Abstract
Background: Stent-assisted coil embolization (SACE) is a common endovascular technique for managing intracranial aneurysms. The permanent presence of a stent inside the cerebral artery necessitates the postoperative use of antiplatelets. However, a consensus about how long to continue on it remains debated. This systematic review aims to discuss and quantify the risk of ischemic complications after antiplatelet discontinuation following SACE. Methods: PubMed, Cochrane Library, Scopus, and Web of Science (WOS) were systematically searched for studies assessing the outcomes after antiplatelet discontinuation following SACE for cerebral aneurysms. The primary outcome was the odds of ischemic complications after antiplatelet discontinuation. Using a random-effects model, the pooled event rate, along with a 95% confidence interval (CI), was calculated. The Comprehensive Meta-Analysis software (CMA) software was used for the analysis. The Newcastle–Ottawa Scale (NOS) was used for the quality assessment. Results: A total of five observational cohort studies were included in this systematic review. The studies recruited cases from 2009 and 2020, predominantly in Korea and Japan. Data from 18,425 cases obtained from four studies were analyzed. The duration of antiplatelet therapy varied widely across the included studies. Additionally, most studies reported a median follow-up of 24 months or more after antiplatelet discontinuation. We extracted and analyzed the odds of thromboembolic complications occurring within 6 to 24 months after the discontinuation of antiplatelets. The pooled rate of thromboembolism after antiplatelet discontinuation in this meta-analysis was 0.01 (95% CI: 0.006 to 0.018). Conclusion: This review demonstrates that the risk of thromboembolic complications after discontinuing antiplatelet therapy post-SACE is low. However, no strong consensus exists on the ideal duration for maintaining dual- or single-antiplatelet therapy. Further prospective studies with longer follow-ups are warranted to clarify the optimal durations needed to balance thromboembolic risk with hemorrhagic complications.
1. Introduction
Cerebral aneurysms stem from arterial wall dilation, often at weak points near bifurcations where hemodynamic stresses and structural vulnerabilities exist [1]. Major risk factors include aging, smoking, and hypertension [2]. They are classified based on their shape into fusiform or saccular categories, with the latter being the most common, accounting for 90% of all cerebral aneurysms [3]. The pooled prevalence of unruptured aneurysms has been estimated to be 3.2%. The majority of these aneurysms are silent and may be incidental imaging findings [4]. The primary concern arises from the potential for aneurysm rupture, leading to subarachnoid hemorrhage (SAH). Although the risk is as low as 0.25%, it is associated with high morbidity and mortality [5]. Ruptured cerebral aneurysms warrant an immediate intervention [6]. However, the decision to intervene in unruptured cerebral aneurysms depends on the assessed risk of rupture, a subject that remains a field of controversy in neurosurgery [7,8].
Stent-assisted coil embolization (SACE) is a widely accepted endovascular treatment for complex, broad-necked aneurysms. These stents are used to help prevent the coils from moving or protruding into the parent artery, ensuring a more secure placement. Due to differences in vessel anatomy and tortuosity, various stents have been developed over time to meet these specific needs [9,10]. Unlike non-stent-assisted coiling, SACE requires long-term postoperative antiplatelet therapy to ensure effective platelet inhibition in the presence of a permanent stent inside the intracranial vessels [11].
The use of antiplatelets is crucial following endovascular stent insertion to prevent stent thrombosis and thromboembolic complications, weighing the risk–benefit ratio with the hemorrhagic risks. Guidelines for coronary stenting recommend DAPT for one year, followed by lifelong SAPT. However, the optimal duration of antiplatelet therapy after cerebral artery stenting remains unclear [12,13]. In SACE, stents are placed in normal, non-atherosclerotic arteries, which lowers the risk of stent thrombosis compared to coronary stenting. However, reintervention in cerebral arteries is challenging. A thrombosed stent would be difficult to manage, making it crucial to avoid stent thrombosis. Discontinuing antiplatelet therapy after a certain period is justified to reduce bleeding risks. However, the optimal duration of therapy remains unclear. The lack of consensus in the literature highlights the need for further research on the appropriate length of treatment [14].
