Abstract
The chemical industry faces major challenges worldwide. Since 1950, production has increased 50-fold and is projected to continue growing, particularly in Asia. It is one of the most energy- and resource-intensive industries, contributing significantly to greenhouse gas emissions and the depletion of finite resources. This development exceeds planetary boundaries and calls for a sustainable transformation of the industry. The key transformation areas are as follows: (1) Non-Fossil Energy Supply: The industry must transition away from fossil fuels. Renewable electricity can replace natural gas, while green hydrogen can be used for high-temperature processes. (2) Circularity: Chemical production remains largely linear, with most products ending up as waste. Sustainable product design and improved recycling processes are crucial. (3) Non-Fossil Feedstock: To achieve greenhouse gas neutrality, oil, gas, and coal must be replaced by recycling plastics, renewable biomaterials, or CO2-based processes. (4) Sustainable Chemical Production: Energy and resource savings can be achieved through advancements like catalysis, biotechnology, microreactors, and new separation techniques. (5) Sustainable Chemical Products: Chemicals should be designed to be “Safe and Sustainable by Design” (SSbD), meaning they should not have hazardous properties unless essential to their function. (6) Sufficiency: Beyond efficiency and circularity, reducing overall material flows is essential to stay within planetary boundaries. This shift requires political, economic, and societal efforts. Achieving greenhouse gas neutrality in Europe by 2050 demands swift and decisive action from industry, governments, and society. The speed of transformation is currently too slow to reach this goal. Science can drive innovation, but international agreements are necessary to establish a binding framework for action.
1. Introduction
The increasing production and use of chemicals pose a significant threat to the stability of the Earth system. As early as 2009, Rockström et al. introduced a novel concept [1], identifying “novel entities” as one of nine processes that puts the Earth system at risk [2,3,4] if the process-specific planetary boundary is exceeded. In 2022, an international research team investigated the extent of the Earth’s exposure to chemicals and other “novel entities”, and they found that the planetary boundary has been significantly exceeded [5]. Chemical pollution is closely linked to the global crises of climate change and biodiversity loss [6]; however, unlike these, it remains largely unregulated at a global level [7]. Many representatives of science and politics now speak of a “triple crisis” [8], in which one problem cannot be overcome without taking action on the others simultaneously. This is also highlighted in the documents of the “Global Framework on Chemicals” (GFC), the successor process to the “Strategic Approach to an International Chemicals Management” (SAICM). Point No. 3 of the Bonn Declaration reads as follows: “The crises of pollution, climate change and biodiversity loss are closely interrelated and need to be addressed in an integrated manner. The sound management of chemicals and waste will contribute significantly to the achievement of the climate objectives of the Paris Agreement and the goals and targets of the Kunming-Montreal Global Biodiversity Framework, and will thereby assure the long-term integrity of vital ecosystem services and their productive capacity” [9].
The chemical industry is a major contributor to environmental degradation, resource depletion, and greenhouse gas emissions, while also causing severe health risks. A fundamental global transformation of the chemical industry toward sustainable, circular, and resource-efficient practices is urgently needed to protect the environment, human health, and ensure social and environmental justice. The “Global Chemicals Outlook II” report impressively documents the problem of the ever-increasing production of chemicals and their growing use and discharge into the environment [10]. It is particularly alarming that —according to an estimate by the World Health Organization (WHO)—2 million deaths per year are attributed to the improper handling of chemicals [11]. The measures taken so far are not enough. Particularly in the countries of the global South, there are gaps in legislative measures and a lack of capacity for the safe handling of chemicals. Better chemicals legislation and its implementation in these countries will also promote health and environmental protection and can counteract the shift of environmental problems from the industrialized countries to the global South. “Business as usual” is not an option; additional measures by all stakeholders, including a global framework, are required [10].
This publication aims to analyze what is necessary for the production and use of chemicals within planetary boundaries. It focuses on data in the EU and Germany, as global data are often lacking. However, due to the global integration of the chemical industry, the conclusions apply to the worldwide development of the chemical industry. Worldwide chemical production has increased 50-fold since 1950. A further tripling is expected by 2050 [10]. According to an estimate of the Nova Institute, the annual use of carbon in products will increase from 710 Mt (2023) to 1500 Mt (2050) [12]. In 1950, only 1.5 Mt of plastics were produced worldwide. In 2022, it was over 400 Mt—an increase of more than 250 times. It has been estimated that around 350,000 chemicals are produced on the global market, including plastics, pesticides, pharmaceuticals and industrial chemicals [13]. The effects on human health and the environment are only known for a small fraction of them. Production volumes, sales, use and the diversity of chemicals are constantly growing, with an increase being predominantly recorded for emerging countries, especially China. In 2023, world chemical sales amounted to EUR 5195 billion [14]. The chemical industry already accounts for around 10% of global energy demand and is the third-largest industrial sector in terms of CO2 emissions [15,16]. The demand for mineral oil is rising faster than in other industrial sectors. In Germany—home to around 30% of the European chemical industry—energy consumption for the manufacture of chemical and pharmaceutical products amounted to 26.5% of manufacturing industry consumption in 2023, more than any other sector. Nearly one-third of this energy was used as raw material for the production of chemicals and materials [17]. Many chemical companies generate their energy using their own power plants with combined heat and power (CHP), whereby natural gas and crude oil are predominantly used as energy sources in Europe, (fracking) gas is widely used in the USA, and coal is used to a large extent in China [18,19]. Unlike other regions, China utilizes coal as both an energy source and chemical feedstuff. In 2017, 180 Mt/a coal were used as chemical feedstock [20].
In principle, energy is not a limited resource, as the sun constantly supplies new energy. In reality, however, even renewable energy can only be used to a limited extent. The limiting factors here are the available area, the resources required for energy crops, and interferences with the protection of biodiversity, e.g., impairment of habitats. As part of the chemical industry’s transformation towards greenhouse gas neutrality, its energy requirements are expected to increase (see Chapter 2). It will have to be covered as completely as possible through renewable sources. Material resources, on the other hand, are finite. The chemical industry is a sector with very high resource requirements. In 2022, the German chemical industry consumed 11.7 Mt of petroleum derivatives and 2 Mt of natural gas for its production [21]. High resource demands apply not only to fossil raw materials for organic chemicals but also increasingly to critical resources like lithium, cobalt, and rare metals [22,23]. Humans currently use almost the entire periodic table. Due to limited deposits, the EU has classified numerous metals and phosphorus as critical raw materials [24]. Recycling rates are often below one percent. The reasons include inter alia a lack of economic incentives and technologically induced material dissipation [25]. The importance of limited resources for sustainable development is still generally underestimated [26]. The United Nations Global Resources Outlook report highlights the problem of unsustainable resource consumption [27].
On the one hand, chemistry provides many tools that help to achieve the United Nations’ Sustainable Development Goals (SDGs) [28]. We need the products of the chemical industry for the generation and storage of renewable energy, clean water, hygiene and health, thermal insulation, mobility, and corrosion protection. A world free from synthetic chemicals is inconceivable and would not be desirable. On the other hand, chemicals can pose a risk to people and the environment and jeopardize the achievement of the SDGs.
To control the risks of chemicals, there is extensive chemicals legislation in the countries of the global North, according to which the manufacture and use of substances with particularly hazardous properties or a high level of exposure can be restricted or banned. REACH, the EU’s chemicals legislation, for example, stipulates that chemicals must be registered and safety data submitted according to the principle ‘no data—no market’ [29]. This has considerably strengthened the idea of prevention. However, there are still prominent gaps in data and knowledge. The EU Commission intends to amend the REACH Regulation to close existing loopholes. However, the submission of a draft has been delayed. Micropollutants continue to be a problem that affects the quality of groundwater and surface water [30,31]. To prevent serious chemical accidents, the Seveso Directive has prescribed stringent measures for plant safety [32]. As part of its “Green Deal” [33] and its follow-up program “Clean Industrial Deal” [34], the EU is aiming for a “non-toxic environment” and “non-toxic material cycles” [35] and has published a communication titled the “Chemicals Strategy for Sustainability” [36].
Obviously, the need to transform the chemical industry concerns both the substitution of hazardous chemicals and the resulting hazardous waste, as well as a reduction in the volume of chemical production, the associated material and energy flows, and the exploitation of resources. A fundamental rethinking of the chemical industry is unavoidable. A sustainable transformation of the chemical industry includes the following challenges, which will be discussed in this publication:
- Energy supply without fossil fuels;
- Circular production instead of prevailing linear production, i.e., closing carbon cycles;
- Transformation to raw material base (feedstock) without fossil resources;
- Switch to sustainable, low-risk production methods;
- Production and use of chemicals that pose no risk to people or environment;
- Reduction in material flows and focus on products that provide social benefits (sufficiency).
Particular attention will have to be paid to the aspect of environmental justice in the planned transition [9,37]. This concerns, for example, the aspect that poor population groups are exposed to hazardous chemicals to a greater extent, e.g., in the vicinity of production facilities, at workplaces, or in residential areas built on contaminated land. The question of justice also arises in the North–South relationship; chemical companies in industrialized countries often relocate their production to developing and emerging countries. They do not always adhere to equal safety standards for both the production and products. Therefore, they also transfer the associated risks. Poorer people, particularly in the South, often suffer from the intensive and often improper use of pesticides [38] and insufficient safety standards in workplaces [39,40,41,42,43].
The chemical industry is faced with several challenges that require a fundamental transformation, including the following [44]:
- (i)
- Climate crisis, resulting in political requirements to achieve greenhouse gas neutrality in Europe by 2050 at the latest;
- (ii)
- Energy crisis (in Europe), as supply of natural gas has become uncertain and more expensive due to Russian war of aggression against Ukraine;
- (iii)
- Unstable value chains for supply of raw materials, driven by COVID-19 pandemic and labile trade relations.
It is obvious that a comprehensive reorientation of the chemical industry is necessary to meet these challenges. Several studies—not only in Europe, but also in the USA and Japan—are focusing on the need for transformation. The goal of reducing the risks from emissions in chemical production and from hazardous chemicals is formulated in the “Louisville Charter for Safer Chemicals” [45] and presented in a corresponding study [46]. In Japan, recent publications shed light on how to achieve the net-zero target for the chemical industry through increased material efficiency and circularity, electrification, reduced consumption, bio-based production, and other measures [47,48].
Several European studies outline scenarios for the development of the chemical industry towards sustainability in the sector, mostly focusing on the defossilization of production. Decarbonization, as in other industrial sectors, cannot be a goal for the chemical industry since organic chemistry is based on carbon. Kloo et al. compared 14 roadmaps of predominantly industrial origin [49]. The chemical industry in Europe, as well as in North America and Japan, is expected to grow only slightly. In the C4C (Chemistry for Climate) scenarios for Germany, for example, a decline of 0.5% p.a. is expected for basic chemicals and a slight growth of 1.1% p.a. for specialty chemicals [50]. In contrast, the forecast growth of the chemical industry will take place primarily in Asian countries and the Middle East. Jiang et al. expected an increase in CO2 emissions of Chinese chemical production from 640 Mt/a (2017) to 1160 Mt/a (2030) in a baseline scenario. Through electrification and the use of “green hydrogen”, they believe a reduction of 242 Mt/a CO2 by 2030 is achievable [20].
2. Sustainable Conversion of the Energy Supply
The chemical industry is one of the largest industrial energy consumers. For example, its total final energy demand in Germany in 2020 amounted to 161 TWh (574 PJ), with the following three processes consuming more than a third (61 TWh/220 PJ): the steam cracking of naphtha to produce alkenes and aromatics as basic chemicals; chlor-alkali electrolysis to produce chlorine and caustic soda; and the production of ammonia using the Haber–Bosch process [18]. Steam cracking and ammonia synthesis are associated with high natural gas consumption and thus high direct greenhouse gas (GHG) emissions.
The chemical industry mainly covers its electricity and heat requirements with natural gas, which is burned directly at energy-intensive production sites or in combined heat and power (CHP) plants. Around 75% of the electricity required is also generated in-house; the remainder is obtained from the public grid. The chemical industry is also increasingly generating its own renewable energy, e.g., BASF, is investing in wind turbines in the North Sea and geothermal energy onsite in Ludwigshafen [51].
According to the EU’s RE Power Plan, European industry must save 43 billion m3 of natural gas by 2030, which means roughly halving the current consumption level [52]. For the generation of heat, alternatives to fossil energy based on natural gas could be, for example, biofuels, green hydrogen, and solar or geothermal energy. However, their availability is limited, so electrification (from renewable sources) may be the method of choice [44,49]. An exemplary step in this direction is the construction of electrically heated steam crackers by some chemical companies. In 2024, BASF connected two such electric cracking furnaces to a conventional steam cracker with eight furnaces [53]. Other options for electrification include high-temperature heat pumps, mechanical steam recompression and electrode boilers [49]. Overall, the German chemical industry expects electricity demand to more than triple, reaching around 440 TWh by 2045 —the year by which Germany aims to achieve GHG neutrality– according to an updated estimate [54]. The industry is also calling for competitive prices of 4–6 EuroCt/kWh [50]. The EU Commission projects that the electricity demand of the chemical industry in the EU will quadruple to 700 TWh by 2050 [55].
In future, hydrogen will play an important role as a raw material base and, in part, as an energy source in high-temperature processes for the chemical industry. However, 96% of hydrogen is currently still produced using the steam reforming process, in which hydrogen (H2) and carbon dioxide (CO2) are produced from methane and water using energy (“gray hydrogen”) [15,56]. The largest industrial consumers are refineries (production of light hydrocarbons through hydrocracking) and the chemical industry (ammonia and methanol production). A global production of 100 Mt/a of H2 is currently assumed [57].
Although electricity could replace natural gas as an energy source in the steam reforming process, “gray hydrogen” production would remain a significant CO2 emitter and will contribute to the global warming. When combined with CCS, the OECD calls such hydrogen “blue hydrogen” [57]. The production of hydrogen through artificial photosynthesis [58,59] or through microbial electrolysis [58] is not yet available on an industrial scale. The future therefore involve “green hydrogen”, which is produced by the electrolysis of water. If the electricity used comes from renewable energies, H2 production is GHG neutral. In Germany, however, 180–245 TWh would be required to generate the current hydrogen demand, roughly the amount of renewable electricity produced in 2019. In addition, the expectations for a significant expansion of hydrogen production are huge, including the following: replacing natural gas as an energy carrier, and using hydrogen in Power-to-X (PtX) processes to produce heat or hydrocarbons from CO2—not only for chemical feedstock, but also for natural gas, gasoline, and other fuels. If hydrocarbons are produced as liquids (PtL), they can be used as basic chemicals. In the literature, CCU (Carbon Capture and Utilization) is used as abbreviation in parallel to PtX for the reaction of hydrogen with carbon dioxide. The abbreviation CCU is used further in this publication. The OECD calculates that global H2 production will increase from the current 100 Mt/a to 700 Mt/a by 2050 [57]. Similar figures are expected by Huo et al., who estimated the global need of the chemical industry alone in 2050 to be 344 Mt/a to 501 Mt/a [60]. Even with a rapid expansion of production capacities in the EU, hydrogen will have to be imported, and a pipeline network with storage facilities will have to be built. In principle, the existing natural gas infrastructure is probably suitable for this. Hydrogen can only be stored under high pressure or at low temperatures, and only with low energy density. Ammonia and methanol are being discussed as storage media. Alternatively, the reversible reaction of potassium hydrogen carbonate (KHCO3) with hydrogen to form potassium formate (KHCO2) could be a suitable system for efficiently storing hydrogen and transporting it over long distances [61]. Competition for the use of hydrogen will arise. Potentially, it will be necessary to limit the use of hydrogen to those areas in which it cannot be replaced, such as iron and steel production, the heating of cement kilns, ammonia synthesis, the production of hydrocarbons, and as a synthesis component in the chemical industry, as well as for fuels in aviation and may be shipping [50].
Numerous documents from the European Commission and plans from the EU member states make it clear that an infrastructure is to be set up to capture emitted CO2 and store it in underground storage facilities (Carbon Capture and Storage—CCS). The greenhouse gases emitted would then have no impact on the climate [62,63,64]. The Intergovernmental Panel on Climate Change (IPCC) also advises removing CO2 from the atmosphere in this way, in view of ongoing global warming [65]. Saline aquifers and depleted oil and gas fields can be considered as storage sites. As CO2 is generally not recovered but stored permanently underground, it should be seen as disposal and not as storage. Apart from residual uncertainties regarding the safety of transporting and storing carbon dioxide, such an option is associated with the development and construction of a complex pipeline structure, which in turn can only be operated profitably if it is utilized with sufficient CO2. Huo et al. expected that a network of several tens of thousands of kilometers would be needed worldwide [60]. Therefore, this could be an incentive to continue current fossil fuel production and avoid determined defossilization. Some scenarios on how greenhouse gas neutrality can be achieved by 2050 do not require CCS, for example, the GreenSupreme scenario in the RESCUE study by the German Environment Agency (UBA) [66], which assumes a significant increase in energy and material efficiency, the establishment of an effective circular economy, an extensive renunciation of fossil raw materials, and a change in the population’s mobility and eating habits. Natural CO2 sinks will be expanded, and technical CO2 sinks such as CCS will be avoided. In addition, you should also consider that storing CO2 in underground storage facilities is not a sustainable long-term solution, as suitable storage sites are limited. However, there will undoubtedly be residual emissions of CO2 even after the transition to a defossilized economy, e.g., from the incineration of waste (including non-recyclable chemicals), the cement industry, and some other processes in the chemical industry [67]. If an infrastructure for the direct extraction of CO2 from the atmosphere (‘direct air capture’ DAC—see Chapter4) is required, extracted CO2 must also be stored.
