has the potential to dramatically change the current clinical practice and also benefit other elective (high-risk) cardiac surgeries that involve patients with high nutritional risk and reduced functional status, respectively. These new and additional therapeutic targets may help us to improve perioperative and long-term outcomes of these patients.
Author Contributions
Conceptualization and methodology: A.H. (Aileen Hill), C.S. and D.K.H., together with R.R., P.M., G.E., V.v.D. A.B., B.N., H.F., M.M., B.P., A.H. (Assad Haneya), R.Z. and A.M. Validation: A.H. (Aileen Hill) and C.S. Investigation: A.H. (Aileen Hill), C.S., A.H. (Aileen Hill), C.S. and D.K.H., together with R.R., P.M., G.E., V.v.D. A.B., B.N., H.F., M.M., B.P., A.H. (Assad Haneya), R.Z. and A.M. Resources: A.H. (Aileen Hill), C.S. and D.K.H. Data curation: A.H. (Aileen Hill), C.S. and V.v.D. Writing—original draft preparation: A.H. (Aileen Hill). Writing—review and editing: A.H. (Aileen Hill), C.S. and D.K.H., together with R.R., P.M., G.E., V.v.D. A.B., B.N., H.F., M.M., B.P., A.H. (Assad Haneya), R.Z. and A.M. Visualization: A.H. (Aileen Hill). Supervision: C.S., R.R., V.v.D., P.M., A.B. and B.N. Project administration: A.H. (Aileen Hill), C.S. and V.v.D. Funding acquisition: A.H. (Aileen Hill) and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This investigator-initiated trial is supported by a grant from Fresenius Kabi Deutschland (Fresenius Kabi Deutschland GmbH, Else-Kröner-Straße 1, 61352 Bad Homburg). The oral nutrition supplements (Fresubin PRO®) are provided by Fresenius Kabi Germany.
Institutional Review Board Statement
The study is conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committees of all participating centers, including RWTH Aachen University (EK 313-19, initial date of approval 3 December 2019) and Bad Oeynhausen (AZ 2021-859_1).
Informed Consent Statement
Written informed consent was and will be obtained from all subjects involved in the study.
Data Availability Statement
The data of this study will be published in a peer-reviewed journal and on clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/NCT04205760 (accessed on 25 September 2022)).
Acknowledgments
We thank Shawna Froese for her help with data management. We thank Elena Laaf, Sebastian Wendt and Jens Schmidt for their help in the project management at the University Hospital RWTH Aachen. We thank Andreas Fruend, as the leading physiotherapist, and Ina-Maria Albrecht and Andrea Schoenbrodt, as well as the clinical study team for their help in the project management and conduct of the study in the Heart and Diabetes Center NRW, Bad Oeynhausen.
Conflicts of Interest
A.H (Aileen Hill) and C.S. report that they have received honoraria for lectures from Fresenius Kabi. Aileen Hill is currently supported by a stipend of the Medical Faculty RWTH Aachen “Habilitationsstipendium”. G.E. reports that he has received honoraria for lectures and advisory activities from Fresenius Kabi. VvD reports to have received travel and lecture fees from Edwards and Orion Pharma. The other authors declare no conflict of interest that may be perceived as inappropriate influence on the study design, conduct, statistical evaluation or on this manuscript. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Members, W.G.; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.-P.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar]
- Berg, T.; Tewarie, L.; Moza, A.; Zayat, R.; Autschbach, R.; Stoppe, C.; Goetzenich, A.; Benstoem, C. Anforderungen an die ambulante Versorgung nach Implantation eines ventrikularen Herzunterstutzungssystems. Herz 2019, 44, 257–264. [Google Scholar] [CrossRef]