Some studies have examined the safety of antiplatelet discontinuation, aiming to determine the optimal duration of DAPT and SAPT for patients without comorbidities requiring ongoing antiplatelet therapy [15,16,17,18,19]. Based on the available data, many clinicians discontinue antiplatelets 3 to 36 months after SACE in clinical practice. However, this approach lacks strong supporting evidence. This systematic review aims to consolidate existing data on the safety of antiplatelet discontinuation after SACE for cerebral aneurysms to help determine the optimal duration of therapy.
2. Methods
This systematic review was conducted in accordance with the guidelines set by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [20].
2.1. Search Strategy and Study Selection
A systematic literature search was carried out using PubMed, Scopus, the Cochrane Library, and the Web of Science (WOS) from their inception until 17 September 2024. The following search terms were used: (“Cerebral aneurysm” OR “Intracranial aneurysm” OR “Brain aneurysm”) AND (“Stent-assisted coiling” OR “SAC” OR “Aneurysm coil embolization” OR “Endovascular aneurysm treatment”) AND (“Antiplatelet therapy” OR “Dual antiplatelet therapy” OR “DAPT” OR “Ticlopidine” OR “Prasugrel” OR “Cangrelor” OR “Ticagrelor” OR “Antithrombotic therapy” OR “Aspirin” OR “Clopidogrel” OR “Platelet inhibition”). Retrieved records were exported to Endnote to detect and remove the duplicates. Relevant studies underwent a comprehensive full-text review to assess whether they fulfilled our pre-specified eligibility criteria. Each step was met by two independent reviewers. In the case of disagreements, a consensus with a third reviewer was taken into consideration.
2.2. Eligibility Criteria
We employed the PICOS framework to identify eligible studies as follows: Population: patients with cerebral aneurysms who underwent stent-assisted coil embolization (SACE); Intervention: antiplatelet discontinuation; Control: both double- and single-arm studies were eligible; Outcomes: safety outcomes, with thromboembolic complications as the primary outcome of interest; Study design: all study designs except case reports, case series, and reviews were included.
2.3. Data Extraction and Quality Assessment
Two independent reviewers extracted relevant data from the included studies. Information on study characteristics and baseline details, including the study design, location, recruitment period, primary outcomes, conclusions, sample size, age, gender, follow-up duration, comorbidities, aneurysm location, stent properties, and antiplatelet regimens, was extracted and tabulated. All safety outcomes were compiled into an Excel sheet. Only one outcome, the risk of thromboembolism, was consistently reported across the studies and subjected to meta-analysis. The odds of thromboembolic complications occurring within 24 months after the discontinuation of antiplatelet therapy were used for the analysis. We assessed the quality of the included studies using the Newcastle–Ottawa Scale (NOS) [21].
2.4. Statistical Analysis
The analysis was conducted using Comprehensive Meta-Analysis (CMA) software version 3.0 (Biostat, Englewood, NJ, USA). A random-effects model was adopted to consider the variability among the included studies. The pooled event rate, along with a 95% confidence interval (CI), was calculated. Heterogeneity was evaluated using the I2 statistic, with values over 50% and a p-value below 0.1, indicating significant heterogeneity [22].
3. Results
3.1. Search Results
Our search yielded 852 records. Of these, 278 were identified as duplicates and removed. After title and abstract screening, 547 articles were found to be irrelevant. A total of 27 studies were subjected to full-text screening, of which 22 were excluded for not fully meeting the inclusion criteria. The reasons for exclusion include studies that did not focus on thromboembolic events, studies comparing DAPT vs. SAPT without discontinuation, studies not related to SACE, and study protocols. Finally, five studies met our pre-specified criteria and were included in this systematic review [15,16,17,18,19]. Reports from four out of these five studies were included in the meta-analysis [15,16,18,19]. The PRISMA flowchart is shown in Figure 1.