However, captured CO2—whether from point sources or obtained by DAC—should preferably not be stored in the underground for a long time but used as a synthesis building block (Carbon Capture and Utilization, CCU). Unlike CCS, CCU does not remove CO2 from the atmosphere but only delays its release from products. However, CCU replaces fossil carbon in products, which is why it can be seen as a climate-friendly alternative [68]. Although CO2 is extremely inert, it can be converted into hydrocarbons with hydrogen and high energy input (see Chapter 4). Therefore, CO2 should not be injected into storage facilities but fed back into production. This would also take into account the low social acceptance of CCS.
The energy supply of the chemical industry must change. The electrification of processes will be a key instrument for greenhouse gas-neutral chemical production. The chemical industry will partly produce renewable electricity itself and partly draw it from the public grid. For high-temperature processes, green hydrogen may replace natural gas, which is also required for the production of non-fossil raw materials (see Chapter 4). The development of a hydrogen network is therefore essential for the chemical industry. CO2 emissions from the chemical industry must be captured and either stored in suitable geological formations (CCS) or better utilized for the synthesis of organic compounds (CCU). This transformation requires considerable investment and an expansion of public networks for electricity, hydrogen, and carbon dioxide.
3. Sustainable Material Flows—Circularity
The chemical industry’s mode of production is largely linear; raw materials are extracted, chemical products are made from them, consumers and industrial users apply and consume them and, finally, they become waste. Being at the beginning of the value chains, the chemical industry has only dealt with the concepts of a circular economy to a limited extent [69]. The issue of recyclability must no longer play only a subordinate role. Take-back systems and innovative concepts, such as chemical leasing—where manufacturers do not offer their customers the largest possible quantities of a chemical but rather take responsibility for the service and have an interest in using the chemical as sparingly as possible—have so far been niche products [7,70].
Some chemical companies are becoming increasingly aware of their responsibility to close material cycles as far as possible, even if the ongoing success of the linear business model leads to more pronounced inertia. A circular economy is also necessary in the chemical sector in order to close material cycles as completely as possible, not only decoupling resource consumption from economic growth but also reducing it in absolute terms [71]. Keijer et al. demonstrated that an orientation of the chemical industry towards the 12 principles of green chemistry [72] can lead to non-sustainable conclusions, as these are based on linear production. Therefore, they formulated 12 principles of circular chemistry, without which chemical production and chemical products cannot be sustainable [73]. A circular economy also helps to reduce dependence on imported raw materials. At a global level, this is reaffirmed in Resolution 11 of UNEA 5.2 in March 2022, which states that a circular economy is essential for sustainable production and consumption (SDG 12) [74]. The European Commission has declared a circular economy to be a central element of its “Green Deal” [33]. In a communication from March 2022, the Commission announced measures to make circular products the norm, e.g., for textiles and construction products, as well as products from chemical industry [75]. Therefore, sustainable, circular material flow management also includes requirements for product design (recyclable, easily separable material mixtures, components that can be dismantled, repairability). This enables the reuse of products and product components. If this is not possible, the materials contained in the products should be recovered as completely as possible (material recycling). Progress to date has been limited. Global consumption of primary resources is growing faster than the gains from reuse and recycling. According to the ‘Circularity Gap Report’, the share of secondary products has declined from 9.1% (2018) to 7.2% (2023) globally, although public awareness of the circular economy has increased during this period [76]. Several countries have developed plans to reduce raw material consumption. For example, the Netherlands adopted a comprehensive circular economy package with binding targets in 2016 [77] and Austria published a circular economy strategy [78]. In its draft national circular economy strategy, the German federal government aims to halve resource consumption to eight tons per capita per year by 2045 [79], which also corresponds to the demand of non-governmental organizations (reduction of the consumption of abiotic primary raw materials to a maximum of 6 tons and reduction in the consumption of biotic primary raw materials to a maximum of 2 tons per person per year) [80].
Regarding resource consumption in the chemical industry, a significant reduction in production waste is also required, i.e., synthesis processes used should have the highest possible yield and as few by-products as possible (atom economy) (see Chapter 5).
Only nature manages to avoid producing non-recyclable waste during biosynthesis. Unfortunately, it is an illusion that technical material cycles can be 100% closed [81,82,83]. The following obstacles make complete recycling impossible:
- Energy and material are lost during the life cycle of a product. Entropy increases, especially when different components are mixed. Recovery then requires high energy input and is never complete.
- Consumers do not fully recycle used products; a proportion ends up in mixed waste.
- Products may contain hazardous substances that can only be separated and eliminated with great effort.
- Recycling companies generally do not have complete substance information, particularly for imported products [84].
- Material flows that are sent for recycling often have varying compositions, complicating the recycling process.
The entropy factor plays a decisive role; the greater the dissipation of a substance in a matrix, the greater the energy required to recover it in the purest possible, reusable form. Therefore, a recycling rate of 100% is not achievable and would also be ecologically disadvantageous [85].
Although completely closed material cycles cannot be achieved, the potential of a circular economy is currently far from being exhausted. Numerous logistical and technical measures are suitable for a significant increase in recycling rates. These include the proposal by Wang et al. to consider the risk of whether the use of a substance can impair the circularity of the products in which it is contained as part of the chemical assessment [86]. With reference to entropy, Kümmerer et al. highlighted the difficulties of successfully recycling highly complex products with a diverse chemical composition into substances that can be reprocessed into high-performance products. They argue in favor of simpler, less complex products and set out rules on how chemistry can be integrated into a circular economy [87]. Another major obstacle to successful recycling is that many products contain hazardous chemicals that can accumulate during recycling. They often also contain legacy substances whose use has been restricted or banned. The EU is aiming for ‘non-toxic material cycles’ [35]. Börjeson and Ågerstrand emphasized the lack of transparency and traceability in the product chains and made initial proposals for a consistent policy and regulation. The avoidance of toxic substances must start at the beginning of the product chain [88].
The concept of the circular economy focuses primarily on cycles within the technosphere. In addition, the question arises as to whether the cycles of the technosphere and the ecosphere are open or closed to each other. Raw materials are extracted from the ecosphere, preferably in a sustainable way. On the other hand, only substances that are as easily degradable and compostable as possible should be introduced into the ecosphere. Persistent substances, toxins, or nutrients that are capable of disrupting the natural material cycles and cannot be assimilated must be kept away from the ecosphere (Figure 1) [88].
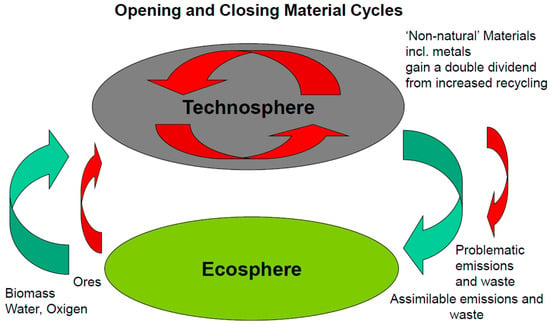
Figure 1.
Opening and closing of transitions between eco- and technosphere (according to Gößling-Reisemann et al. [89]).
Plastic production is particularly important for the circular economy [90]. Some calculations predict that the increase in the amount of plastic waste over the next 20 years will be far greater than the success of increasing the recycling rates [91]. In 2021, 34.6% of the plastic waste collected separately in Germany was recycled. The recyclates—often of inferior quality (downcycling)—were mainly used in the construction sector and for packaging [92]. The amount of plastic packaging waste in Germany has more than doubled in the past 20 years [68]. In 2019, 59% of plastic waste in Germany (corresponding to 3.16 Mt) was packaging [93]. Globally, 90% of the plastic produced is virgin, non-recycled plastic, which is also due to the fact that around 32% of plastic waste is not collected but instead diffusely released into the environment [94]. Improving collection and sorting logistics, avoiding or banning the use of unnecessary and short-lived products, designing for recycling, substituting with other materials such as paper, and restricting toxic or recycling-disruptive additives (e.g., carbon black) can help to mitigate the constant increase in the flood of plastic waste. New technologies for cleaning, sorting, and collecting mixed plastic waste may help to significantly improve the quality and quantity of mechanical recycling (see Supplementary Materials [95,96,97,98,99]). Furthermore, business models that completely dispense with packaging and are geared towards reuse or refilling should be strengthened.
Regulatory requirements, e.g., requirements in accordance with the EU Ecodesign Regulation [100], and economic measures can achieve extensive mechanical recycling of thermoplastics and other materials. The remaining problem of mechanical recycling is that the polymer chains shorten during use and due to the heat involved in the recycling process, which can lead to a loss of quality [101].
Even if a significant increase in mechanical recycling can be achieved, there will still be plastic waste that cannot be recovered in this way or cannot be recovered in sufficient quality, including multilayer films, thermosets, cross-linked structures such as tire rubber, residues from sorting plants, and silicones. Chemical recycling is being discussed as a supplement to mechanical recycling for this waste [102,103,104,105]. The chemical industry associates chemical recycling with high hopes and promises that, through this approach, feedstock recycling can be achieved, and the carbon cycle can be closed [106].
The three main types of procedures for chemical recycling of plastic waste (see Supplementary Materials [107,108,109,110,111,112,113,114,115,116,117,118]) are as follows:
- Solvent-based processes: Plastic waste is dissolved in an organic solvent, any insoluble fillers are filtered off, and the polymer is precipitated again.
- Solvolysis: Polymers are broken down into monomers or oligomers (depolymerization) at elevated temperatures by adding alkali hydroxide, ethylene glycol, ammonia, or similar chemicals [105,119]. Polymer plastics can be resynthesized using these monomers and oligomers. The process is limited to plastics with heteroatoms in the polymer chain such as polyamides, polyurethanes, and polyesters.
- Thermal depolymerization: This includes gasification at 750–1500 °C [120,121], as well as pyrolysis in the absence of oxygen at 420–850 °C, and innovative processes such as liquefaction at normal pressure at 250–350 °C [122,123] or hydrothermal plastic recycling (splitting with supercritical water under pressure) [124,125,126,127]. The resulting products are intended to serve as starting materials for the synthesis of new polymers or other chemical products (see Chapter 4).
There is still limited experience with liquefaction and hydrothermal recycling in terms of the suitability of the reaction products as chemical raw materials. Gasification has been implemented on an industrial scale and is generally suitable for plastic mixtures, but it has a high energy requirement and tends to form tar and char.
Pyrolysis, an old process [128] in which pyrolysis oil, synthesis gas, and coal are produced in a radical reaction, is currently being promoted most intensively. However, a breakthrough has not yet been achieved; many projects have also failed for technical reasons [129]. Apparently, only a few percent of the pyrolysis oil can currently be added to the naphtha in steam crackers without risk [130]. Surprisingly, several publications, especially from the industry, give the impression that the technical problems have already been solved [106,131]. Considerations of using pyrolysis oils for less valuable purposes, e.g., as fuel, make little sense, as it is much more thermodynamically sound to use the energy content of the plastics directly through combustion and to utilize the resulting CO2 as a building block for synthesis (CCU—see Chapter 4).
Although the chemical recycling of plastic waste is expected to be technically successful and economically viable in the future, it is still not an equivalent alternative to mechanical recycling. Quicker et al. compared pyrolysis and liquefaction with mechanical recycling and incineration. Thermochemical recycling processes have clear disadvantages compared to mechanical recycling in terms of energy consumption and greenhouse gas emissions, but they are associated with lower greenhouse gas emissions than incineration [123]. In principle, thermochemical processes are not yielding new plastic but raw material for chemical production. Therefore, they should not be regarded as recycling but as chemical utilization of plastic waste. Chemical and mechanical recycling should not be placed on the same level in the waste hierarchy. Whenever mechanical recycling is possible and leads to satisfactory results, it is preferable. It should also be noted that the development of a larger infrastructure for chemical recycling deprives recyclable plastics of mechanical recycling and should therefore be avoided. Saputra Lase et al. carried out a material flow analysis of Europe, according to which 41–46% of plastic waste could be mechanically recycled and 15–35% chemically processed into either new polymers or chemicals in 2030 with improved collection and sorting [132]. Still, the handling of contaminants and impurities plays an important role in both mechanical and chemical recycling [50].
Overall, the combination of mechanical and chemical recycling could contribute to recycling carbon and to tackling the plastic problem. A “cascade use” approach can also be useful here, starting from high-quality mechanical recycling to chemical recycling or energy recovery. PlasticsEurope, an association of manufacturers, acknowledges these challenges and presents proposals and solutions; however, it does not specify the concrete specific measures that the plastic industry intends to take in order to transition to an effective circular economy [133]. In view of the C4C study, a successful circular economy with plastics also requires a change in mentality when considering plastic waste as a valuable raw material [50]. The proposal to create a market for recycled products, e.g., in the European Union, is also under discussion [90].
The chemical industry must change from a linear to a circular mode for its production. This means that the chemicals and products made from them must be reusable or recyclable. Several countries have already developed circular economy strategies aimed at a significant increase in the currently low recycling rates. While complete recycling is impossible, there exists great potential for increasing circularity rates in the chemical industry. Business models, such as chemical leasing, must grow out of their infancy. Applications of chemicals must be assessed as to whether they affect the recyclability of a product. Interestingly, Soeteman-Hernández et al. developed a proposal to link the EU concept of “Safe and Sustainable by Design” (SSbD) for chemicals (see Chapter 6 and Supplementary Materials) at product level with the criterion of recyclability (SSRbD). They tested their approach using three case studies of polymer applications [134]. Developments in the recycling of plastics are particularly important. Mechanical recycling can be significantly increased through new technologies and improved logistics. Chemical recycling could complement the preferable mechanical recycling if the current technical problems are solved. However, sustainability goals can only be achieved if there is a significant reduction in the production, use, and waste of plastics. Efficiency gains and progress in recycling (consistency) are important. Without sufficiency, i.e., reductions, the planetary boundaries will continue to be exceeded (see Section 7).
4. Change to Non-Fossil Feedstock
In 2019, science journalist Robert F. Service posed the following question: “Can the world make the chemicals it needs without oil?” [135]. The chemical industry uses fossil raw materials not only to cover its energy requirements but also as basic materials for its production. Mineral oil and natural gas currently dominate as the material basis for organic chemical production, accounting for around 90%. As early as 2013, the chemical industry used 676 Mt/a of fossil raw materials worldwide [16]. In terms of sustainability and climate protection, this cannot be sustainable, as carbon-containing chemicals from fossil sources ultimately contribute to the emission of greenhouse gases at the end of their product life. Coal—still a major source of basic chemicals in China today and once widely used globally—is not an option, as the CO2 emissions per unit of energy are even greater and toxic by-products are generated. The recent fossil fuel crisis in Europe has particularly affected the chemical industry and highlighted its dependence on raw material imports. For the same reasons, the chemical industry in Europe and North America is only partially relocating basic chemical production to Asia or the Middle East in order to secure the supply of more complex chemical products.
Changing to a non-fossil feedstock is particularly challenging in regions where the chemical industry is growing rapidly like China.
The chemical industry essentially produces the following seven basic substances from crude oil and natural gas: ethene, propene, butenes, benzene, toluene, and xylenes, as well as methanol. Ammonia must be also included, as it is still produced with “gray hydrogen” obtained from natural gas by steam reforming (see Chapter 2). A special feature of the chemical industry is the strong networking of processes at efficient integrated sites, with the syntheses of basic chemicals forming the core. Therefore, the substitution of these central processes would have effects that go beyond the actual process [50].
Chemistry without carbon is not possible. The chemical industry in Germany currently requires approx. 11 Mt of carbon per year to produce basic chemicals [45]. The following three options exist for establishing a non-fossil carbon base. Presumably, all three options will be necessary in the future to meet the required demand:
- Production of basic organic chemicals through chemical recycling of polymers;
- Production of chemicals from renewable raw materials (bioeconomy);
- Production of basic chemicals from CO2 via reaction with “green” hydrogen (CCU).
The chemical recycling of plastic waste can complement mechanical recycling through solvolysis, liquefaction, pyrolysis, or gasification. Pyrolysis, in particular, is currently being discussed as an option (see Chapter 3). Pyrolysis oil is intended to be an alternative to naphtha in steam crackers for the production of base materials. However, the chemical composition of the oil is very different from naphtha, such that only small quantities of pyrolysis oil can be added [130,136,137,138]. It is not yet possible to predict when the calculated quantities for raw material recycling will be able to replace the fossil base.
Great hopes for climate-neutral chemical production are associated with the bioeconomy [46,139,140]. Bio-substances benefit from using nature’s synthesis processes. In addition, natural materials such as wood are often a sensible alternative to concrete as a building material, for example, or to plastics.