- Laribi, S.; Aouba, A.; Nikolaou, M.; Lassus, J.; Cohen-Solal, A.; Plaisance, P.; Pavillon, G.; Jois, P.; Fonarow, G.C.; Jougla, E.; et al. Trends in death attributed to heart failure over the past two decades in Europe. Eur. J. Heart Fail. 2012, 14, 234–239. [Google Scholar] [CrossRef]
- Stoerk, S.; Handrock, R.; Jacob, J.; Walker, J.; Calado, F.; Lahoz, R.; Hupfer, S.; Klebs, S. Epidemiology of heart failure in Germany: A retrospective database study. Clin. Res. Cardiol. Off. J. Ger. Card. Soc 2017, 106, 913–922. [Google Scholar] [CrossRef]
- Beckmann, A.; Meyer, R.; Lewandowski, J.; Markewitz, A.; Gummert, J. German Heart Surgery Report 2020: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac. Cardiovasc. Surg. 2021, 69, 294–307. [Google Scholar] [CrossRef]
- Cotts, W.G.; McGee, E.C.; Myers, S.L.; Naftel, D.C.; Young, J.B.; Kirklin, J.K.; Grady, K.L. Predictors of hospital length of stay after implantation of a left ventricular assist device: An analysis of the INTERMACS registry. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2014, 33, 682–688. [Google Scholar] [CrossRef]
- Bottle, A.; Faitna, P.; Aylin, P.P.; Cowie, M.R. Five-year outcomes following left ventricular assist device implantation in England. Open Heart 2021, 8, e001658. [Google Scholar] [CrossRef]
- Czermak, T.; Seitelberger, V.; Hagl, C.; Samson-Himmelstjerna, P.N.; Gross, S.; Sadoni, S.; Heyn, O.; Kellnar, A.; Hartrampf, B.; Lemmermohle, E.; et al. Survival after left ventricular assist device implantation correlates with a novel device-based measure of heart rate variability: The heart rate score. Interact. Cardiovasc. Thorac. Surg. 2021, 33, 309–315. [Google Scholar] [CrossRef]
- Warren, O.J.; Smith, A.J.; Alexiou, C.; Rogers, P.L.B.; Jawad, N.; Vincent, C.; Darzi, A.W.; Athanasiou, T. The inflammatory response to cardiopulmonary bypass: Part 1-mechanisms of pathogenesis. J. Cardiothorac. Vasc. Anesth. 2009, 23, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Wragge, T.; Pasque, C. Determinants of mortality and multiorgan dysfunction in cardiac surgery patients requiring prolonged mechanical ventilation. Chest 1995, 107, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Stoppe, C.; McDonald, B.; Benstoem, C.; Elke, G.; Meybohm, P.; Whitlock, R.; Fremes, S.; Fowler, R.; Lamarche, Y.; Jiang, X.; et al. Evaluation of Persistent Organ Dysfunction Plus Death As a Novel Composite Outcome in Cardiac Surgical Patients. J. Cardiothorac. Vasc. Anesth. 2016, 30, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Nesterova, E.; Lomivorotov, V.; Efremov, S.; Goetzenich, A.; Benstoem, C.; Zamyatin, M.; Chourdakis, M.; Heyland, D.; Stoppe, C. Current Evidence about Nutrition Support in Cardiac Surgery Patients-What Do We Know? Nutrients 2018, 10, 597. [Google Scholar] [CrossRef]
- Hill, A.; Arora, R.C.; Engelman, D.T.; Stoppe, C. Preoperative Treatment of Malnutrition and Sarcopenia in Cardiac Surgery: New Frontiers. Crit. Care Clin. 2020, 36, 593–616. [Google Scholar] [CrossRef]
- Hoogeboom, T.J.; Dronkers, J.J.; Hulzebos, E.H.J.; van Meeteren, N.L.U. Merits of exercise therapy before and after major surgery. Curr. Opin. Anaesthesiol. 2014, 27, 161–166. [Google Scholar] [CrossRef]
- Grover, A.; Gorman, K.; Dall, T.M.; Jonas, R.; Lytle, B.; Shemin, R.; Wood, D.; Kron, I. Shortage of cardiothoracic surgeons is likely by 2020. Circulation 2009, 120, 488–494. [Google Scholar] [CrossRef]
- Humphrey, R.; Malone, D. Effectiveness of preoperative physical therapy for elective cardiac surgery. Phys. Ther. 2015, 95, 160–166. [Google Scholar] [CrossRef][Green Version]
- Han, J.J.; Acker, M.A.; Atluri, P. Left Ventricular Assist Devices. Circulation 2018, 138, 2841–2851. [Google Scholar] [CrossRef]
- Parry, S.M.; Huang, M.; Needham, D.M. Evaluating physical functioning in critical care: Considerations for clinical practice and research. Crit. Care 2017, 21, 249. [Google Scholar] [CrossRef]