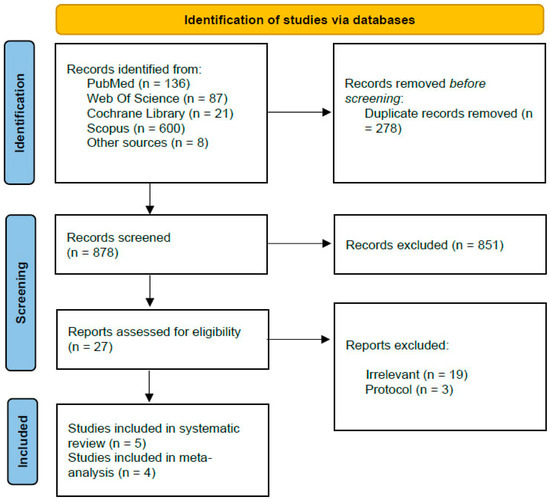
Figure 1.
Prisma flowchart.
3.2. Study Characteristics and Narrative Synthesis
All studies included in this systematic review were observational cohort studies, with recruitment periods ranging from 2009 to 2020. Four studies were conducted in Korea [15,17,19], and one study was conducted in Japan [16]. Approximately 72% of the patients were females.
Baik and colleagues conducted a retrospective cohort study to explore the safety of antiplatelet discontinuation after the SACE of unruptured brain aneurysms, recruiting cases from the National Health Insurance Service (NHIS) database, which covers a large data set, reaching 17,692 patients. In this study, the authors assessed the odds of ischemic and bleeding events one month after SACE, recorded over a mean follow-up period of 4.2 years. At one year, 20.5% of patients were not on antiplatelet therapy, while 41.7% discontinued antiplatelet use two years after SACE. A time-dependent Cox proportional hazards regression model was used to evaluate the outcomes during three distinct periods following SACE: within the first year, within the second year, and after two years. However, the study did not provide detailed information regarding aneurysm location or stent types. They reported that the use of antiplatelet was associated with a 44% reduction in the risk of ischemic events in the first year after SACE, with a hazard ratio (HR) of 0.56 (95% CI 0.35 to 0.89). However, beyond the first year, this protective effect was not observed, together with an increased risk of bleeding after 2 years (HR 1.76 [95% CI 1.11 to 2.87]). After 2 years of SACE, a total number of 5147 patients were on no antiplatelets at all. Following them up for more than 24 months, only 64 patients experienced cerebral infarction (1.2%). This is the value used in our meta-analysis [19].
Hong and colleagues analyzed data from 120 patients who had antiplatelet therapy discontinued following the SACE procedure over a 10-year period. The primary outcome was thromboembolic complications related to antiplatelet discontinuation within 6 months, with lesions linked to the stented artery. Of the 120 patients, 74 (61.6%) stopped antiplatelet therapy between 18 and 36 months after SACE. During the 6-month follow-up, no patients experienced stent-related cerebral ischemia. However, transient ischemia occurred in one patient 46 months after discontinuing aspirin, though it was not related to the stented artery. This study is limited by the small sample size, which may raise concerns about potential underreporting [15].
Goto and colleagues conducted a long-term, single-center retrospective study investigating ischemic and hemorrhagic events following the discontinuation of SAPT after SACE during the period from 2010 to 2020. They included 240 patients, and the average follow-up was 46.7 months. Two years after SACE, 71.7% of patients had been taken off antiplatelets, and ischemic events were not observed at a median of 24 months, with a range from 1 to 84 months after SAPT discontinuation. They reported nine cases of ischemic complications; however, all of them occurred while the patients were on antiplatelets (SAPT or DAPT). Four out of these nine cases had Y- or T-stents, which has been reported as a significant risk factor for ischemic complications in this study group. Although ischemic events in these patients occurred while on antiplatelets, the discontinuation of antiplatelets in this subgroup of patients still warrants more caution.