Quantitatively more important than modifying biological substances (see Supplementary Materials [141,142,143]) is that biomass can also be converted into basic chemical substances by hydrogenation (Fischer–Tropsch synthesis). However, this destroys the valuable complexity level of natural products. Preferably, only residual materials and waste biomass should be used for this purpose. The raw materials obtained (bio-naphtha) can then be fed back into the usual synthesis routes as basic chemicals. When collecting residual materials (e.g., wood or straw), care must be taken to ensure that a significant proportion remains in the forest or on the field in order to ensure humus formation [44]. Several authors advocate cascading use in biorefineries, i.e., the bio-product initially used as a material, such as wood or paper, gradually becomes a raw material for chemical syntheses [44].
The extent of biomass for utilization as chemical feedstock is limited (see Supplementary Materials [144,145,146,147,148,149,150,151,152]). Currently, around 4.3 Gt per year of fresh biomass are used globally for bioenergy. It has also been estimated that 7 Gt/a would be needed to meet the additional demand for chemical production [140]. In Germany, the current biomass quota of 13% (2.6 dm Mt) is overall limited and likely overestimated in several studies [153]. The Agora study, for example, calculated an additional 14–48 dm Mt of biomass potential for Germany [44]. The C4C study expects around 26 Mt dm of mobilizable technical potential of residual biomass for Germany by 2045 [50]. Other estimates have included 31 dm Mt [154] or 27.7 dm Mt [155]—both being on a similar scale. The use of biomass on a large scale for chemical production is only viable if the biological raw materials are permanently available in constant quality. However, despite all efforts to establish biorefineries, this is currently not assured.
The third possible carbon source is CO2. Since CO2 is a very inert gas, it requires a lot of energy to be converted into organic chemicals. The large-scale use of CO2 is possible by using the CCU technology through a reaction with hydrogen. The following two synthesis routes have been proven in several plants: Fischer–Tropsch synthesis, with the formation of hydrocarbons (naphtha), which are then further processed into olefins or aromatics in steam crackers, and the methanol-to-olefin (MTO) route or methanol-to-aromatics (MTA) route (see Supplementary Materials [68,156,157,158,159,160,161,162,163,164,165]). In a comparison of the Fischer–Tropsch route with steam crackers with the MTO/MTA route, the latter performed better in terms of energy requirements by 30–40% [153].
It has been calculated that 2.5–4.7 Gt/a CO2 will be needed in 2050 for the production of basic chemicals [166]. Huo et al. expect a global CO2 demand of the chemical industry of 2.2–3.1 Gt/a in 2050 based on figures from the International Energy Agency (IEA) [60,167]. The long-term availability of CO2 needs to be clarified. For the time being, industrial point sources that can be used via Carbon Capture and Utilization (CCU) are available for this purpose (see Chapter 2). The calculations by the IEA and Huo et al. are based on the assumption that power plants and pulp mills will continue to be available as CO2 point sources in 2050, and that there will therefore be no shortage of CO2 [60,167]. However, if the chemical industry is largely decarbonized, the remaining point sources such as waste incineration plants and cement kilns will not be sufficient in the long term. The CO2 must then be filtered out of the atmosphere (CO2 concentration approx. 400 ppm) (Direct Air Capture, DAC), also a process that requires a lot of energy, especially for the release of CO2 from the sorbents (1.4–2.8 MWh per ton of CO2) [168,169,170]. DAC is being tested in several pilot plants. A life cycle analysis of the process is still pending.
One advantage of using CO2 as a synthesis building block in the CCU process is that the hydrocarbon mixtures obtained can be used in the same way as virgin naphtha. Therefore, it would not be necessary to change the basic chemistry routes. However, the energy and cost requirements are high.
The three options to achieve net-zero emissions for the non-fossil production of chemical feedstock do not compete with but rather complement each other. Meys et al. calculated that 1471 Mt/a of carbon will be needed worldwide by 2050, 39% of which could come from biomass, 18% from CCU, and 24% from chemical recycling. The remaining 19% will be recovered through mechanical recycling [171]. In the report “Chemistry for Climate”, the authors presented three scenarios on how chemistry in Germany could become GHG neutral by 2045 [50]. While two scenarios are based only on the use of electricity, hydrogen, and the CCU process, the third scenario includes plastic waste and biomass as feedstock (see Supplementary Materials).
A new study by DECHEMA presents three scenarios on how the chemical industry in Germany and the EU can be supplied without fossil carbon by 2050. The share of biomass and recycling differs little between the scenarios, which assume significant growth or a considerable reduction in chemical production, because the potentials of these two sources are limited. The energy-intensive use of CO2 as a raw material, on the other hand, is heavily dependent on the further development of the chemical industry in Europe. In the (medium) scenario with little change in production volume, 16.3 Mt C of 28 Mt C will be obtained from CO2 in the EU by 2050 [172].
The chemical industry must change its material base for organic substances. Today, it is not possible to predict the exact future ratio of each alternative feedstock type. The mix will change over time and region [140]. All three processes also have clear limits, as follows: Chemical plastic recycling must be technically mature, and the raw materials must be available in sufficient quantity and quality. Biological raw materials will only be available as feedstock in limited quantities and should be of consistent quality. Biomass will always be subject to competing utilization interests. The production of organic chemicals from carbon dioxide requires a very high energy input and well-developed logistics for the delivery of CO2 and H2. Therefore, there is no way around reducing chemical production worldwide (see Chapter 7).
5. Sustainable Chemical Production
Today’s chemical production at large industrial sites is highly interconnected. In its own interest, the chemical industry is making considerable efforts to use energy efficiently and generate less waste by linking production. This complexity is an advantage, but it also makes changes more difficult, as improvements in one area of production can result in disadvantages in others. This interconnection has been developed and optimized over the past decades on the existing fossil raw material base of coal, oil, and natural gas. The question arises as to whether this networked co-production should be reconsidered, at least in part, when switching to a regenerative raw material base. Unfortunately, there has been an almost complete lack of thought and innovation in this direction so far.
Principles of sustainable chemical production have been established, for example, in Annex III of the EU Industrial Emissions Directive (IED) [173], the principles for green chemistry [72], and the principles for green engineering [174], which include low resource and energy use, waste avoidance, minimal emissions, and low human and ecotoxicity.
Issues of energy and resource efficiency, as well as the potential for changing the raw material base, have already been discussed in Chapters 2–4. Avoiding toxic emissions into air and water is another requirement of sustainable chemical production. Reducing production waste is also a key objective. The so-called E-factor, which describes the ratio of kilograms of waste and by-products to kilograms of desired product, is expected to be particularly high for pharmaceuticals (25 to 100), but is often close to 1 for basic materials [175]. In some cases, it is possible to completely redesign a synthesis pathway to achieve higher atom economy and fewer synthesis steps, while also avoiding derivatization.
Another important element of sustainability in the chemical industry is to construct and operate plants in an accident-proof manner. It is essential to carry out chemical production at low temperatures and low pressure. Biotechnical processes and the use of catalysts (see below) help us to meet these requirements.
The German Environment Agency (UBA) has named a number of innovative technological approaches that are suitable for making chemical production more sustainable, particularly regarding the production of fine and specialty chemicals [153,176]. The IUPAC identified the following ten emerging technologies in chemistry that can contribute to sustainability, some of which relate to further developments in chemical synthesis [177]:
- Avoidance of volatile organic solvents (VOCs): Chemical syntheses can often be carried out with significantly less solvents, or the VOCs can be completely replaced by supercritical fluids, such as carbon dioxide or ionic liquids [178,179]. Some ionic liquids, which have a low vapor pressure, are easily degradable [180]; however, there is still a need for clarification regarding human and ecotoxicity.
- Optimized separation processes: Processes for the separation and purification of substance mixtures consume more than 40% of the total energy in chemical production. Membrane technologies and processes such as reactive rectification and extraction reduce the consumption of energy and materials and often increase yield and selectivity.
- Energy input through new processes: Usually, the energy required for chemical reactions is input by electricity or the combustion of natural gas. New processes use plasma, ultrasound, or microwaves, which can lead to modified reactions, higher selectivity, and increased yields [153].
- Catalysis: Catalysts reduce the energy requirements of syntheses, as they generally run at lower temperatures and avoid waste due to their higher selectivity. Organocatalysts, which are often used immobilized on carrier materials, have the advantage of abstaining from critical metals [153]. Of particular interest are chiral catalysts, which preferentially produce the desired enantiomer in drug syntheses and thus almost double the yield [177].
- New processes for chemical synthesis: Fine and specialty chemicals in particular can be produced in high yields and under controlled conditions using new processes. These include continuous flow chemistry processes, e.g., in microreactors, where temperature, mixing, and other reaction parameters can be easily controlled. The selectivity increases, as do resource and energy productivity. Also, in solvent-free reactive extrusion processes and click chemistry applications, side reactions can be avoided, resource requirements can be reduced, and new synthesis routes can be explored [177].
- Biotechnology: Industrial biotechnology uses selective enzymes, bacteria, yeasts, or fungi to produce or process chemicals. Algae cultures can also be used to produce chemical products and plastics [181,182,183]. The advantages of these processes include their operation under normal pressure and at low temperatures, as well as the potential to replace multi-stage chemical syntheses with single-stage, selective processes. Biological residues such as molasses or whey are often suitable as culture media. A disadvantage is that the reactions take place in a diluted aqueous medium, which increases the amount of wastewater and can lead to high energy-intensive costs for separating and isolating the products. Although “white” biotechnology is now viewed by some chemical companies as a technology of the future, it was not attractive in the past due to low energy prices and the availability of natural gas. BASF, for example, is building a new large fermentation plant in 2025 [53]. While most biotechnological processes take place in closed systems, chemical syntheses in genetically modified plants or animals pose other risks, such as uncontrolled spread. Examples include the “amylose potato”, which only contains amylopectin and not the second starch component amylose, or the production of pharmacological active ingredients using milk of transgenic animals, which should be viewed critically, but have not yet left the experimental scale [184].
The water requirements of the chemical industry—partly for cooling, partly as process water or steam generation—are also essential for the sustainability of chemical proeduction. The chemical industry is a major industrial user of surface and groundwater. This is an apparent problem in water-scarce regions and, increasingly, in countries with ample water resources such as Germany [15]. With more frequent periods of water scarcity due to climate change, these uses are becoming increasingly questionable. At Currenta’s chemical sites in Krefeld, Leverkusen, and Dormagen on the river Rhine, for example, annual withdrawals of 182,950,000 m3, 236,142,000 m3, and 116,000,000 m3 are permitted. There is an urgent need for the chemical industry to introduce and expand water conservation options such as recycling and multiple use, for which water law permits should be appropriately adapted and water abstractions made transparent [185].
While the chemical industry is successful in networking its production, the potential for saving water and energy and reducing waste, particularly in the case of fine and specialty chemicals, is far from exhausted. Various emerging technologies can contribute to this goal. However, no innovative approach is free of risks. Therefore, it is necessary to examine the environmental benefits associated with the introduction of such processes on a case-by-case basis.
6. Sustainable Chemical Products
The chemical industry is only sustainable if its products, i.e., the chemicals, also meet the sustainability criteria. The chemicals legislation of the global North stipulates that the manufacture and use of hazardous chemicals can be restricted or banned if safe handling is not guaranteed. REACH, the EU’s chemicals legislation, obligates manufacturers and importers to register their substances along with their safety data if their volume exceeds 1 t/a [29]. If there is a risk, i.e., if potential exposure of humans or the environment exceeds the effect threshold, risk reduction measures are required. Substances with serious hazard characteristics, e.g., carcinogenic substances or substances with PBT properties (persistent, bioaccumulative, and toxic) are designated as substances of very high concern (SVHC). Their use is subject to authorization and the aim is to ‘phase out’ such chemicals. Therefore, regulation is based on a hazard assessment. There is an ongoing discussion between authorities and NGOs (non-governmental organizations), together with the chemical industry, insisting that a risk assessment should be carried out. Classic risk assessments are based on the comparison of adverse effects and exposure. This procedure may lead to incorrect results for persistent and mobile chemicals or chemicals with delayed, irreversible toxicity. In these cases, hazard-based assessments justify the need for action (see Supplementary Materials).
The EU intends to amend the REACH regulation, closing some gaps and eliminating weaknesses [36]. One new element will be the “essential uses” concept. Following the example of the Montreal Protocol, applications are designated as essential if they are necessary for health, safety, or the functioning of society [186,187]. In the case of particularly hazardous substances, only applications for which no technically and economically suitable alternatives are available should be permitted. This assessment approach is not only being tested in Europe—using the example of per- and polyfluorinated alkyl substances (PFAS) [188,189]—but is now also recommended for use in the USA and Canada [190]. The approach is linked to the proposal of “functional substitutions” by Tickner et al. [191,192,193]. The authors differentiate between the following three types of functional substitution: replacement with another chemical, achieving the desired function through other materials or processes (end use function), or a change in system (function as a service, e.g., possible avoidance of antimicrobials). Therefore, it is necessary to examine not only the substitution of a hazardous substance with a possibly similarly hazardous one. Van Dijk et al. tested the combination of ‘essential uses’ with ‘functional substitutions’ using the example of some persistent and mobile cosmetic ingredients [194].
There is no such thing as a sustainable chemical per se. The decisive factor is rather the function that this chemical is intended to fulfill. Is it possible to achieve the same goal in other sustainable, non-chemical ways (see above: functional substitution)? Does the application even make sense? The answer depends on factors such as the raw material base, the amount of energy and resources consumed (or saved) during production and use, potential issues arising during the waste phase, and whether the chemical can be recycled after use, among other criteria.
The function must always be the starting point for sustainability considerations, prompting the following question: Is this purpose necessary, and how can it best be achieved [195]? It is important to consider non-chemical alternatives and alternative business models, such as the leasing of chemicals. Only when it is clear that a chemical compound is needed for a certain purpose do the questions of ecological molecular design and possible risks in the life cycle arise. The unlimited consumption of “harmless” chemicals for purposes of questionable use is not sustainable. With such a critical approach to the use of chemicals and other materials, there is an opportunity to reverse the increase in chemical production and consumption and thus answer the question of sufficiency (see Chapter 7).
Sustainable chemical products need an ecological molecular design when applied; they should be “benign by design”, i.e., have no undesirable effects and exhibit low stability in the environment before they are broken down into harmless substances [196,197]. They should have a short range in time and space, which emphasizes their low persistence and low mobility [198]. Some hazardous properties are inextricably linked to function, e.g., the flammability of fuels or the toxic effect on target organisms of disinfectants. However, hazardous properties unrelated to function should be avoided as far as possible. Many currently registered chemicals can only be handled safely if extensive safety measures are observed—a requirement that is often not met in small- and medium-sized enterprises and outside industrialized countries. In its Chemicals Strategy for Sustainability [36], the European Commission has set the goal of favoring safe and sustainable chemicals (SSbD, see Supplementary Materials [199,200,201,202,203,204,205,206,207,208,209]). In 2022, the Commission published a corresponding concept and recommended that it be tested by industry and academia [199]. Therefore, the chemical industry should consistently strive for an ecological molecular design when developing new and alternative substances. Numerous in vitro methods (high throughput analyses) and in silico methods (computational chemistry) help to identify eligible chemicals with comparatively little effort [210,211]. These methods are also suitable for grouping and regulating chemicals in view of the sheer number of inadequately investigated substances [36,212].
In any case, it is essential for the ecological molecular design that the chemical is not persistent. Persistence has become increasingly important in the assessment of chemicals in recent decades—primarily in the EU and USA, but also internationally in the Stockholm Convention—usually in conjunction with other hazard characteristics such as toxicity and/or bioaccumulation. However, persistent substances have hazard potential even without known negative effects, because it is often too late to stop their use when hazardous properties are detected many years after they have entered the market. They can remain in the environment for a long time, spread widely, accumulate in certain compartments, and lead to unexpected interactions with different substances and organisms [213,214,215,216]. Harmful effects often only become known at a late stage, as was the case with chlorofluorocarbons (CFCs) or some PFAS (per- and polyfluorinated alkyl substances) [217]. Therefore, the consequences of not knowing about unrecognized effects can be particularly great in the case of persistent substances. Not all chemicals can be short-lived. There may be a conflict of objectives with the desired durability of materials. The lifetime of a chemical in the ecosphere should be balanced with the desired lifetime of the product’s function in the technosphere [218]. However, “eternal pollutants” with extreme persistence must be avoided in any case.
The requirements for ecological molecular design relate in particular to chemicals in consumer-related products and chemicals that are intentionally or unintentionally released into the environment. Some authors also call for changes in chemical production concerning the hazardousness of basic chemicals such as benzene, toluene, and xylenes, as well as methanol and many derivatives such as styrene, vinyl chloride, phosgene, or isocyanates [46,219]. The concerns mostly relate to chemical accidents within plants and during transportation. However, it should be borne in mind that the production of basic chemicals takes place in an interconnected and rather efficient system. Changes can lead to significant interactions elsewhere. Therefore, the options for action involve imposing stringent requirements on plant safety and transportation (see Chapter 5). However, production not based on oil and natural gas may also lead to opportunities to establish new, less hazardous networks. Biotechnological production, for example, is generally not based on hazardous basic chemicals (see Chapter 5).