- Silberman, S.; Bitran, D.; Fink, D.; Tauber, R.; Merin, O. Very prolonged stay in the intensive care unit after cardiac operations: Early results and late survival. Ann. Thorac. Surg. 2013, 96, 15–21; discussion 21–22. [Google Scholar] [CrossRef] [PubMed]
- Paccagnella, A.; Calò, M.A.; Caenaro, G.; Salandin, V.; Jus, P.; Simini, G.; Heymsfield, S.B. Cardiac cachexia: Preoperative and postoperative nutrition management. J. Parenter. Enter. Nutr. 1994, 18, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Tepaske, R.; Velthuis, H.; Oudemans-van Straaten, H.M.; Heisterkamp, S.H.; van Deventer, S.J.; Ince, C.; Eysman, L.; Kesecioglu, J. Effect of preoperative oral immune-enhancing nutritional supplement on patients at high risk of infection after cardiac surgery: A randomised placebo-controlled trial. Lancet 2001, 358, 696–701. [Google Scholar] [CrossRef]
- Aquilani, R.; Opasich, C.; Gualco, A.; Verri, M.; Testa, A.; Pasini, E.; Viglio, S.; Iadarola, P.; Pastoris, O.; Dossena, M.; et al. Adequate energy-protein intake is not enough to improve nutritional and metabolic status in muscle-depleted patients with chronic heart failure. Eur. J. Heart Fail. 2008, 10, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Rozentryt, P.; von Haehling, S.; Lainscak, M.; Nowak, J.U.; Kalantar-Zadeh, K.; Polonski, L.; Anker, S.D. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: A randomized, double-blind pilot study. J. Cachexia Sarcopenia Muscle 2010, 1, 35–42. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995, 332, 556–562. [Google Scholar] [CrossRef]
- LeBlanc, A.D.; Schneider, V.S.; Evans, H.J.; Pientok, C.; Rowe, R.; Spector, E. Regional changes in muscle mass following 17 weeks of bed rest. J. Appl. Physiol. 1992, 73, 2172–2178. [Google Scholar] [CrossRef]
- Griffiths, R.D.; Palmer, T.E.; Helliwell, T.; MacLennan, P.; MacMillan, R.R. Effect of passive stretching on the wasting of muscle in the critically ill. Nutrition 1995, 11, 428–432. [Google Scholar]
- Preiser, J.; Prato, C.; Harvengt, A.; Peters, L.; Bastin, M. Passive cycling limits myofibrillar protein catabolism in unconscious patients: A pilot study. J. Nov. Physiother. 2014, 4, 225. [Google Scholar]
- Flynn, K.E.; Pina, I.L.; Whellan, D.J.; Lin, L.; Blumenthal, J.A.; Ellis, S.J.; Fine, L.J.; Howlett, J.G.; Keteyian, S.J.; Kitzman, D.W.; et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009, 301, 1451–1459. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Whellan, D.J.; Lee, K.L.; Keteyian, S.J.; Cooper, L.S.; Ellis, S.J.; Leifer, E.S.; Kraus, W.E.; Kitzman, D.W.; Blumenthal, J.A.; et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009, 301, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Kozarez, I.; Adams, V.; Mangner, N.; Hoellriegel, R.; Erbs, S.; Linke, A.; Moebius-Winkler, S.; Thiery, J.; Kratzsch, J.; et al. Age-related effects of exercise training on diastolic function in heart failure with reduced ejection fraction: The Leipzig Exercise Intervention in Chronic Heart Failure and Aging (LEICA) Diastolic Dysfunction Study. Eur. Heart J. 2012, 33, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Lenk, K.; Erbs, S.; Hoellriegel, R.; Beck, E.; Linke, A.; Gielen, S.; Winkler, S.M.; Sandri, M.; Hambrecht, R.; Schuler, G.; et al. Exercise training leads to a reduction of elevated myostatin levels in patients with chronic heart failure. Eur. J. Prev. Cardiol. 2012, 19, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Hoellriegel, R.; Beck, E.B.; Linke, A.; Adams, V.; Moebius-Winkler, S.; Mangner, N.; Sandri, M.; Gielen, S.; Gutberlet, M.; Hambrecht, R.; et al. Anabolic effects of exercise training in patients with advanced chronic heart failure (NYHA IIIb): Impact on ubiquitin-protein ligases expression and skeletal muscle size. Int. J. Cardiol. 2013, 167, 975–980. [Google Scholar] [CrossRef]