Kim and colleagues conducted a multicenter retrospective study on 373 patients who had undergone SACE and discontinued antiplatelet therapy between 12 and 24 months after the procedure. DAPT maintenance durations ranged from 3 to 12 months based on different institutions. The average duration for antiplatelet use was 15.8 months, while the median clinical follow-up period after discontinuation was 24 months. No ischemic complications were observed within this follow-up, except for one high-risk patient with multiple risk factors for thromboembolism [18].
Another retrospective study involving 214 cases of patients who underwent SACE found that the discontinuation of antiplatelet therapy had no significant effect on the odds of cerebral infarction or intracranial hemorrhage. However, in terms of survival, patients who continued DAPT had better overall survival rates than those who did not. Notably, the highest mortality risk was observed in the group that stopped DAPT within 3 months after SACE, while those who continued on DAPT for 1 year had the highest survival rates. Herein, the authors recommended that DAPT should be continued for 12 months. No data were available to conclude the optimum duration of SAPT. Additionally, the study did not provide data on follow-up periods for those on or off antiplatelet therapy. Hence, this study was excluded from this meta-analysis [17]. The summary and baseline data of the included studies are shown in Table 1 and Table 2. We excluded studies that did not assess the complete discontinuation of all antiplatelet therapy (both DAPT and SAPT), as some focused only on the effects of switching from DAPT to monotherapy, which did not meet our inclusion criteria [23,24].

Table 1.
Summary of the included studies.

Table 2.
Baseline characteristics of the included studies.
3.3. Meta-Analysis
Four studies reported the events of thromboembolism. The pooled event rate of thromboembolism after antiplatelet discontinuation in this meta-analysis was 0.01 (95% CI: 0.006 to 0.018), as shown in Figure 2.
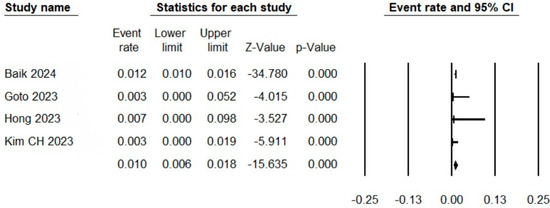
Figure 2.
Forest plot showing the rates of thromboembolic complications after antiplatelet discontinuation.
3.4. Quality Assessment
The quality of each study was evaluated using the Newcastle–Ottawa Scale (NOS), which assesses the selection, comparability, and outcome. Based on this assessment, all studies were found to be of good quality (Table 3).

Table 3.
Quality assessment using the NOS tool.
4. Discussion
In this systematic review, we summarize five studies investigating the safety outcomes after the discontinuation of antiplatelets following the SACE for cerebral aneurysms. Although the studies adopted heterogenous methodologies, the results were consistent, confirming the safety of this practice in terms of no identified risk for stent-related ischemic complications. We pooled the odds of thromboembolic complications after a follow-up of 6 to 24 months after antiplatelet discontinuation. The pooled rate in this meta-analysis was 1% (0.6% to 1.8%).
In the studies included in this systematic review, most patients discontinued antiplatelet agents 18 to 36 months after SACE. Baik and colleagues [19] reported a 41.7% discontinuation rate after 2 years, while Hong and colleagues [15] stated that 61.6% of their cases stopped antiplatelet therapy within 18 to 36 months. Goto and colleagues [16] reported the discontinuation of SAPT in 71.7% of their cohort after 24 months. Kim and colleagues [18] reported a mean duration for antiplatelet therapy of 15.8 months. One study suggested maintaining dual-antiplatelet therapy (DAPT) for at least 12 months, citing improved survival rates compared to those who received DAPT for only 3 months [17]. This recommendation aligns with the findings by Hwang et al. [23], who argued that delaying the transition from DAPT to SAPT beyond 9 months may reduce the risk of ischemic stroke. However, longer DAPT use has been associated with an increased risk of hemorrhagic events [25]. Conversely, Ozaki et al. [26] found no significant difference in the risk of ischemic stroke between patients who received 12 months of DAPT and those who had only 3 months of therapy. In another study, Ozaki et al. [27] reported no association between the duration of DAPT and the risk of ischemic stroke within 15 months post-SACE. The significant variation in the optimal duration of antiplatelet therapy across the studies highlights the lack of consensus regarding the ideal treatment duration. Some studies advocate for extended durations of up to 3 years, while others suggest that the duration should be determined based on individual patient evaluation by healthcare providers [15]. The debate is still ongoing to optimize the balance between preventing thromboembolic events and minimizing the risk of hemorrhagic complications. Baik et al. reported that in the first year, antiplatelets reduced the risk of ischemic events, with no detected difference after 2 years, together with an increased risk of bleeding [19]. Despite the variability in antiplatelet durations, discontinuing antiplatelet therapy at certain time points is a safe practice in otherwise fit patients. The pooled risk of ischemic complications in low-risk patients reported in this meta-analysis is equivalent to the estimated stroke incidence in low-risk atrial fibrillation patients who did not receive anticoagulation therapy. This translates into a low risk that does not necessitate prophylaxis as long as the patients are free from other risk factors [28].