A major problem for sustainable chemicals and materials flow management is the complex composition of products with an almost immeasurable variety of chemicals. Olisah et al. analyzed the contents of 57 databases on products and articles. They concluded that the databases were very heterogeneous and inconsistent. Only a few databases provide data beyond regulatory requirements. In many cases, the producers claim confidential business information (CBI) [220]. Commercial users, recyclers, and consumers generally do not have sufficient information. This is increasingly becoming a problem for the circular economy and hinders the acceptance of secondary raw materials (see Chapter 3). Therefore, Kümmerer et al. advocated the restriction of material diversity [84]. Various methods are being discussed as to how transparency in ingredients can be improved, e.g., chemical and digital tracers, electronic labeling. The new EU Ecodesign Regulation specifies in detail what information a digital product passport should contain [100]. This includes not only hazardous ingredients but also additives that make recycling more difficult. Therefore, the chemical industry should take up the cause of limiting the variety of chemicals in certain product types, increasing transparency and playing its part in making products fit for recycling. It is necessary to examine the possibilities in accordance with the world trade law (WTO) to limit the diversity of substances in products.
The products of the chemical industry should meet the sustainability criteria. Beyond the current legal requirements, the chemical industry is called upon to develop and manufacture chemicals that have an ecological design. The Safe and Sustainable by Design (SSbD) concept is an essential basis for this. In particular, persistence must be avoided with regard to irreversible adverse long-term effects. When evaluating chemicals and products, greater attention should be paid to the necessity of use, the substitutability of function, and the avoidance of complex product compositions that hinder circularity.
7. Reduction of Material Flows—Sufficiency
Material flows must become sustainable; the following three complementary strategies help to achieve this [7]:
- The aim of the efficiency strategy is to use energy and materials as sparingly as possible, to optimize existing technologies, and to develop new, suitable technologies.
- The central goal of the consistency strategy within the technosphere is the circular use of materials (see Chapter 3).
- The sufficiency strategy seeks answers to the question of what a conscious and frugal use of limited resources means.
It has been pointed out several times in the previous Chapters that the defossilization of energy use and the raw materials base is not enough. Rather, an absolute reduction in the production and consumption of chemicals and an assessment of the benefits of chemicals are required in order not to exceed the Earth’s limits. The German Advisory Council on Environment (SRU) has published a statement, entitled “Sufficiency as a Strategy of the Enough: A Necessary Debate”, in which 11 theses clarify that efficiency and consistency are not enough to prevent material flows from compromising the planetary boundaries [221]. In order to realize sufficiency social change is needed to decouple economic growth from the growth of material streams. Therefore, sufficiency will not only affect personal lifestyles but also imply a political–structural change and, ultimately, also a cultural level. In an essay, Mederake emphasized that the technocratic view of the circular economy is not sufficient for a sustainable plastic economy and that an absolute, sufficient reduction in material flows is required [222].
This also applies to the chemical industry. For example, the Louisville Charter for Safer Chemicals calls in its declaration “to limit the output to produce chemicals shown to be necessary and safe throughout their lifecycle” (plank3) [45]. The chemical industry’s studies on the possibilities of achieving greenhouse gas neutrality in the German and European chemical industry assume unabated chemical production and do not address the question of which and how many chemicals are needed. Therefore, it is necessary to generate a sufficiency scenario for the chemical industry, which is outlined in Box 1.
Box 1. Outline a sufficiency scenario for the chemical industry.
Compared to the scenarios of the C4C study discussed in Chapter 4 (see Supplementary Materials), this outline assumes that it is not only necessary but also possible to reduce chemical production in Germany by a third by 2045 without compromising the supply of chemical products essential for social well-being, human health, and societal needs. However, this requires that the process takes place not only in Germany but also in other regions of the world with high levels of chemical production, particularly Asia. Shifting production to emerging and developing countries and importing chemicals from these countries would not help, on the contrary.
It is assumed that chemical production will be completely defossilized, energy will be provided by direct electrification, and the raw material base will be a mixture of biomass, plastic waste, and CCU (reaction of CO2 with hydrogen).
The following measures are required for a sufficiency scenario, among others:
- Ecological molecular design of chemicals (benign by design) and their economical use only in cases where function cannot be fulfilled in a sustainable way without chemicals;
- New chemical production processes (e.g., biotechnology, microreactors, catalysis, biomimetics) reduce energy and resource requirements and amount of waste produced;
- Expansion of circular business models, e.g., chemical leasing [223];
- Halving resource requirements in industrialized countries to 8 tons per person annually;
- Complex products must be modularly assembled and repairable;
- Reduction in plastic waste, in particular by avoiding single-use plastic;
- Increase in high-quality mechanical recycling of plastics;
- Avoiding products with complex chemical compositions that cannot be mechanically recycled;
- Chemical recycling only for plastics that cannot be mechanically recycled and only for raw materials designed for chemical production;
- Political, social, and cultural changes aiming to reduce consumption behavior.
A reduction in chemical production by a third on the basis of the three options for producing non-fossil base materials would also mean a reduction in the demand for electricity, hydrogen, and CO2 by around one-third. It is not possible to forecast and determine the proportions to which they contribute to the mix of raw materials. In any case, the pressure on the use of biomass will be reduced, and the demand for electricity will be lower. The use of chemical plastics recycling also depends on whether it is possible to obtain products that are suitable for steam cracking.
In 2021, the German Environment Agency published a ‘Thought Starter’, titled “Towards sustainable chemical intensity”, in which the increasing production of chemicals is problematized [224]. It proposed to define a “corridor” between the chemical intensity necessary for social well-being, human health, and the functioning of ecosystems and excessive, harmful chemical pollution (Figure 2). There is an obvious need to limit the per capita use of chemicals to a sustainable measure [225]. In Europe and North America, the chemical industry is hardly growing at all, in contrast to even more challenging situation in many Asian and Middle East countries. Here, and in the countries of the South, a development strategy is needed that will enable economic participation without emulating the wasteful consumption of energy and resources in the North [226]. Questions of environmental and climate justice between the North and South are affected. Okereke emphasized that de-growth strategies are not suitable for Africa and advocated a green growth strategy that is largely decoupled from resource consumption and enables the development of a sustainable infrastructure [227].
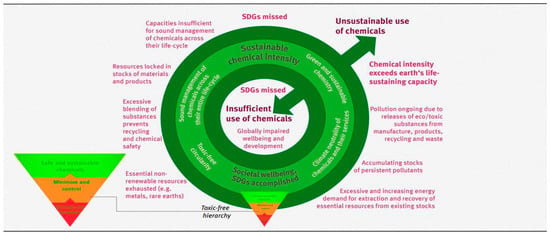
Figure 2.
Sustainable chemical intensity as safe operating space for use of chemicals (according to German Environment Agency [224]).
The chemical industry is not yet fully committed to switching to renewable energy sources and changing its material basis. The key question arises as to whether “business as usual” is the right strategy, or whether a reduction in chemical production is more likely to lead to sustainable solutions. It will take time to design and build the necessary plants; however, 25 years until 2050 is a short period, which is why waiting and hesitating is certainly not the right strategy. Interestingly, the EU document “Transition Pathway for the Chemical Industry” contains the following sentence: “Some stakeholders stress the need to reconsider our energy needs and move towards a sufficiency approach” [54].
8. Discussion and Conclusions
We are facing the necessity of a fundamental change in the production and use of chemicals. Thus, the chemical industry is facing a huge challenge, but it has a very high level of innovative strength that—as has already been shown in the past—will enable it to overcome these issues and become fit for the future. According to the previous Chapters, the transformation of the chemical industry must take place in the following six interconnected areas: Change in energy supply to renewable resources; switch to circular chemistry; a non-fossil basis of raw materials; sustainable chemical production and products and; a reduction in the production and use of chemicals by a sufficiency approach.
The following guiding principles may help to successfully transform the chemical industry:
- -
- The precautionary principle is a globally recognized guiding principle for politics and business. The Rio Declaration of 1992 states the following: “In view of the risk of irreversible environmental damage, lack of full scientific certainty should not be used as an excuse for delaying action that is justified in itself” [228]. Accordingly, action is already required if there are serious indications for concern but without conclusive proof of a causal link between substance exposure and negative effects on the environment and health [229]. For precautionary reasons, it is necessary to reduce material flows and the range of chemicals in space and time in order to avoid exceeding the planetary boundaries (see Chapter 7). However, it is not only the quantity of chemical production and consumption that is unsustainable but also the diversity of chemicals in products, which effectively prevents many products from being integrated into a circular economy (see Chapter 3) [87]. Increased transparency regarding the chemical composition of products—particularly concerning hazardous ingredients—would provide additional improvement (see Chapter 6). Solutions that are compatible with commercial legislation and guarantee protection for legitimate confidential business information (CBI) still need to be developed.
- -
- To achieve the United Nations’ Sustainable Development Goals (SDGs), chemistry must become sustainable by avoiding irreversible harm to human health and the environment. An international committee defines sustainable chemistry as the development and application of chemicals, processes, and products that benefit the current and future generations without harming humans or ecosystems, identifying the following five action areas: equity and justice, transparency, climate and ecosystem impacts, health and safety impacts, and circularity [230]. In 2020, the UNEP published ten goals and principles for green and sustainable chemistry to align production with ethical and social standards [231]. Similarly, ISC3 emphasized holistic, systemic thinking and social and ethical responsibility alongside scientific and technical requirements [195,232]. The German Environment Agency outlined objectives and principles for the transition to sustainable chemistry [233]. The “Global Framework on Chemicals” (GFC), launched at the ICCM5 in Bonn in 2023, also promoted the sustainable use of chemicals across their life cycle, including products and waste [9]. In 2021, the German Environment Agency published six major goals for sustainable chemical management [234] (see Supplementary Materials). Friege et al. reviewed indicators for measuring progress toward sustainable chemistry, proposing 23 indicators to assess the implementation of chemical and waste management goals [235]. In this sense, indicators are proposed for the following, among others:
- ○
- a reduction in use of hazardous chemicals and resulting damage to health and the environment;
- ○
- a support for climate protection through production and products from chemical industry;
- ○
- a renunciation of subsidies for fossil-based materials;
- ○
- sustainable management of nutrients (especially nitrogen);
- ○
- careful use of resources for extraction of raw materials;
- ○
- an effective recycling of products and waste;
- ○
- measures taken by chemical industry for greater safety of products and in production.
Transforming the chemical industry is not solely its own challenge. According to the Global Chemicals Outlook II, the state, commercial and industrial users, trade, science, and consumers must all contribute to a sustainable transition [10]. Governments are tasked with creating legal frameworks, using taxes, levies, and subsidies, and setting clear political goals. Commercial and industrial users, along with retailers, can pressure the industry by demanding sustainable products and improving transparency in supply chains. Consumers and NGOs also exert growing pressure for safer, more sustainable solutions, supported by environmental labels like the “Blue Angel” and the EU Ecolabel. Science plays a key role in developing, communicating, and implementing sustainable alternatives. A global science policy panel on chemicals, waste, and pollution prevention (SPP), modeled after the IPCC and IPBES, is being negotiated to advise political leaders and industry on sustainable chemical production [236,237]. Such a science policy panel should address all issues of sustainable chemical production. This should include the following, among other topics:
- High-quality recycling of chemicals and products;
- Use of chemicals that are as sustainable as possible with lowest possible harmful effects and exclusion of highly persistent substances;
- New, regenerative raw material base for chemicals needed for social well-being, health and to achieve Sustainable Development Goals (SDGs);
- Pathways to a new networked chemical production based on sufficiency, consistency and efficiency;
- Strategies to minimize waste from chemical production and to dispose of chemicals in waste in an environmentally compatible manner;
- Analyzing social benefits of chemicals, chemical products, and sufficiency.
Different instruments are needed to enable the transformation. Regulatory provisions, as well as market-based incentives and state investment subsidies, can support the transition to sustainability. Some companies have complained about the excessive regulatory requirements [238] and threatened to move to regions with fewer regulations. However, previous publications have revealed that strict environmental laws only influence the choice of location to a limited extent. On the contrary, regulatory reliability can often be a positive argument [239,240]. But unnecessary and too time-consuming bureaucracy should be avoided in any case.
CIEL and IPEN have proposed a low international tax on fossil basic chemicals (feedstock) to finance the transformation and support the transition from fossil energy and feedstock to sustainable energy and chemical products [241]. The OECD has discussed “extended producer responsibility” (EPR) as a way of holding producers accountable for their products throughout the entire lifecycle [242]. A fee paid by producers is used to finance the environmentally sound treatment of products; experience has shown that EPR increases transparency in the product chain and raises recycling rates. Mandatory EPR regimes have proven to be more effective than voluntary ones.
An effective market-based system to reduce the consumption of resources and chemicals could be the introduction of a maximum quantity (CAP) [5], thereby limiting production quantities (e.g., of plastic) [49], resource consumption, or waste quantities and continuously reducing them, e.g., with the help of certificate trading.
A fundamental rethinking is necessary. The chemical industry has so far been hesitant to address sustainable transformation [49,68]. In the US, Tickner et al. criticized the industry’s adherence to the status quo, lacking sustainable investment and innovation [243]. Only recently has the European chemical industry engaged in studies on decarbonization and defossilization [50,244], focusing mainly on sustainable energy supply, hydrogen, and raw materials for organic chemicals. These “supply side” studies often neglect “demand side” issues like the actual need for chemicals, circular approaches, sufficiency, and the avoidance of hazardous substances and processes. Moreover, the industry has shown reluctance in implementing its own study results, waiting for government support and fearing market share losses. Discussions focus on investment barriers and energy costs, often defending the status quo rather than pursuing change. Only a few frontrunners are embracing fundamental transformation [10]. Given global competition, regional efforts alone are insufficient. Binding global guidelines and agreements are needed to replace voluntary declarations like the Global Framework on Chemicals. A comprehensive global framework convention covering all aspects of sustainable chemical production and use is essential [7].
The foreword to the Global Resources Outlook states the following: “The messages from this report could not be clearer: It is no longer whether a transformation towards global sustainable resource consumption and production is necessary, but how to urgently make it happen” [27]. This message has not yet really reached the chemical industry, politicians, or society. If the sustainable transformation is to succeed, it will require business leadership, political will, and public pressure.
Supplementary Materials
The supporting information can be downloaded at the following link: https://www.mdpi.com/article/10.3390/suschem6020016/s1.
Author Contributions
The authors (M.G.O. and K.G.S.) contributed equally to this work. K.G.S. coordinated the conceptualization and preparation of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data presented in this review are publicly available and can be found under the references listed below.
Acknowledgments
We thank the Working group Environmental chemicals and toxicology of FoE Germany, in particular Henning Friege, Arnim von Gleich, Klaus Kümmerer, and Wolfgang Körner, for helpful comments and critical discussions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| C4C | Chemistry for Climate |
| CBI | Confidential Business Information |
| CCS | Carbon Capture and Storage |
| CCU | Carbon Capture and Utilization |
| CFC | Chlorofluorocarbon |
| CHP | Combined Heat and Power |
| CIEL | Center for International Environmental Law |
| DAC | Direct Air Capture |
| DECHEMA | Gesellschaft für Chemische Technik und Biotechnologie e. V. |
| dm | Dry Matter |
| EPR | Extended Producer Responsibility |
| EU | European Union |
| GFC | Global Forum on Chemicals |
| ICCM5 | International Conference on Chemicals Management (Fifth Meeting) |
| IEA | International Energy Agency |
| IED | Industrial Emissions Directive |
| IPBES | Intergovernmental Platform on Biodiversity and Ecosystem Services |
| IPCC | Intergovernmental Panel on Climate Change |
| IPEN | International Pollutants Elimination Network |
| ISC3 | International Sustainable Chemistry Collaborative Centre |
| JRC | Joint Research Centre |
| LCA | Lifecycle Analysis |
| LDPE | Low-Density Polyethylene |
| MTA | Methanol-to-Aromatics |
| MTO | Methanol-to-Olefines |
| NGO | Non-Governmental Organization |
| OECD | Organization for Economic Cooperation and Development |
| PCW | Post-Consumer Waste |
| PE | Polyethylene |
| PET | Polyethylene Terephthalate |
| PFAS | Per- and Polyfluorinated Alkyl Substances |
| PP | Polypropylene |
| PtX | Power-to-X |
| PVC | Polyvinyl Chloride |
| REACH | Registration, Evaluation, and Authorization of Chemicals |
| SAICM | Strategic Approach to International Chemicals Management |
| SDG | Sustainable Development Goal |
| SPP | Science Policy Panel |
| SRU | German Advisory Council on Environment |
| SSbD | Safe and Sustainable by Design |
| SVHC | Substance of Very High Concern |
| UBA | German Environment Agency |
| UNEA | United Nations Environment Assembly |
| UNEP | United Nations Environment Program |
| VOC | Volatile Organic Carbon |
| WHO | World Health Organization |
References
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S., III; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 6223. [Google Scholar] [CrossRef]
- Richardson, K.; Steffen, W.; Lucht, W.; Bendtsen, J.; Cornell, S.E.E.; Donges, J.F.; Druke, M.; Fetzer, I.; Bala, G.; von Bloh, W.; et al. Earth beyond six of nine planetary boundaries. Sci. Adv. 2023, 9, eadh2458. [Google Scholar] [CrossRef]
- Caesar, L.; Sakschewski, B.; Andersen, L.S.; Beringer, T.; Braun, J.; Dennis, D.; Gerten, D.; Heilemann, A.; Kaiser, J.; Kitzmann, N.H.; et al. Planetary Health Check Report 2024; Potsdam Institute for Climate Impact Research: Potsdam, Germany; Available online: https://www.planetaryhealthcheck.org/storyblok-cdn/f/301438/x/a4efc3f6d5/planetaryhealthcheck2024_report.pdf (accessed on 25 May 2025).