- Arthur, H.M.; Daniels, C.; McKelvie, R.; Hirsh, J.; Rush, B. Effect of a preoperative intervention on preoperative and postoperative outcomes in low-risk patients awaiting elective coronary artery bypass graft surgery. A randomized, controlled trial. Ann. Intern. Med. 2000, 133, 253–262. [Google Scholar] [CrossRef]
- Herdy, A.H.; Marcchi, P.L.B.; Vila, A.; Tavares, C.; Collaco, J.; Niebauer, J.; Ribeiro, J.P. Pre- and postoperative cardiopulmonary rehabilitation in hospitalized patients undergoing coronary artery bypass surgery: A randomized controlled trial. Am. J. Phys. Med. Rehabil. 2008, 87, 714–719. [Google Scholar] [CrossRef]
- Rosenfeldt, F.; Braun, L.; Spitzer, O.; Bradley, S.; Shepherd, J.; Bailey, M.; van der Merwe, J.; Leong, J.-Y.; Esmore, D. Physical conditioning and mental stress reduction--a randomised trial in patients undergoing cardiac surgery. BMC Complementary Altern. Med. 2011, 11, 20. [Google Scholar] [CrossRef]
- Tung, H.-H.; Shen, S.-F.; Shih, C.-C.; Chiu, K.-M.; Lee, J.-Y.; Liu, C.-Y. Effects of a preoperative individualized exercise program on selected recovery variables for cardiac surgery patients: A pilot study. J. Saudi Heart Assoc. 2012, 24, 153–161. [Google Scholar] [CrossRef][Green Version]
- Burtin, C.; Clerckx, B.; Robbeets, C.; Ferdinande, P.; Langer, D.; Troosters, T.; Hermans, G.; Decramer, M.; Gosselink, R. Early exercise in critically ill patients enhances short-term functional recovery. Crit. Care Med. 2009, 37, 2499–2505. [Google Scholar] [CrossRef]
- Pires-Neto, R.C.; Kawaguchi, Y.M.F.; Hirota, A.S.; Fu, C.; Tanaka, C.; Caruso, P.; Park, M.; Carvalho, C.R.R. Very early passive cycling exercise in mechanically ventilated critically ill patients: Physiological and safety aspects-a case series. PLoS ONE 2013, 8, e74182. [Google Scholar] [CrossRef]
- Rahimi, R.A.; Skrzat, J.; Reddy, D.R.S.; Zanni, J.M.; Fan, E.; Stephens, R.S.; Needham, D.M. Physical rehabilitation of patients in the intensive care unit requiring extracorporeal membrane oxygenation: A small case series. Phys. Ther. 2013, 93, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kho, M.E.; Martin, R.A.; Toonstra, A.L.; Zanni, J.M.; Mantheiy, E.C.; Nelliot, A.; Needham, D.M. Feasibility and safety of in-bed cycling for physical rehabilitation in the intensive care unit. J. Crit. Care 2015, 30, 1419.e1–1419.e5. [Google Scholar] [CrossRef] [PubMed]
- Fossat, G.; Baudin, F.; Courtes, L.; Bobet, S.; Dupont, A.; Bretagnol, A.; Benzekri-Lefevre, D.; Kamel, T.; Muller, G.; Bercault, N.; et al. Effect of In-Bed Leg Cycling and Electrical Stimulation of the Quadriceps on Global Muscle Strength in Critically Ill Adults: A Randomized Clinical Trial. JAMA 2018, 320, 368–378. [Google Scholar] [CrossRef]
- Nickels, M.R.; Aitken, L.M.; Barnett, A.G.; Walsham, J.; King, S.; Gale, N.E.; Bowen, A.C.; Peel, B.M.; Donaldson, S.L.; Mealing, S.T.J.; et al. Effect of in-bed cycling on acute muscle wasting in critically ill adults: A randomised clinical trial. J. Crit. Care 2020, 59, 86–93. [Google Scholar] [CrossRef]
- Waldauf, P.; Hruskova, N.; Blahutova, B.; Gojda, J.; Urban, T.; Krajcova, A.; Fric, M.; Jiroutkova, K.; Rasova, K.; Duska, F. Functional electrical stimulation-assisted cycle ergometry-based progressive mobility programme for mechanically ventilated patients: Randomised controlled trial with 6 months follow-up. Thorax 2021, 76, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Berney, S.; Hopkins, R.O.; Rose, J.W.; Koopman, R.; Puthucheary, Z.; Pastva, A.; Gordon, I.; Colantuoni, E.; Parry, S.M.; Needham, D.M.; et al. Functional electrical stimulation in-bed cycle ergometry in mechanically ventilated patients: A multicentre randomised controlled trial. Thorax 2021, 76, 656–663. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).