Unlike simple coil embolization (non-stent-assisted coiling), SACE increases thrombogenicity and requires long-term postoperative antiplatelet therapy to ensure effective platelet inhibition in the presence of a permanent stent inside the intracranial vessels [11]. As a response to stent deployment inside an artery, many dynamic sequential changes occur [29].
These changes typically occur within one month, with the vessel wall reaching its maximal thickness at two months following stenting. This results in angiographically visible stent stenosis, which gradually resolves as the vessel wall becomes thinner over time. In the context of SACE, in-stent restenosis (ISR) has been reported in 2.3% to 7.8% of cases, with resolution occurring in 12.5% to 66.6% of cases within 24 months [9,30]. Based on these findings, the discontinuation of antiplatelet after 1–2 years was deemed safe in selected low-risk patients. The risk of IRS after using bare-metal stents in coronaries was reported to be 30% at 6 months [31]. The lower risks of IRS after SACE can be explained by the fact that the stents deployed in diseased stenosed arteries like coronaries expose the vessel wall to higher degrees of injury than those faced by patent cerebral vessels. This theoretically lowers the concern for stent thrombosis compared to coronary stenting. Therefore, when weighing the risks of bleeding associated with antiplatelet therapy against the potential for unnecessary lifelong use, there is a rationale for discontinuing antiplatelet treatment after a certain duration [14].
Some risk factors have been reported to be significant contributors to ischemic events after SACE. Hwang et al. reported that incomplete aneurysm occlusion after SACE was a long-term source of delayed thromboembolic complications [23]. Goto et al. reported nine cases of ischemic complications while the patients were on antiplatelet therapy (SAPT or DAPT). Four out of these nine cases had Y- or T-stents, which has been reported as a significant risk factor for delayed ischemic complications in this study group [16]. Song et al. showed that smokers and patients with aneurysms with a maximum parent artery diameter of more than 4.5 mm had more thromboembolic complications [32]. Another study by Li et al. found that a pre-SACE clinical condition and a higher dome-to-neck ratio were independent risk factors for thromboembolism [33].
The individualization of antiplatelet therapy is a critical aspect of managing patients undergoing stent implantation. Considering coronary stenting as a reference, although guidelines are well-established, clinical practice should tailor treatment to patient-specific factors. For example, in certain high-risk cases, such as patients with a higher risk of bleeding complications, DAPT may be discontinued earlier than the standard recommendation. On the other hand, some patients, particularly those with complex comorbidities, may require triple therapy (combining antiplatelets with anticoagulation), even though this regimen significantly increases the risk of bleeding complications [12,34]. Similarly, an individualized approach is essential in SACE. Based on this systematic review, low-risk patients do not require lifelong antiplatelet therapy. A duration of 12 months of DAPT may suffice, followed by 6 to 12 months of SAPT. The timing of this transition should be individualized, considering the patient’s clinical condition, comorbidities, and any adverse events during DAPT. Further studies are needed to identify the risk factors for adverse events and explore the predictive role of factors like early platelet inhibition response in guiding therapy.