- Persson, L.; Carney Almroth, B.M.; Collins, C.D.; Cornell, S.; de Wit, C.A.; Diamond, M.L.; Fantke, P.; Hassellöv, M.; MacLeod, M.; Ryberg, M.W.; et al. Outside the Safe Operating Space of the Planetary Boundary for Novel Entities. Environ. Sci. Technol. 2022, 56, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Minovi, D. The Chemical Industry: An Overlooked Driver of Climate Change. Policy Paper #1 for the Louisville Charter. 2022. Available online: https://comingcleaninc.org/assets/media/images/Louisville%20Charter%20content/plank%201%20policy%20paper.pdf (accessed on 27 February 2025).
- Steinhäuser, K.G.; von Gleich, A.; Große Ophoff, M.; Körner, W. The Necessity of a Global Binding Framework for Sustainable Management of Chemicals and Materials–Interactions with Climate and Biodiversity. Sustain. Chem. 2022, 3, 205–237. [Google Scholar] [CrossRef]
- Andersen, I. The Triple Planetary Crisis: Forging a New Relationship Between People and the Earth. UNEP. 14 July 2020. Available online: https://www.unep.org/news-and-stories/speech/triple-planetary-crisis-forging-new-relationship-between-people-and-earth (accessed on 5 February 2025).
- UNEP. The Global Framework on Chemicals–For a Planet Free of Harm from Chemicals and Waste. Available online: https://www.unep.org/resources/global-framework-chemicals-planet-free-harm-chemicals-and-waste (accessed on 17 October 2024).
- UNEP. Global Chemicals Outlook II–From Legacies to Innovative Solutions: Implementing the 2030 Agenda for Sustainable Development–Synthesis Report (GCO II Synthesis Report); Châtelaine: Geneva, Switzerland, 2019; ISBN 978-92-807-3745-5. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/27651/GCOII_synth.pdf?sequence=1&isAllowed=y (accessed on 25 May 2025).
- World Health Organization (WHO). The Public Impact of Chemicals–Knowns and Unknowns, Data Addendum for 2019. Available online: https://iris.who.int/bitstream/handle/10665/342273/WHO-HEP-ECH-EHD-21.01-eng.pdf?sequence=1 (accessed on 5 February 2025).
- Kähler, F.; Porc, O.; Carus, M. RCI Carbon Flows Report: Compilation of Supply and Demand of Fossil and Renewable Carbon on a Global and European Level; Renewable Carbon Initiative, Ed.; Nova Institute: Hürth, Germany, 2023. [Google Scholar] [CrossRef]
- Wang, Z.; Walker, G.W.; Muir, D.C.G.; Yoshida, K.N. Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories. Environ. Sci. Technol. 2020, 54, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- CEFIC. The European Chemical Industry–A Vital Part of Europe’s Future–Facts and Figures; The European Chemical Industry Council: Brussels, Belgium, 2024; Available online: https://cefic.org/a-pillar-of-the-european-economy/facts-and-figures-of-the-european-chemical-industry/ (accessed on 25 May 2025).
- IEA (International Energy Agency). The Future of Petrochemicals–Towards More Sustainable Plastics and Fertilisers; IEA Publications: Paris, France,, 2018; Available online: https://iea.blob.core.windows.net/assets/bee4ef3a-8876-4566-98cf-7a130c013805/The_Future_of_Petrochemicals.pdf (accessed on 25 May 2025).
- Levi, P.G.; Cullen, J.M. Mapping Global Flows of Chemicals: From Fossil Fuel Feedstocks to Chemical Products. Environ. Sci. Technol. 2018, 52, 1725–1734. [Google Scholar] [CrossRef]
- Destatis. Energieverbrauch der Industrie 2023 um 7,8% geringer als im Vorjahr, Pressemitteilung Nr. 413 vom 4. November 2024. Available online: https://www.destatis.de/DE/Presse/Pressemitteilungen/2024/11/PD24_413_435.html (accessed on 5 February 2025).
- BUND (FoE Germany). Blackbox Chemieindustrie-Die Energieintensivste Industrie Deutschlands; BUND: Berlin, Germany, 2023; Available online: https://www.bund.net/fileadmin/user_upload_bund/publikationen/chemie/blackbox-chemieindustrie-die-energieintensivste-industrie-deutschlands-studie-bund.pdf (accessed on 25 May 2025).
- WWF. Deutschland: Dirty Dozen: Chemie–Emissionen der 12 Größten Chemieparks in Deutschland; WWF: Berlin, Germany, 2024; Available online: https://www.oeko.de/fileadmin/oekodoc/WWF_DirtyDozen_Chemie.pdf (accessed on 25 May 2025).
- Jiang, M.; Cao, Y.; Liu, C.; Chen, D.; Zhou, W.; Wen, Q.; Yu, H.; Jiang, J.; Ren, Y.; Hu, Y.; et al. Tracing fossil-based plastics, chemicals and fertilizers production in China. Nat. Commun. 2024, 15, 3854. [Google Scholar] [CrossRef]
- Statista Deutschlands Chemieindustrie. 2022. 2024. p. 51. Available online: https://de.statista.com/statistik/kategorien/kategorie/7/themen/59/branche/chemieindustrie/#overview (accessed on 26 November 2024).
- Bringezu, S.; Kümmerer, K. Nachhaltiges Ressourcen- und Stoffstrommanagement- Zwischen Gigatonnen und Mikrogramm. GAIA 2012, 21, 69–72. [Google Scholar] [CrossRef][Green Version]
- Exner, A.; Held, M.; Kümmerer, K. Kritische Metalle in der Großen Transformation; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-44838-0/978-3-662-44839-7. (eBook). [Google Scholar] [CrossRef]
- EU Regulation (EU) 2024/1252 of the European Parliament and of the Council of 11 April 2024 Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) No 168/2013, (EU) 2018/858, (EU) 2018/1724 and (EU) 2019/1020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L_202401252 (accessed on 25 May 2025).
- Zimmermann, T.; Gößling-Reisemann, S. Recycling Potentials of Critical Metals-Analyzing Secondary Flows from Selected Applications. Resources 2014, 3, 291–318. [Google Scholar] [CrossRef]
- Remenyi, C. Interview Henning Hopf “Das Stoffliche Problem ist ernst”, Nachrichten aus der Chemie, 2024, 72, Juli/August 2024, 8–11. Available online: https://www.pedocs.de/zeitschriften.php?la=en&zst=7133 (accessed on 25 May 2025).
- UNEP. Global Resources Outlook–Bend the Trend, Pathways to a Liveable Planet as Resource Use Spikes; International Resource Panel: Nairobi, Kenya, 2024; ISBN 978-92-807-4128-5. Available online: https://www.unep.org/resources/Global-Resource-Outlook-2024 (accessed on 25 May 2025).
- United Nations Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals (accessed on 5 February 2025).
- EU: Regulation (EC) No. 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Last Amended by Commission Regulation (EU) 2022/586 of 8 April 2022, Official Journal of the European Union of 11.04.2022, L 112, p. 6. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02006R1907-20221217&qid=1682101077968 (accessed on 25 May 2025).
- BUND (FoE Germany). Mikroschadstoff-Strategie, BUND-Standpunkt 11, BUND Berlin, Germany. 2017. Available online: https://www.bund.net/fileadmin/user_upload_bund/publikationen/fluesse/fluesse_mikroschadstoffe_standpunkt.pdf (accessed on 25 May 2025).
- German Environment Agency (UBA). Recommendations for Reducing Micropollutants in Waters, Background Paper, Dessau-Roßlau, Germany. 2018. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/1410/publikationen/180709_uba_pos_mikroverunreinigung_en_bf.pdf (accessed on 25 May 2025).
- EU: Directive 2012/18/EU of the European Parliament and of the Council of 4 July 2012 on the Control of Major-Accident Hazards Involving Dangerous Substances, Amending and Subsequently Repealing Council Directive 96/82/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012L0018 (accessed on 25 May 2025).
- European Commission. A European Green Deal—Striving to Be the First Climate-Neutral Continent. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 4 March 2025).
- European Commission. A Clean Industrial Deal for Competitiveness and Decarbonization in the EU. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_25_550 (accessed on 22 April 2025).
- European Commission. Pathway to a Healthy Planet for All–EU Action Plan: ‘Towards Zero Pollution for Air, Water and Soil’, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions of 12.05.2021, COM (2021) 400 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52021DC0400&qid=1682412866082 (accessed on 25 May 2025).
- European Commission. Chemicals Strategy for Sustainability–Towards a Toxic-Free Environment, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions of 14.10.2020, COM (2020) 667 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52020DC0667&qid=1682504977252 (accessed on 25 May 2025).
- OECD. Environmental Justice: Context, Challenges and National Approaches; OECD Publishing: Paris, France, 2024. [Google Scholar] [CrossRef]
- Zinyemba, C.; Archer, E.; Rother, H.-A. Climate Change Pesticides Health: Considering the Risks Opportunities of Adaptation for Zimbabwean Smallholder Cotton Growers. Int. J. Environ. Res. Public Health 2021, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, P.; Wiese, K. Pushed to the Wastelands–Environmental Racism Against Roma Communities in Central and Eastern Europe; European Environmental Bureau (EEB): Brussels, Belgium, 2020; Available online: https://eeb.org/wp-content/uploads/2020/04/Pushed-to-the-Wastelands.pdf (accessed on 25 May 2025).
- BUND (FoE Germany). Profits Without Borders–How Companies Disregard Environmental Protection and Human Rights All over the World; BUND: Berlin, Germany, 2021; Available online: https://www.bund.net/fileadmin/user_upload_bund/publikationen/chemie/chemie_Profite_ohne_Grenzen.pdf (accessed on 25 May 2025).
- Ituen, I.; Tatu Hey, L. The Elephant in the Room–Environmental Racism in Germany, Studies, Knowledge Gaps, and Their Relevance to Environmental and Climate Justice, Brief Study; Heinrich Böll Foundation, Ed.; Berlin, Germany, 2021; Available online: https://www.boell.de/sites/default/files/2021-12/E-Paper_The_Elephant_in_the_Room.pdf (accessed on 25 May 2025).
- Berlin Senate Department for the Environment; Urban Mobility, Consumer Protection and Climate Action (Eds.) Die Umweltgerechte Stadt–Umweltgerechtigkeitsatlas Aktualisierung; Senatsverwaltung für Mobilität, Verkehr, Klimaschutz und Umwelt: Berlin, Germany, 2021/2022; Available online: https://www.berlin.de/sen/uvk/umwelt/nachhaltigkeit/umweltgerechtigkeit/ (accessed on 5 February 2025).
- Sheats, N.; Baptista, A. Addressing Environmental Injustice Through the Adoption of Cumulative Impacts Policy, Policy paper #2 for the Louisville Charter. 2022. Available online: https://comingcleaninc.org/assets/media/images/Louisville%20Charter%20content/plank%202%20policy%20brief.pdf (accessed on 25 May 2025).
- Agora Industry Chemicals in Transition—The Three Pillars for Transforming Chemical Value Chains, Agora Industry, Berlin (Germany). 2023. Available online: https://www.agora-industry.org/fileadmin/Projekte/2022/2022-02_IND_Climate_Positive_Chemistry_DE/A-EW_300_Chemicals_in_transition_EN_WEB.pdf (accessed on 25 May 2025).
- The Louisville Charter for Safer Chemicals. 2021. Available online: https://comingcleaninc.org/assets/media/images/Louisville%20Charter%20content/Louisville%20Charter%202021.pdf (accessed on 26 February 2025).
- Thorpe, B. Transforming the Chemical Industry: Safer Substitutes and Solutions for a Non-Toxic Economy, Policy Paper #3 for the Louisville Charter. 2022. Available online: https://comingcleaninc.org/assets/media/images/Louisville%20Charter%20content/plank%203%20policy%20paper.pdf (accessed on 25 May 2025).
- Kanazawa, D.; Wagner, A.; Kremer, A.B.; Leung, J.L.; Lingeswaran, S.; Goult, P.; Herrmann, S.; Ishii, N.; Kikuchi, Y. Scope 1, 2, and 3 Net Zero Pathways for the Chemical Industry in Japan. J. Chem. Eng. Jpn. 2024, 57, 2360900. [Google Scholar] [CrossRef]
- Hata, S.; Nansai, K.; Shigetomi, Y.; Kito, M.; Nakajima, K. Material Efficiency and Circularity Goals to Achieve a Carbon-Neutral Society by 2050. Environ. Sci. Technol. 2025, 59, 6025–6036. [Google Scholar] [CrossRef] [PubMed]
- Kloo, Y.; Nilsson, L.J.; Palm, E. Reaching net-zero in the chemical industry—A study of roadmaps for industrial decarbonization. Renew. Sustain. Energy Transit. 2024, 6, 100075. [Google Scholar] [CrossRef]
- VCI; VDI. Chemistry for Climate–Wie Die Transformation der Chemie Gelingen Kann; Verband der Chemischen Industrie e.V., Verband Deutscher Ingenieure e.V., Eds.; Frankfurt: Main, Germany, 2023; Available online: https://www.vci.de/vci/downloads-vci/publikation/broschueren-und-faltblaetter/final-c4c-broschure-langfassung.pdf (accessed on 25 May 2025).
- BASF. Offshore Wind Farm Hollandse Kust Zuid Inaugurated, Joint News Release. 29 September 2023. Available online: https://www.basf.com/global/en/media/news-releases/2023/09/p-23-318 (accessed on 18 November 2024).
- European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions, REPowerEU Plan, COM(2022) 230 Final. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:fc930f14-d7ae-11ec-a95f-01aa75ed71a1.0001.02/DOC_1&format=PDF (accessed on 25 May 2025).
- Neubauer, U. Petrochemie unter Strom, Nachrichten aus der Chemie, 2024, 72, Juli/August 2024, 36–38. Available online: https://www.pedocs.de/zeitschriften.php?la=en&zst=7133 (accessed on 25 May 2025).
- VCI; VDI. Chemistry for Climate–Wie Die Transformation der Chemie Gelingen Kann-Ein Update Verband; der Chemischen Industrie e.V., Verband Deutscher Ingenieure e.V., Eds.; Frankfurt: Main, Germany, 2024; Available online: https://www.vci.de/vci/downloads-vci/publikation/broschueren-und-faltblaetter/2024-11-07-c4c-update-publikation-kurzfassung.pdf (accessed on 25 May 2025).
- European Commission. Transition Pathway for the Chemical Industry; EU Publications: Brussels, Belgium, 2020; ISBN 978-92-76-61690-0. [Google Scholar] [CrossRef]
- Engel, K.M. Hoffnungsträger der Zukunft. Spektrum Spez. 2024, 3, 66–73. [Google Scholar]
- ESMAP (Energy Sector Management Assistance Program); Organization for Economic Co-operation and Development (OECD); Global Infrastructure Facility and Hydrogen Council. Scaling Hydrogen Financing for Development. ESMAP Paper 2023, Washington, DC: World Bank. License: Creative Commons Attribution CC BY 3.0 IGO. Available online: https://www.esmap.org/sites/default/files/2022/2023/H4D/Full_Slide_deck_Scaling%20Hydrogen%20Financing%20for%20Development_November17.pdf (accessed on 25 May 2025).
- Machin, A.; Cotto, M.; Ducongé, J.; Márquez, F. Artificial photosynthesis: Current advancements and future prospects. Biomimetics 2023, 8, 298. [Google Scholar] [CrossRef]
- Landesagentur. KoalAplan–Die Kläranlage der Zukunft; Landesagentur für Umwelttechnik und Ressourceneffizienz Baden-Württemberg: Stuttgart, Germany, 2024; Available online: https://www.umwelttechnik-bw.de/sites/default/files/2024-09/20241001_KoalAplan_Abschlussbericht_web.pdf (accessed on 25 May 2025).