We acknowledge several limitations in this systematic review and meta-analysis. All included studies were observational retrospective cohort studies, which are inherently prone to selection bias and confounding factors. Additionally, most studies were conducted in Korea and Japan, which may limit the generalizability of our findings to other populations with different genetic and lifestyle factors. The included studies exhibited variability in the durations of antiplatelet use, their definitions of thromboembolic events, and follow-up periods after discontinuation. Given that our primary aim was to systematically summarize the available evidence rather than conduct an extensive quantitative meta-analysis, we did not perform subgroup analyses for different antiplatelet regimens or durations. Each study was analyzed as a standalone data set due to the heterogeneity in study designs, and separate subgroup analyses would not have added substantial value given the limited number of studies included. Furthermore, there were no additional eligible studies that met our inclusion criteria, underscoring the need for further research in this area. Future studies should focus on larger, multicenter comparative studies with long-term follow-up, incorporating subgroup analyses based on demographic variables, stent types, comorbidities, and aneurysm characteristics. The development of risk stratification models may also enhance individualized antiplatelet management, ultimately improving patient outcomes while minimizing thromboembolic risks.
5. Conclusions
This systematic review demonstrates that the risk of thromboembolic complications after discontinuing antiplatelet therapy post-SACE is low. However, no strong consensus exists on the ideal duration for maintaining dual- or single-antiplatelet therapy (DAPT or SAPT). Further prospective studies with longer follow-ups are needed to clarify the optimal durations necessary to balance thromboembolic risk with hemorrhagic complications.
Author Contributions
Conceptualization, M.M.A.-S., M.S.A.-J. and A.A.E.; Methodology, M.M.A.-S., A.A.E. and R.S.; Formal analysis, M.M.A.-S., R.S. and A.S.; Investigation, M.M.A.-S., A.S. and F.S.; Writing—original draft, M.M.A.-S., F.S. and A.A.; Writing—review and editing, M.M.A.-S. and A.A.; Supervision, M.M.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All materials related to this meta-analysis, including data collection forms, extracted data, and analysis files, will be made publicly available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Toth, G.; Cerejo, R. Intracranial aneurysms: Review of current science and management. Vasc. Med. 2018, 23, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Vlak, M.H.M.; Rinkel, G.J.E.; Greebe, P.; Algra, A. Independent risk factors for intracranial aneurysms and their joint effect: A case-control study. Stroke 2013, 44, 984–987. [Google Scholar] [CrossRef]
- Jersey, A.M.; Foster, D.M. Cerebral Aneurysm. StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Vlak, M.H.; Algra, A.; Brandenburg, R.; Rinkel, G.J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Miller, J.M.; Samaniego, E.A. Clinical Scales in Aneurysm Rupture Prediction. SVIN 2024, 4, e000625. [Google Scholar] [CrossRef]
- Hammer, A.; Steiner, A.; Kerry, G.; Ranaie, G.; Baer, I.; Hammer, C.M.; Kunze, S.; Steiner, H.-H. Treatment of ruptured intracranial aneurysms yesterday and now. PLoS ONE 2017, 12, e0172837. [Google Scholar] [CrossRef]
- Cho, K.-C.; Yang, H.; Kim, J.-J.; Oh, J.H.; Kim, Y.B. Prediction of rupture risk in cerebral aneurysms by comparing clinical cases with fluid–structure interaction analyses. Sci. Rep. 2020, 10, 18237. [Google Scholar] [CrossRef]
- Greving, J.P.; Wermer, M.J.; Brown, R.D.; Morita, A.; Juvela, S.; Yonekura, M.; Ishibashi, T.; Torner, J.C.; Nakayama, T.; Rinkel, G.J.E.; et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014, 13, 59–66. [Google Scholar] [CrossRef]
- Biondi, A.; Janardhan, V.; Katz, J.M.; Salvaggio, K.; Riina, H.A.; Gobin, Y.P. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: Strategies in stent deployment and midterm follow-up. Neurosurgery 2007, 61, 460–468, discussion 8–9. [Google Scholar] [CrossRef]
- Zhang, J.; He, L.; Xia, X.; Zhang, L.; Yu, K. Braided stent-assisted coil embolization versus laser engraved stent-assisted coil embolization in patients with unruptured complex intracranial aneurysms. Clinics 2023, 78, 100202. [Google Scholar] [CrossRef]
- Zahid, M.B.A.; Memon, M.S.; Tappiti, M.; Kumar, V.S.; Nazir, A.M.; Koganti, B.; Gupta, K.; Mostafa, J.A. Duration of Dual Antiplatelet Therapy After Stent Implantation, Still an Enigma: A Systematic Review of Randomized Clinical Trials. Cureus 2021, 13, e19549. [Google Scholar]
- Angiolillo, D.; Galli, M.; Collet, J.-P.; Kastrati, A.; O’Donoghue, M. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention 2022, 17, e1371. [Google Scholar] [CrossRef]
- Thompson, B.G.; Brown, R.D.; Amin-Hanjani, S.; Broderick, J.P.; Cockroft, K.M.; Connolly, E.S.; Duckwiler, G.R.; Harris, C.C.; Howard, V.J.; Johnston, S.C.C.; et al. Guidelines for the Management of Patients With Unruptured Intracranial Aneurysms: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015, 46, 2368–2400. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Taoka, T.; Myouchin, K.; Wada, T.; Nakagawa, H.; Nakagawa, I.; Miyasaka, T.; Sakamoto, M.; Kurokawa, S.; Kichikawa, K. Enterprise Stent-assisted Cerebral Aneurysm Coiling: Can Antiplatelet Therapy be Terminated after Neointima Formation with the Enterprise Stent? J. Neuroendovasc. Ther. 2016, 10, 201–205. [Google Scholar] [CrossRef]
- Hong, N.; Cho, Y.D.; Kim, H.S.; Pang, C.H.; Yoo, D.H.; Kim, J.E.; Kim, K.M.; Cho, W.-S.; Lee, S.H.; Kang, H.-S. Is it safe to discontinue antiplatelet medication after stent-assisted coil embolization? If so, when is the best time? J. Neuroradiol. 2023, 50, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Izumi, T.; Nishihori, M.; Imai, T.; Araki, Y.; Kanamori, F.; Uda, K.; Yokoyama, K.; Saito, R. Antiplatelet therapy discontinuation after stent-assisted coil embolization for intracranial aneurysms: A single-center, long-term, retrospective, observational study. J. Neurosurg. 2023, 138, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G. Discontinuation of antiplatelet therapy after stent-assisted coil embolization for cerebral aneurysms. J. Cerebrovasc. Endovasc. Neurosurg. 2023, 25, 132–142. [Google Scholar] [CrossRef]
- Kim, C.H.; Hong, N.; Rhim, J.-K.; Mun, J.H.; Lim, J.; Choi, H.H.; Kim, Y.H.; Lee, S.W.; Cho, Y.D. Safety of discontinuing antiplatelet therapy 12–24 months after stent-assisted coil embolization: A multicenter retrospective study. J. Neurosurg. 2023, 139, 1311–1316. [Google Scholar] [CrossRef]
- Baik, M.; Jeon, J.; Kim, J.; Yoo, J. Discontinuation of antiplatelet therapy after stent-assisted coil embolisation of cerebral aneurysm: A nationwide cohort study. Stroke Vasc. Neurol. 2024, 9, 560–567. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 October 2024).