- Huo, J.; Wang, Z.; Oberschelp, C.; Guillén-Gosálbez, G.; Hellweg, S. A Net-zero transition of the global chemical industry with CO2-feedstock by 2050: Feasible yet challenging. Green Chem. 2023, 25, 415. [Google Scholar] [CrossRef]
- Sang, R.; Stein, C.A.M.; Schareina, T.; Hu, Y.; Léval, A.; Massa, J.; Turan, V.; Sponholz, P.; Wei, D.; Jackstell, R.; et al. Development of a practical formate/bicarbonate energy system. Nat. Commun. 2024, 15, 7268. [Google Scholar] [CrossRef]
- BMWK (Federal Ministry for Economic Affairs and Climate Action). Eckpunkte der Bundesregierung Für eine Carbon Management-Strategie. Berlin, Germany. 2024. Available online: https://www.bmwk.de/Redaktion/DE/Downloads/E/eckpunkte-der-bundesregierung-fuer-eine-carbon-management-strategie.pdf?__blob=publicationFile&v=2 (accessed on 25 May 2025).
- BMWK (Federal Ministry for Economic Affairs and Climate Action). Entwurf Eines Gesetzes zur Änderung des Kohlendioxid-Speicherungsgesetzes. Available online: https://www.bmwk.de/Redaktion/DE/Downloads/E/entwurf-eines-gesetzes-zur-aenderung-des-kohlendioxid-speicherungs-gesetzes.pdf?__blob=publicationFile&v=2 (accessed on 25 May 2025).
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, Towards an Ambitious Industrial Carbon Management for the EU, COM(2024) 62 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52024DC0062 (accessed on 25 May 2025).
- IPCC. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: New York, NY, USA, 2018; Available online: https://www.ipcc.ch/site/assets/uploads/2018/03/srccs_wholereport.pdf (accessed on 25 May 2025).
- German Environment Agency (UBA). Resource-Efficient Pathways towards Greenhouse Gas Neutrality (RESCUE)—How Germany Can Achieve Greenhouse Gas Neutrality by 2050 and Resulting Raw Materials Demand, Scientific Opinion Paper, 2020, Dessau-Roßlau, Germany. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/1410/publikationen/2020_sciopap_rescue_final_0.pdf (accessed on 25 May 2025).
- Nestler, R. Ein Endlager für Kohlenstoffdioxid. Spektrum Spez. 2024, 3, 50–55. [Google Scholar]
- Erlach, B.; Gierds, J.; Fischedick, M.; Matthies, E.; Pittel, K.; Sauer, D.U. CO2 als Rohstoff. Baustein einer klimaneutralen Kohlen-stoffwirtschaft (Impuls). Schriftenreihe Energiesysteme Der Zuk. (ESYS) 2024. [Google Scholar] [CrossRef]
- Wilts, H. Chemische Produktion als Kreislaufwirtschaft–Wege aus der Sackgasse der Linearität. In Politische Ökologie; oekom-Verlag: München, Germany, 2022; Volume 171, pp. 84–89. [Google Scholar]
- UNIDO. Chemical Leasing–The Performance Based Business Model for Sustainable Chemicals Management. 2021. Available online: https://www.unido.org/our-focus-safeguarding-environment-resource-efficient-and-low-carbon-industrial-production/chemical-leasing (accessed on 5 February 2025).
- German Council for Sustainable Development (Rat für Nachhaltige Entwicklung, RNE). Circular Economy: Leveraging a Sustainable Transformation, Statement of 05.10.2021, Berlin, Germany. Available online: https://www.nachhaltigkeitsrat.de/wp-content/uploads/2022/02/20211005_RNE-Statement_Circular-Economy-1.pdf (accessed on 25 May 2025).
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; Reprint edition of 25 May 2000; ISBN 978-0198506980. [Google Scholar]
- Keijer, T.; Bakker, V.; Slootweg, J.C. Circular chemistry to enable a circular economy. Nat. Chem. 2019, 11, 190–195. [Google Scholar] [CrossRef] [PubMed]
- UNEP. Enhancing Circular Economy as a Contribution to Achieving Sustainable Consumption and Production, Resolution 5/11 (UNEP/EA.5/Res.11), Adopted by the 5th United Nations Environmental Assembly on 2 March 2022, Nairobi, Kenya. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/39920/ENHANCING%20CIRCULAR%20ECONOMY%20AS%20A%20CONTRIBUTION%20TO%20ACHIEVING%20SUSTAINABLE%20CONSUMPTION%20AND%20PRODUCTION.%20English.pdf?sequence=1&isAllowed=y (accessed on 25 February 2025).
- European Commission. On Making Sustainable Products the Norm, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions of 30 March 2022, COM(2022) 140 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52022DC0140&qid=1682432763402 (accessed on 25 May 2025).
- Circle Economy Foundation. The Circularity Gap Report 2024, Amsterdam, The Netherlands. 2024. Available online: https://www.circularity-gap.world/2024 (accessed on 18 November 2024).
- Langsdorf, S. Ressourcenschonungspolitik in der EU-Eine Zusammenschau Politischer Strategiepapiere von den Anfängen bis Heute, Ecologic, Institut 2021, Berlin, Germany. Available online: https://www.ecologic.eu/sites/default/files/publication/2021/3554-Langsdorf-Ressourcenschonung-in-der-EU-Bericht.pdf (accessed on 25 May 2025).
- BMK (Federal Ministry for Climate Protection, Environment, Energy, Mobility, Innovation and Technology). Austria on the Path to a Sustainable and Circular Society–The Austrian Circular Economy Strategy, Vienna, Austria. 2022. Available online: https://www.bmk.gv.at/dam/jcr:427f6f36-1d5a-4ef2-bc84-52a1792ad3db/Austrian_CES.pdf (accessed on 25 May 2025).
- BMUV (Federal Ministry for the Environment, Nature Conservation, Nuclear Safety and Consumer Protection). Nationale Kreislaufwirtschaftsstrategie–Entwurf. Berlin, Germany. 17 June 2024. Available online: https://www.bmuv.de/fileadmin/Daten_BMU/Download_PDF/Abfallwirtschaft/nkws_entwurf_bf.pdf (accessed on 25 May 2025).
- BUND (FoE Germany). Resource Protection Means Drastic Reduction of Resource Consumption, BUNDposition 74, Berlin, Germany. 2023. Available online: https://www.bund.net/fileadmin/user_upload_bund/publikationen/ressourcen_und_technik/resource-protection-means-drastic-reduction-of-resource-consumption-position-paper-bund.pdf (accessed on 25 May 2025).
- Friege, H. Chancen und Grenzen der “Circular Economy”: Erkenntnisse aus der BMBF-Fördermaßnahme ReziProK. Müll Und Abfall 2022, 54, 606–619. [Google Scholar] [CrossRef]
- Friege, H.; Kümmerer, K. Practising circular economy: Stumbling blocks for circulation and recycling. In The Impossibilities of the Circular Economy—Separating Aspirations from Reality; Lehmann, H., Hinske, C., de Margerie, V., Nikolova, A.S., Eds.; Routledge: London, UK; New York, NY, USA, 2022; pp. 259–271. ISBN 978-1-032-15443-5 (hbk). [Google Scholar] [CrossRef]
- Lehmann, H.; Hinske, C.; de Margerie, V.; Slaveikova Nikolova, A. (Eds.) The Impossibilities of the Circular Economy—Separating Aspirations from Reality; Routledge: Abingdon, UK, 2023; ISBN 978-1-032-15443-5 (hbk)/978-1-032-15446-6 (pbk)/978-1-003-24419-6 (ebk). [Google Scholar] [CrossRef]
- Friege, H.; Kummer, B.; Steinhäuser, K.G.; Wuttke, J.; Zeschmar-Lahl, B. How should we deal with the interfaces between chemicals, product and waste legislation? Environ. Sci. Eur. 2019, 31, 51. [Google Scholar] [CrossRef]
- Bunge, R. Recycling Ist Gut, Mehr Recycling Ist Besser–Oder Nicht? Proceedings Zur Berliner Rohstoff-Und Recyclingkonferenz, 79–91. 2016. Available online: https://scholar.google.de/scholar?hl=de&as_sdt=0%2C5&q=Rainer+bunge+recycling+ist+gut&btnG= (accessed on 25 May 2025).
- Wang, Z.; Hellweg, S. First Steps Toward Sustainable Circular Uses of Chemicals: Advancing the Assessment and Management Paradigm. ACS Sustain. Chem. Eng. 2021, 9, 6939–6951. [Google Scholar] [CrossRef]
- Kümmerer, K.; Clark, H.J.; Zuin, V.G. Rethinking chemistry for a circular economy—Chemical complexity complicates product recycling and manufacturing sustainability. Science 2020, 367, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Börjeson, N.; Ågerstrand, M. The Problems that we have Today, are Yesterday’s Solutions: Enabling Circular Non-toxic Supply Chains. Circ. Econ. Sustain. 2025. [Google Scholar] [CrossRef]
- Gößling-Reisemann, S.; von Gleich, A. Ressourcen Kreislaufwirtschaft und Entropie am Beispiel der Metalle. In Müll-Handbuch–Sammlung und Transport, Behandlung und Ablagerung Sowie Vermeidung und Verwertung von Abfällen; Hösel, G., Bilitewski, B., Schenkel, W., Schnurer, H., Zeschmar-Lahl, B., Eds.; Erich Schmidt Verlag: Berlin, Germany, 2009; pp. 1–27. [Google Scholar]
- European Commission. A European Strategy for Plastics in a Circular Economy, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions of 16.01.2018, COM(2018) 28 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52018DC0028&qid=1682503065040 (accessed on 25 May 2025).
- The Pew Charitable Trusts; SYSTEMIQ (Eds.) Trusts Breaking the Plastic Wave–A Comprehensive Assessment of Pathways towards Stopping Ocean Plastic Pollution; 2020; Available online: https://www.systemiq.earth/wp-content/uploads/2020/07/BreakingThePlasticWave_MainReport.pdf (accessed on 25 May 2025).
- German Environment Agency (UBA). Kunststoffabfälle. Available online: https://www.umweltbundesamt.de/daten/ressourcen-abfall/verwertung-entsorgung-ausgewaehlter-abfallarten/kunststoffabfaelle#unterschiede-bei-der-stofflichen-verwertung (accessed on 24 October 2024).
- Betz, J.; Bachmann, H.; Huber, I.; Schleicher, T.; Achilles, L.-S. Kunststoffabfälle aus Deutschland: Handlungsempfehlungen Zu einer Umweltgerechten Behandlung Im In- und Ausland, Texte 47/2024, Umweltbundesamt Dessau Roßlau, Germany. 2024. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/11850/publikationen/47_2024_texte_kunststoffabfall.pdf (accessed on 25 May 2025).
- Ellen McArthur Foundation. The New Plastics Economy–Rethinking the Future of Plastics. 2016. Available online: https://www.ellenmacarthurfoundation.org/the-new-plastics-economy-rethinking-the-future-of-plastics (accessed on 25 May 2025).
- Bahr, L.; Katzy, V.; Scholz, O. Abschlussbericht DBU-AZ37088/01, “Prototypanlage zur Felderprobung des Hochdynamischen Quantifizierten Metallrecyclings (ready2sort)”. 2023. Available online: https://www.dbu.de/projektdatenbank/37088-01/ (accessed on 5 February 2025).
- Krieg, G. UNISENSOR Sensorsysteme GmbH, Food to Food Recycling von PET mittels Prozess Laser Fluoreszenz und Prozess Raman Spektroskopie, Abschlussbericht über ein Entwicklungsprojekt, Eefördert unter dem Az: 25507 von der Deutschen Bundesstiftung Umwelt. 2009. Available online: https://www.dbu.de/projektdatenbank/25507-01/ (accessed on 25 May 2025).
- Spilok, K. Kunststoffrecycling nach dem Aschenputtelprinzip, VDI-Nachrichten. 2015. Available online: https://www.vdi-nachrichten.com/technik/umwelt/kunststofftrennung-nach-dem-aschenputtelprinzip/ (accessed on 5 February 2025).
- Unisensor Sensorsysteme GmbH: UNISPECTRE 400, Hocheffizientes refPET Sniffer-System zum Aufspüren von Fremdstoffen und Verunreinigungen. 2023. Available online: https://www.unisensor.de/produkte/product-details/getraenkeindustrie/unispectre-400.html (accessed on 5 February 2025).
- Ciupek, M. Hightech-Sortieranlage Identifiziert Metall blitzschnell per Laser, VDI-Nachrichten. 2023. Available online: https://www.vdi-nachrichten.com/technik/umwelt/hightech-sortieranlage-identifiziert-metall-blitzschnell-per-laser/ (accessed on 5 February 2025).
- EU Regulation (EU) 2024/1781 of the European Parliament and of the Council of 13 June 2024 Establishing a Framework for the Setting of Ecodesign Requirements for Sustainable Products, Amending Directive (EU) 2020/1828 and Regulation (EU) 2023/1542 and Repealing Directive 2009/125/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L_202401781 (accessed on 25 May 2025).
- Vohlídal, J. Polymer degradation: A short review. Chem. Teach. Int. 2021, 3, 213–220. [Google Scholar] [CrossRef]
- Eunomia. Chemical Recycling: State of Play–Report for ChemTrust. 2020. Available online: https://chemtrust.org/wp-content/uploads/Chemical-Recycling-Eunomia.pdf (accessed on 25 May 2025).
- Puig-Arnavat, M.; Hindsgaul, C.; Kamuk, B. Means to Improve Circularity of Plastic Waste. In Proceedings of the ISWA World Congress, Athens, Greece, 4–7 October 2021; Available online: https://www.researchgate.net/publication/355209694_Means_to_improve_circularity_of_plastic_waste (accessed on 25 May 2025).
- NABU. Chemisches Recycling von Kunststoffen–Potenziale, Risiken und viele offene Fragen, Chemisches Recycling von Kunststoffen-NABU Chemisches Recycling von Kunststoffen—NABU. Available online: https://www.nabu.de/umwelt-und-ressourcen/abfall-und-recycling/recycling/27543.html (accessed on 12 September 2024).
- Rizos, V.; Urban, P.; Righetti, E.; Kassab, A. Chemical Recycling of Plastics–Technologies, Trends and Policy Implications. CEPS Brussels, Belgium. 2023. Available online: https://cdn.ceps.eu/wp-content/uploads/2023/07/CEPS-In-depth-analysis-2023-11_Chemical-recycling-of-plastics.pdf (accessed on 25 May 2025).
- BASF. Factsheet ChemCycling. Available online: https://www.basf.com/global/de/who-we-are/sustainability/we-drive-sustainable-solutions/circular-economy/mass-balance-approach/chemcycling (accessed on 5 February 2025).
- Altnau, G.; Hamann, J.; Dias Fonseca, J.M. CreaSolv- Process—Material Recycling in a Circular Economy for Plastics—A Critical Assessment. 2024. Available online: https://www.creasolv.de/images/2021.04.15_CreaSolv_Positionspapier_EN.pdf (accessed on 25 May 2025).
- LyondellBasell (2024): Circulen Brochure—Everyday Circularity. Available online: https://cdn.brandfolder.io/EZH48IU3/at/bsv7wh4f3k9s47bx8qxwq4ts/7187_2024_Jan_CLCS_CirculenBrochure_Update_LH.pdf (accessed on 26 February 2025).
- SOLVAY: VINYLOOP—A New Process to Regenerate PVC Compounds from Composite Residues. 2001. Available online: https://p2infohouse.org/ref/38/37438.pdf (accessed on 16 September 2024).
- VINYLOOP: Closure of Operation in Italy—Phthalates Issue Under REACH Brings Down European PVC Recycling Project. Published on 04.07.2018. Available online: https://www.plasteurope.com/news/Closure_of_operation_in_Italy_Phthalates_issue_under_REACH_brings_do_t240095/ (accessed on 16 September 2024).
- Groß, M. Enzyme gegen die Plastikflut, Nachrichten aus der Chemie, 2024, 72, März 2024, 78–79. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/nadc.20244141920 (accessed on 25 May 2025).
- Payne, J.M.; Kamran, M.; Davidson, M.G.; Jones, M.D. Versatile Chemical Recycling Strategies: Value-Added Chemicals from Polyester and Polycarbonate Waste. ChemSusChem 2022, 15, e202200255. [Google Scholar] [CrossRef]
- Kusenberg, M.; Eschenbacher, A.; Delva, L.; de Meester, S.; Delikonstantis, E.; Stefanidis, G.D.; Ragaert, K.; van Geem, K.M. Towards high-quality petrochemical feedstocks from mixed plastic packaging waste via advanced recycling: The past, present and future. Fuel Process. Technol. 2022, 238, 107474. [Google Scholar] [CrossRef]
- Kusenberg, M.; Eschenbacher, A.; Djokic, M.R.; Zayoud, A.; Ragaert, K.; de Meester, S.; van Geem, K.M. Opportunities and challenges for the application of post-consumer plastic waste pyrolysis oils as steam cracker feedstocks: To decontaminate or not to decontaminate? Waste Manag. 2022, 138, 83–115. [Google Scholar] [CrossRef]
- Kusenberg, M.; Roosen, M.; Doktor, A.; Casado, L.; Abdulrahman, A.J.; Parvizi, B.; Eschenbacher, A.; Biadi ELaudou, N.; Jänsch, D.; de Meester, S.; et al. Contaminant removal from plastic waste pyrolysis oil via depth filtration and the impact on chemical recycling: A simple solution with significant impact. Chem. Eng. 2023, 473, 145259. [Google Scholar] [CrossRef]
- Öko-Institut: Climate Impact of Pyrolysis of Waste Plastic Packaging in Comparison with Reuse and Mechanical Recycling, 2022, Darmstadt, Germany. Available online: https://zerowasteeurope.eu/wp-content/uploads/2022/09/zwe_2022_report_climat_impact__pyrolysis_plastic_packaging.pdf (accessed on 25 May 2025).
- Garcia-Gutierrez, P.; Amadei, A.M.; Klenert, D.; Nessi, S.; Tonini, D.; Tosches, D.; Ardente, F.; Saveyn, H. Environmental and Economic Assessment of Plastic Waste Recycling, EUR 31423 EN; Publications Office of the European Union: Luxembourg, 2023; ISBN 978-92-76-99528-9. JRC132067; Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC132067 (accessed on 25 May 2025). [CrossRef]
- Klotz, M.; Oberschelp, C.; Salah, C.; Subal, C.; Hellweg, S. The role of chemical and solvent-based recycling within a sustainable circular economy for plastics. Sci. Total Environ. 2024, 906, 167586. [Google Scholar] [CrossRef] [PubMed]
- Asueta, A.; Arnaiz, S.; Miguel-Fernández, R.; Leivar, J.; Amundarain, I.; Aramburu, B.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Viability of Glycolysis for the Chemical Recycling of Highly Coloured and Multi-Layered Actual PET Wastes. Polymers 2023, 15, 4196. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.H.; Amin, M.; Iqbal, A.; Nadeem, I.; Kalin, M.; Soomar, A.M.; Galal, A.M. A review on gasification and pyrolysis of waste plastics. Front. Chem. 2023, 10, 960894. [Google Scholar] [CrossRef]
- Saebea, D.; Ruengrit, P.; Arpornwichanop, A.; Patcharavorachot, Y. Gasification of plastic waste for synthesis gas production. Energy Rep. 2020, 6, 202–207. [Google Scholar] [CrossRef]
- Biessey, P.; Vogel, J.; Seitz, M.; Quicker, P. Plastic Waste Utilization via Chemical Recycling: Approaches, Limitations, and the Challenges Ahead. Chem. Ing. Tech. 2023, 95, 1199–1214. [Google Scholar] [CrossRef]
- Quicker, P.; Seitz, M. Abschätzung der Potenziale und Bewertung der Techniken des thermochemischen Kunststoffrecyclings, 2024, Texte 154/2024, Umweltbundesamt Dessau-Roßlau Germany. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/11850/publikationen/154_2024_texte_thermochemisches_kunststoffrecycling.pdf (accessed on 25 May 2025).
- ZfK (Zeitung Für kommunale Wirtschaft). Kunststoff: Innovative Recyclingmethode vor dem Durchbruch? 9 April 2021. Available online: https://www.zfk.de/entsorgung/abfallwirtschaft/kunststoff-innovative-recyclingmethode-vor-dem-durchbruch (accessed on 5 February 2025).
- Mura Technologies. The Next Generation of Advanced Plastic Recycling; Mura Technology Ltd.: London, UK, 2024; Available online: https://muratechnology.com/app/uploads/2024/04/Mura-Technology-2024-Brochure-English.pdf (accessed on 17 November 2024).
- Matera, E. Chemisches Recycling: Fortschritt oder Greenwashing? RiffReporter. 30 November 2022. Available online: https://www.riffreporter.de/de/umwelt/plastik-kunststoffe-recycling-chemie-dow-mura-kreislaufwirtschaft (accessed on 13 September 2024).
- Laredo, G.C.; Reza, J.; Meneses Ruiz, E. Hydrothermal liquefaction processes for plastics recycling: A review. Clean. Chem. Eng. 2023, 5, 100094. [Google Scholar] [CrossRef]
- Neubauer, U. Chemisches Recycling ist keine neue Idee, Interview mit Kerstin Kuchta. Nachrichten aus der Chemie 2023, 71, 32–33. Available online: https://www.pedocs.de/zeitschriften.php?la=en&zst=7133 (accessed on 25 May 2025). [CrossRef]
- Reuters. The Recycling Myth—Big Oil’s Solution for Plastic Waste Littered with Failure, Joe Brock, Valerie Volkovici, John Geddie. 29 July 2021. Available online: https://www.reuters.com/investigates/special-report/environment-plastic-oil-recycling/ (accessed on 12 September 2024).
- Rollinson, A. Leaky Loop “Recycling”—A Technical Correction on the Quality of Pyrolysis Oil Made from Plastic Waste, Zero Waste Europe. 2023. Available online: https://zerowasteeurope.eu/wp-content/uploads/2023/10/Leaky-Loop-Recycling_-A-Technical-Correction-on-the-Quality-of-Pyrolysis-Oil-made-from-Plastic-Waste-.docx.pdf (accessed on 25 May 2025).
- THINKTANK Industrielle Ressourcenstrategien. Unternehmerforum Chemisches Recycling (UFCR) Handlungsfelder der Politik Für Die Rohstoffwende und Die Transformation Zu Einer Zirkulären Wirtschaft Mittels Chemischen Recyclings in Deutschland. 2022. Available online: https://www.thinktank-irs.de/wp-content/uploads/2023/07/RZ_THINKTANK_Broschuere_Unternehmerforum-Chemisches-Recycling_DE_Web.pdf (accessed on 10 October 2024).
- Saputra Lase, I.; Tonini, D.; Caro, D.; Albizzati, P.F.; Cristóbal, J.; Roosen, M.; Kusenberg, M.; Ragaert, K.; van Geem, K.M.; Dewulf, J.; et al. How much can chemical recycling contribute to plastic waste recycling in Europe? An assessment using material flow analysis modeling. Resour. Conserv. Recycl. 2023, 192, 106916. [Google Scholar] [CrossRef]
- PlasticsEurope Deutschland e.V. KreislaufwirtschaftPLUS: Handlungsempfehlungen Für Eine Nationale Kreislaufwirtschaftsstrategie. 2022. Available online: https://plasticseurope.org/de/wp-content/uploads/sites/3/2022/11/2022-10-13-Handlungsempfehlungen-Nationale-Kreislaufwirtschaftsstrategie.pdf (accessed on 25 May 2025).
- Soeteman-Hernández, L.; Cabrera, G.; Huegun, A.; Outón, P.R.; Artous, S.; Desrousseaux, S.; Staal, Y.; Cazzagon, V.; Delpivo, C.; Ganszky, D.; et al. Safe, sustainable and recyclable by design (SSRbD): A qualitative integrated approach applied to polymeric materials early in the innovation process. Sustain. Circ. NOW 2025, 2, a25547325. [Google Scholar] [CrossRef]
- Service, R.F. Can the World Make the Chemicals it Needs Without Oil?-with Solar and Wind Booming, the Chemical Industry Dabbles with Forgoing Petroleum as Its Source. Sci. Advis. 2019. [Google Scholar] [CrossRef]
- Erkmen, B.; Ozdogan, A.; Ezdesir, A.; Celik, G. Can Pyrolysis Oil Be Used as a Feedstock to Close the Gap in the Circular Economy of Polyolefins? Polymers 2023, 15, 859. [Google Scholar] [CrossRef] [PubMed]
- Eunomia: Feedstock Quality Guidelines for Pyrolysis of Plastic Waste–Report for the Alliance to End Plastic Waste. 2022. Available online: https://eunomia.eco/reports/feedstock-quality-guidelines-for-pyrolysis-of-plastic-waste/ (accessed on 25 May 2025).
- Kusenberg, M.; Zayoud, A.; Roosen, M.; Dao Thi, H.; Abbas-Abadi, M.S.; Eschenbacher, A.; Kresovic, U.; de Meester, S.; van Geem, K.M. A comprehensive experimental investigation of plastic waste pyrolysis oil quality and its dependence on the plastic waste composition. Fuel Process. Technol. 2022, 227, 107090. [Google Scholar] [CrossRef]
- BioÖkonomieRat: Bioökonomie Gemeinsam Eine Nachhaltige Zukunft Gestalten 1 Arbeitspapier Des, III. Bioökonomierats, Berlin, Germany. 2022. Available online: https://www.biooekonomierat.de/media/pdf/arbeitspapiere/1._Arbeitspapier_des_BOER_-_Gemeinsam_eine_nachhaltige_Zukunft_gestalten.pdf?m=1657008309& (accessed on 25 May 2025).
- The Royal Society. Catalysing Change: Defossilising the Chemical Industry—Policy Briefing; The Royal Society: London, UK, 2024; ISBN 978-1-78252-705-3. Available online: https://royalsociety.org/-/media/policy/projects/defossilising-chemicals/defossilising-chemical-industry-report.pdf (accessed on 25 May 2025).
- Ehrensberger, C. Weniger Ethen, mehr Milchsäure. Nachrichten Aus Der Chemie 2022, 70, 40–43. [Google Scholar] [CrossRef]
- Kamm, B.; Kamm, M.; Schmidt, M.; Hirth, T.; Schulze, M. Lignocellulose-based Chemical Products and Product Family Trees. In Biorefineries—Industrial Processes and Products. Status quo and Future Directions; Kamm, B., Gruber, P.R., Kamm, M., Eds.; Wiley-VCH: Weinheim, Germany, 2006; pp. 67–84. ISBN 3-527-31027-4. [Google Scholar]
- Wong, S.S.; Shu, R.; Zhang, J.; Liu, H.; Yan, N. Downstream processing of lignin derived feedstock into end products. Chem. Soc. Rev. 2020, 15, 5510–5560. Available online: https://pubs.rsc.org/en/content/articlelanding/2020/cs/d0cs00134a (accessed on 25 May 2025). [CrossRef]
- Piotrowski, S.; Essel, R.; Carus, M.; Dammer, L.; Engel, L. Nachhaltig nutzbare Potenziale für Biokraftstoffe in Nutzungskonkurrenz zur Lebens- und Futtermittelproduktion, Bioenergie sowie zur Stofflichen Nutzung in Deutschland, Europa und der Welt, 2015, Nova-Institut GmbH, Hürth, Germany. Available online: https://renewable-carbon.eu/publications/download-confirmation-page/?somdn_rrpage=somdn_rrpage&somdn_rrtdid=298550&somdn_rrdkey=Mjk4NTUw&somdn_rrskey=MTczODc1NTQ3OQ=&somdn_rrpkey=MTk3NA=&somdn_rrukey=MA=&somdn_rrtype=cmVkaXJlY3Q (accessed on 17 November 2024).
- Spangenberg, J.H.; Kuhlmann, W. Bioökonomie im Lichte der Planetaren Grenzen und des Schutzes der Biologischen Vielfalt, Study for BUND and Denkhaus Bremen, 2020, Cologne, Bremen, Germany. Available online: https://www.bund.net/fileadmin/user_upload_bund/publikationen/ressourcen_und_technik/ressourcen_technik_biooekonomie_projekt_studie_spangenberg.pdf (accessed on 25 May 2025).
- Wydra, S.; Reyhani, M.N.; Hüsing, B.; Schwarz, A. Exploring Innovative Technology Fields for a Circular Bio-Based Economy. Fraunhofer-Institut für System- und Innovationsforschung ISI, 2023, Karlsruhe, Germany. Available online: https://publica-rest.fraunhofer.de/server/api/core/bitstreams/159d78f0-af08-443c-8a36-39b8c25f59b2/content (accessed on 25 May 2025).
- Institut für Ökologische Wirtschaftsforschung (IÖW); Bund für Umwelt und Naturschutz Deutschland (BUND) (Eds.) Wie Nachhaltig ist die Bioökonomie Wirklich? Anregungen für einen Perspektivwechsel—Damit eine Sozial und Ökologisch Gerechte Wirtschaftsweise Gelingen kann; Berlin, Germany, 2020; Available online: https://www.bund.net/fileadmin/user_upload_bund/publikationen/ressourcen_und_technik/ressourcen_technik_biooekonomie_broschuere.pdf (accessed on 25 May 2025).
- German Environment Agency (UBA): Sustainable Use of Global Land and Biomass Resources, UBA-Position, 2013, Dessau Roßlau, Germany. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/419/publikationen/130617_englisch_lang_web.pdf (accessed on 25 May 2025).
- Searchinger, T.; James, O.; Dumas, P.; Kastner, T.; Wirsenius, S. Klimaschutz auf Kosten der Natur. Spektrum Spez. 2024, 3, 74–80. [Google Scholar]
- Priefer, C.; Jörissen, J.; Frör, O. Pathways to Shape the Bioeconomy. Resources 2017, 6, 10. [Google Scholar] [CrossRef]
- Greenhill, L.; Sundnes, F.; Karlsson, M. Towards sustainable management of kelp forests: An analysis of adaptive governance in developing regimes for wild kelp harvesting in Scotland and Norway. Ocean. Coast. Manag. 2021, 212, 105816. [Google Scholar] [CrossRef]
- UNEP: Into the Blue: Securing a Sustainable Future for Kelp Forests. 2023. Available online: https://www.researchgate.net/publication/371133448 (accessed on 17 October 2024).
- Rosental, M.; Fröhlich, T.; Liebich, A.; Deprie, T.; Hartleif, K.; Schock, M. Prozessintegrierte Maßnahmen und Alternative Produktionsverfahren Für Eine Umweltschonendere Herstellung von Chemikalien; Umweltbundesamt, Ed.; Texte 140/2024; Dessau-Roßlau, Germany, 2024; Available online: https://www.umweltbundesamt.de/sites/default/files/medien/11850/publikationen/140_2024_texte_upchem.pdf (accessed on 25 May 2025).
- Fachagentur Nachwachsende Rohstoffe (FNR). Biomassepotenziale von Rest-Und Abfallstoffen: Status Quo in Deutschland. Fachagentur Nachwachsende Rohstoffe. Gülzow-Prüzen, Germany. 2015. Available online: https://mediathek.fnr.de/downloadable/download/sample/sample_id/1251/ (accessed on 25 May 2025).
- Brödner, R.; Cyffka, K.-F.; Fais, A.; Günther, S.; Kalcher, J.; Kazmin, S.; Naegeli de Torres, F.; Radtke, K.S.; Selig, M.; Sittaro, F.; et al. Biomassepotenziale aus Abfällen und Reststoffen-Hintergrundpapier Stand und Perspektiven der DBFZ Ressourcendatenbank und der Aktuellen Datenlage (12/2023), DBFZ, Leipzig, Germany. 2023. Available online: https://www.dbfz.de/fileadmin/user_upload/Referenzen/Statements/StatusQuo_Abfall_und_Reststoffpotenziale.pdf (accessed on 25 May 2025).
- Erb, T.J. Photosynthesis 2.0: Realizing New-to-Nature CO2-Fixation to Overcome the Limits of Natural Metabolism, Cold Spring Harbor Perspectives in Biology, 2023, a041669. Available online: https://cshperspectives.cshlp.org/content/16/2/a041669 (accessed on 25 May 2025).
- Bierbaumer, S.; Winkler, C.K.; Tinzl, M.; Glueck, S.M. Enzymatic Conversion of CO2: From Natural to Artificial Utilization. Chem. Rev. 2023, 123, 5702–5754. [Google Scholar] [CrossRef]
- Behr, A.; Neuberg, S. Katalytische Kohlendioxid-Chemie, Aktuelle Wochenschau, Hrsg. GDCh. 2008. Available online: https://archiv.aktuelle-wochenschau.de/2008/woche20/woche20.html (accessed on 25 May 2025).
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. Available online: https://pubmed.ncbi.nlm.nih.gov/29220170/ (accessed on 25 May 2025). [CrossRef]
- Weidlich, T.; Kamenická, B. Utilization of CO2-Available Organocatalysts for Reactions with Industrially Important Epoxides. Catalysts 2022, 12, 298. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, J.; Wang, Y.; Li, J.; Wang, N.; Harding, J.; Mo, S.; Chen, L.; Chen, P.; Fu, M.; et al. Plasma-Catalytic CO2 Hydrogenation over a Pd/ZnO Catalyst: In Situ Probing of Gas-Phase and Surface Reactions. JACS Au 2022, 2, 1800–1810. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Y.; Guo, H.; Tu, X. Atmospheric Pressure and Room Temperature Synthesis of Methanol through Plasma-Catalytic Hydrogenation of CO2. ACS Catal. 2018, 8, 90–100. [Google Scholar] [CrossRef]
- Koch, C. Methanol und Ethylen aus Kohlendioxid, Nachrichten aus der Chemie, 2023, Vol. 71, pp. 33–34. Available online: https://gdch.app/article/methanol-und-ethylen-aus-kohlendioxid-4132285 (accessed on 25 May 2025).