- Higgins, J.P.T. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Hwang, G.; Kim, J.G.; Song, K.S.; Lee, Y.J.; Villavicencio, J.B.; Suroto, N.S.; Park, N.-M.; Park, S.J.; Jeong, E.-A.; Kwon, O.K. Delayed Ischemic Stroke after Stent-assisted Coil Placement in Cerebral Aneurysm: Characteristics and Optimal Duration of Preventative Dual Antiplatelet Therapy. Radiology 2014, 273, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Rossen, J.D.; Chalouhi, N.; Wassef, S.N.; Thomas, J.; Abel, T.J.; Jabbour, P.M.; Kung, D.K.; Hasan, D.M. Incidence of cerebral ischemic events after discontinuation of clopidogrel in patients with intracranial aneurysms treated with stent-assisted techniques: Clinical article. J. Neurosurg. 2012, 117, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Shoda, K.; Enomoto, Y.; Egashira, Y.; Kinoshita, T.; Mizutani, D.; Iwama, T. Long-term complications after stent assist coiling dependent on clopidogrel response. BMC Neurol. 2021, 21, 247. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T.; Yamagami, H.; Morimoto, M.; Hatano, T.; Oishi, H.; Haraguchi, K.; Yoshimura, S.; Sugiu, K.; Iihara, K.; Matsumaru, Y.; et al. Short- versus long-term Dual AntiPlatelet Therapy for Stent-Assisted treatment of CErebral aneurysm (DAPTS ACE): A multicenter, open-label, randomized clinical trial. J. Neurointervent. Surg. 2024, 16, 171–176. [Google Scholar] [CrossRef]
- Ozaki, T.; Yamagami, H.; Morimoto, M.; Imamura, H.; Hatano, T.; Oishi, H.; Haraguchi, K.; Yoshimura, S.; Satow, T.; Sugiu, K.; et al. Relation between duration of dual antiplatelet therapy and risk of ischemic stroke after stent-assisted treatment of cerebral aneurysm (DAPTS ACE-registry). J. Neurointervent. Surg. 2024, 16, 691–697. [Google Scholar] [CrossRef]
- Abdel-Qadir, H.; Singh, S.M.; Pang, A.; Austin, P.C.; Jackevicius, C.A.; Tu, K.; Dorian, P.; Ko, D.T. Evaluation of the Risk of Stroke Without Anticoagulation Therapy in Men and Women With Atrial Fibrillation Aged 66 to 74 Years Without Other CHA2DS2-VASc Factors. JAMA Cardiol. 2021, 6, 918–925. [Google Scholar] [CrossRef]
- Edelman, E.R.; Rogers, C. Pathobiologic responses to stenting. Am. J. Cardiol. 1998, 81, 4E–6E. [Google Scholar] [CrossRef]
- Fiorella, D.; Albuquerque, F.C.; Woo, H.; Rasmussen, P.A.; Masaryk, T.J.; McDougall, C.G. Neuroform in-stent stenosis: Incidence, natural history, and treatment strategies. Neurosurgery 2006, 59, 34–42. [Google Scholar] [CrossRef]
- Pelliccia, F.; Zimarino, M.; Niccoli, G.; Morrone, D.; De Luca, G.; Miraldi, F.; De Caterina, R. In-stent restenosis after percutaneous coronary intervention: Emerging knowledge on biological pathways. Eur. Heart J. Open 2023, 3, oead083. [Google Scholar] [CrossRef]
- Song, J.; Yeon, J.Y.; Kim, J.-S.; Hong, S.-C.; Kim, K.-H.; Jeon, P. Delayed thromboembolic events more than 30 days after self expandable intracranial stent-assisted embolization of unruptured intracranial aneurysms. Clin. Neurol. Neurosurg. 2015, 135, 73–78. [Google Scholar] [CrossRef]
- Li, G.; Xing, H.; Mao, G.; Cai, J.; Jin, D.; Tian, Y.; Zhang, X.; Zhao, B. Predictors of thromboembolic complications after stent-assisted coiling of acutely ruptured intracranial aneurysms: A retrospective multicenter study. Front. Cardiovasc. Med. 2022, 9, 922858. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Minhas, A.; Sarwar, U.; Tahir, H. Triple Antithrombotic Therapy (Triple Therapy) After Percutaneous Coronary Intervention in Chronic Anticoagulation: A Literature Review. Cureus 2022, 14, e21810. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).