- Gao, J.; Jia, C.; Liu, B. Direct and selective hydrogenation of CO2 to ethylene and propene by bifunctional catalysts. Catal. Sci. Technol. 2017, 7, 5602–5607. [Google Scholar] [CrossRef]
- Ni, Y.; Chen, Z.; Fu, Y.; Liu, Y.; Zhu, W.; Liu, Z. Selective conversion of CO2 and H2 into aromatics. Nat. Commun. 2018, 9, 3457. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.; Keiner, D.; Fasihi, M.; Koiranen, T.; Breyer, C. From fossil to green chemicals: Sustainable pathways and new carbon feedstocks for the global chemical industry. Energy Environ. Sci. 2023, 16, 2879. [Google Scholar] [CrossRef]
- IEA (International Energy Agency). Net Zero Roadmap: A Global Pathway to Keep the 1.5 °C Goal in Reach-2023 Update, 2023, IEA Publications Paris France. Available online: https://iea.blob.core.windows.net/assets/8ad619b9-17aa-473d-8a2f-4b90846f5c19/NetZeroRoadmap_AGlobalPathwaytoKeepthe1.5CGoalinReach-2böll023Update.pdf (accessed on 25 May 2025).
- Viebahn, P.; Scholz, A.; Zelt, O. Entwicklungsstand und Forschungsbedarf von Direct Air Capture—Ergebnis einer multidimensionalen Analyse. Energ. Tagesfr. 2019, 69, 30–33. [Google Scholar]
- Heinrich Böll-Stiftung. Direct Air Capture (DAC), Geoengineering Monitor, Heinrich Böll Foundation Berlin, Germany. 2021. Available online: https://www.boell.de/sites/default/files/2021-01/GM_DAC_de.pdf (accessed on 25 May 2025).
- IEA (International Energy Agency). Direct Air Capture—Tracking Report November 2021; IEA Publications: Paris, France, 2021; Available online: https://iea.blob.core.windows.net/assets/9766b4da-a5e3-4d76-874d-ea286e333956/DirectAirCapture_Akeytechnologyfornetzero.pdf (accessed on 25 May 2025).
- Meys, R.; Kätelhön, A.; Bachmann, M.; Winter, B.; Zibunas, C.; Suh, S.; Bardow, A. Achieving net-zero greenhouse gas emission plastics by a circular carbon economy. Science 2021, 374, 71–76. [Google Scholar] [CrossRef]
- Krämer, D. Verfahren zur Klimaneutralen Bereitstellung und Verarbeitung von Kohlenstoff, Vortrag beim Symposium “CO2 als Rohstoff in einer Klimaneutralen Kohlenstoffwirtschaft”, Lecture Held in an Online Workshop 17 December 2024, DECHEMA Frankfurt am Main, Germany. Available online: https://energiesysteme-zukunft.de/fileadmin/user_upload/Veranstaltungen/Ergebnispraesentationen/DECHEMA_CCU_Kohlenstofffluesse.pdf (accessed on 25 May 2025).
- EU Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on Industrial Emissions (Integrated Pollution Prevention and Control) (Recast), Last Amended by Corrigendum in the Official Journal of the European Union of 19.06.2012, L 158, p. 25. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02010L0075-20110106&qid=1682338299491 (accessed on 25 May 2025).
- Anastas, P.T.; Zimmerman, J.B. Through the twelve principles of green engineering. Environ. Sci. Technol. 2003, 37, 95A–101A. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor at 30: A passion for pollution prevention. Green Chem. 2023, 25, 1704–1728. [Google Scholar] [CrossRef]
- German Environment Agency (UBA). Sustainable Chemistry—Positions and Criteria of the Federal Environment Agency, Background Paper, Dessau-Roßlau, Germany. 2009. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/publikation/long/3798.pdf (accessed on 25 May 2025).
- Gomollón Bel, F. Ten chemical innovations that will change our world–IUPAC identifies emerging technologies in chemistry with potential to make our world more sustainable. Chem. Int. 2019, 41, 12–17. [Google Scholar] [CrossRef]
- Brennecke, J.F.; Maginn, E.J. Ionic Liquids: Innovative Fluids for Chemical Processing. AIChE J. 2001, 47, 2384–2389. [Google Scholar] [CrossRef]
- Ranke, J.; Mölter, K.; Stock, F.; Bottin-Weber, U.; Poczobutt, J.; Hoffmann, J.; Ondruschka, B.; Filser, J.; Jastorff, B. Biological effects of imidazolium ionic liquids with varying chain lengths in acute Vibrio fischeri and WST-1 cell viability assays. Ecotoxicol. Environ. Saf. 2004, 58, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Suk, M.; Kümmerer, K. Towards greener and sustainable ionic liquids using naturally occurring and nature-inspired pyridinium structures. Green Chem. 2023, 25, 365. [Google Scholar] [CrossRef]
- DECHEMA. Mikroalgen-Biotechnologie—Gegenwärtiger Stand, Herausforderungen, Ziele, DECHEMA 2016; Frankfurt am Main, Germany. Available online: https://dechema.de/dechema_media/Downloads/Positionspapiere/PP_Algenbio_2016_ezl.p016_ezl.pdf (accessed on 25 May 2025).
- Onen Cinar, S.; Kai Chong, Z.; Kucuker, M.A.; Wieczorek, N.; Cengiz, U.; Kuchta, K. Bioplastic Production from Microalgae: A Review. Int. J. Environ. Res. Public Health 2020, 17, 3842. [Google Scholar] [CrossRef]
- Pleissner, D.; Smetana, S. Conversion of organic waste to food and feed. Curr. Opin. Green Sustain. Chem. 2020, 26, 100394. [Google Scholar] [CrossRef]
- Echalard, Y.; Ziomek, C.A.; Meade, H.M. Production of Recombinant Therapeutic Proteins in the Milk of Transgenic Animals; BioPharm International, 2006; Volume 19, Issue 8; Available online: https://www.biopharminternational.com/view/production-recombinant-therapeutic-proteins-milk-transgenic-animals (accessed on 25 May 2025).
- BUND NRW (FoE Germany). Chemieriesen verbrauchen enorme Wassermengen, Press Release. 17 August 2023. Available online: https://www.bund.net/service/presse/pressemitteilungen/detail/news/chemieriesen-verbrauchen-enorme-wassermengen/ (accessed on 5 February 2025).
- Figuière, R.; Borchert, F.; Cousins, I.T.; Ågerstrand, M. The essential use concept: A valuable tool to guide decision making on applications for authorisation under REACH? Environ. Sci. Eur. 2023, 35, 5. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission: Guiding Criteria and Principles for the Essential Use Concept in EU Legislation Dealing with Chemicals, C(2024) 1995 Final. Available online: https://environment.ec.europa.eu/document/download/fb27e67a-c275-4c47-b570-b3c07f0135e0_en?filename=C_2024_1995_F1_COMMUNICATION_FROM_COMMISSION_EN_V4_P1_3329609.PDF (accessed on 25 May 2025).
- Cousins, I.T.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Patton, S.; Scheringer, M.; Trier, X.; Vierke, L.; et al. The concept of essential use for determining when uses of PFASs can be phased out. Environ. Sci. Process Impacts. 2019, 21, 1803–1815. [Google Scholar] [CrossRef]
- INERIS (Institut National de l’Environnement Industriel et des Risques). Review of Scientific and Methodological Data on the Concept of Essential Use, with PFAS as a Case Study, Verneuil-en-Halatte: Ineris-229262-2816719-v1.0. 2024. Available online: https://www.ineris.fr/sites/ineris.fr/files/contribution/Documents/Ineris-229262_2816719_PFAS_usages_essentiels_en.pdf (accessed on 25 May 2025).
- Bǎlan, S.A.; Andrews, D.Q.; Blum, A.; Diamond, M.L.; Rojello Fernandez, S.; Harriman, E.; Lindstrom, A.B.; Reade, A.; Richter, L.; Sutton, R.; et al. Optimizing Chemicals Management in the United States and Canada through the Essential-Use Approach. Environ. Sci. Technol. 2023, 57, 1568–1575. [Google Scholar] [CrossRef]
- Tickner, J.A.; Dorman, D.C.; Shelton-Davenport, M. Answering the Call for Improved Chemical Alternatives Assessments (CAA). Environ. Sci. Technol. 2015, 49, 1995–1996. [Google Scholar] [CrossRef][Green Version]
- Tickner, J.A.; Schifano, J.N.; Blake, A.; Rudisill, C.; Mulvihill, M.J. Advancing Safer Alternatives Through Functional Substitution. Environ. Sci. Technol. 2015, 49, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.A.; Cousins, I.; Harriman, E.; Scheringer, M.; Tickner, J.A.; Wang, Z. Combined Application of the Essential-Use and Functional Substitution Concepts: Accelerating Safer Alternatives. Environ. Sci. Technol. 2022, 56, 9842–9846. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, J.; Figuière, R.; Dekker, S.C.; van Wezel, A.P.; Cousins, I.T. Managing PMT/vPvM substances in consumer products through the concepts of essential-use and functional substitution: A case-study for cosmetics. Environ. Sci. Process. Impacts 2023, 25, 1067. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K.; Große Ophoff, M. Nachhaltige Chemie–Für eine neue Ganzheitlichkeit. Politische Ökologie. 2022, 171, 90–96, oekom-Verlag München Germany. [Google Scholar]
- Kümmerer, K. Sustainable from the very beginning: Rational design of molecules by life cycle engineering as an important approach for green pharmacy and green chemistry. Green Chem. 2007, 9, 899–907. [Google Scholar] [CrossRef]
- Moermond, C.T.; Puhlmann, N.; Brown, A.R.; Owen, S.F.; Ryan, J.; Snape, J.; Venhuis, B.J.; Kümmerer, K. Greener Pharmaceuticals for More Sustainable Healthcare. Environ. Sci. Technol. Lett. 2022, 9, 699–705. [Google Scholar] [CrossRef]
- Scheringer, M. Persistence and Spatial Range of Environmental Chemicals: New Ethical and Scientific Concepts for Risk Assessment; Wiley-VCH Verlag: Weinheim, Germany, 2002; ISBN 9783527305278. Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/3527607463 (accessed on 25 May 2025).
- European Commission. Commission Recommendation (EU) 2022/2510 of 8 December 2022 Establishing a European Assessment Framework for ‘Safe and Sustainable by Design’ Chemicals and Materials. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022H2510 (accessed on 25 May 2025).
- ChemSec: Safe and Sustainable by Design Chemicals, 2021-06-29, Gothenburg, Sweden. 2021. Available online: https://chemsec.org/app/uploads/2023/03/ChemSec-Safe-and-Sustainable-by-Design-Chemicals-2021-06-29.pdf (accessed on 25 May 2025).
- CEFIC: Safe and Sustainable by Design: A Guidance to Unleash the Transformative Power of Innovation, Brussels, Belgium. 2024. Available online: https://cefic.org/app/uploads/2024/03/Safe-and-Sustainable-by-Design-a-guidance-to-unleash-the-transformative-power-of-innovation.pdf (accessed on 25 May 2025).
- OECD. Sustainability and Safe and Sustainable by Design: Working Descriptions for the Safer Innovation Approach, OECD Series on the Safety of Manufactured Nanomaterials and Other Advanced Materials; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- EEA: Designing Safe and Sustainable Products Requires a New Approach for Chemicals, EEA 2021, Copenhagen, Denmark. Available online: https://www.eea.europa.eu/publications/designing-safe-and-sustainable-products-1 (accessed on 5 February 2025).
- Caldeira, C.; Farcal, R.; Moretti, C.; Mancini, L.; Rasmussen, K.; Rauscher, H.; Riego Sintes, J.; Sala, S. Safe and Sustainable by Design Chemicals and Materials—Review of Safety and Sustainability Dimensions, Aspects, Methods, Indicators, and Tools, European Commission, Joint Research Centre, JRC Technical Report JRC127109, Publications Office of the European Union, Luxembourg, (PDF). 2022. Available online: https://data.europa.eu/doi/10.2760/879069 (accessed on 5 February 2025).
- Abbate, E.; Garmendia Aguirre, I.; Bracalente, G.; Mancini, L.; Tosches, D.; Rasmussen, K.; Bennett, M.J.; Rauscher, H.; Sala, S. Safe and Sustainable by Design chemicals and materials—Methodological Guidance; European Commission, Joint Research Centre Publications Office of the European Union: Luxembourg, 2024. [Google Scholar] [CrossRef]
- Caldeira, C.; Garmendia Aguirre, I.; Tosches, D.; Mancini, L.; Abbate, E.; Farcal, R.; Lipsa, D.; Rasmussen, K.; Rauscher, H.; Riego Sintes, J.; et al. Safe and Sustainable by Design Chemicals and Materials—Application of the SSbD Framework to Case Studies; European Commission: Joint Research Centre, Publications Office of the European Union: Luxembourg, 2023; Available online: https://data.europa.eu/doi/10.2760/329423 (accessed on 5 February 2025).
- PARC (Partnership for the Assessment of Risks from Chemicals): PARC SSbD Toolbox Version 0.1 Guidebook. 2024. Available online: https://www.parc-ssbd.eu/wp-content/uploads/2024/06/SSbD-toolbox-guidebook-v2.0-1.pdf (accessed on 17 November 2024).
- Apel, C.; Kümmerer, K.; Sudheshwar, A.; Nowack, B.; Som, C.; Colin, C.; Walter, L.; Breukelaar, J.; Meeus, M.; Ildefonso, B.; et al. Safe-and-sustainable-by-design: State of the art approaches and lessons learned from value chain perspectives. Curr. Opin. Green Sustain. Chem. 2024, 45, 100876. [Google Scholar] [CrossRef]
- Sudheshwar, A.; Apel, C.; Kümmerer, K.; Wang, Z.; Soeteman-Hernández, L.G.; Valsami-Jones, E.; Som, C.; Nowack, B. Learning from Safe-by-Design for Safe-and-Sustainable-by-Design: Mapping the current landscape of Safe-by-Design reviews, case studies, and frameworks. Environ. Int. 2024, 183, 108305. [Google Scholar] [CrossRef]
- Kostal, J.; Voutchkova-Kostal, A.; Anastas, P.T.; Beth Zimmerman, J. Identifying and designing chemicals with minimal acute aquatic toxicity. Proc. Natl. Acad. Sci. USA 2015, 112, 6289–6294. [Google Scholar] [CrossRef]
- Escher, B.I.; Altenburger, R.; Blüher, M.; Colbourne, J.K.; Ebinghaus, R.; Fantke, P.; Hein, M.; Köck, M.; Kümmerer, K.; Leipold, S.; et al. Modernizing persistence–bioaccumulation–toxicity (PBT) assessment with high throughput animal free methods. Arch. Toxicol. 2023, 97, 1267–1283. [Google Scholar] [CrossRef]
- Fenner, K.; Scheringer, M. The Need for a Chemical Simplification as a Logical Consequence of the Ever-Increasing Chemical Pollution. Environ. Sci. Technol. 2021, 55, 14470–14472. [Google Scholar] [CrossRef] [PubMed]
- Scheringer, M. Persistence and Spatial Range as Endpoints of an Exposure-Based Assessment of Organic Chemicals. Environ. Sci. Technol. 1996, 30, 1652–1659. [Google Scholar] [CrossRef]
- Cousins, I.T.; Ng, C.A.; Wang, Z.; Scheringer, M. Why is high persistence alone a major cause of concern? Env. Sci Process Impacts 2019, 21, 781. [Google Scholar] [CrossRef] [PubMed]
- Cousins, I.T.; DeWitt, J.C.; Gluge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Scheringer, M.; Wang, Z. The high persistence of PFAS is sufficient for their management as a chemical class. Env. Sci Process Impacts 2020, 22, 2307–2312. [Google Scholar] [CrossRef]
- Scheringer, M.; Johansson, J.J.; Salter, M.E.; Sha, B.; Cousins, I.T. Stories of Global Chemical Pollution: Will We Ever Understand Environmental Persistence? Environ. Sci. Technol. 2022, 56, 17498–17501. [Google Scholar] [CrossRef]
- Brunn, H.; Arnold, G.; Körner, W.; Steinhäuser, K.G.; Valentin, I. PFAS: Forever chemicals–persistent, bioaccumulative and mobile–Reviewing the status and the need for their phase out and remediation of contaminated sites. Environ. Sci. Eur. 2023, 35, 20. [Google Scholar] [CrossRef]
- Kümmerer, K. The ecological impact of time. Time Soc. 1996, 5, 209–235. [Google Scholar] [CrossRef]
- Tickner, J.A.; Jacobs, M.; Brody, C.H. Chemistry Urgently Needs to Develop Safer Materials, Scientific American. 25 February 2023. Available online: https://www.scientificamerican.com/article/chemistry-urgently-needs-to-develop-safer-materials/ (accessed on 25 May 2025).
- Olisah, C.; Melymuk, L.; Vestergren, R.; Rumar, K.; Wickman, T.; Melander, N.; Talasniemi, P.; Brandsma, S.; Boije af Gennas, U.; Scheringer, M. Toward Product Safety and Circularity: Understanding the Information Structure of Global Databases on Chemicals in Products and Articles. Environ. Sci. Technol. 2025, 59, 1897–1908. [Google Scholar] [CrossRef]
- Advisory Council on Environment (SRU). Sufficiency as a “Strategy of the Enough”: A Necessary Debate, Sachverständigenrat für Umweltfragen, Berlin, Germany. 2024. Available online: https://www.umweltrat.de/SharedDocs/Downloads/EN/04_Statements/2020_2024/2024_06_Sufficiency_as_a_Strategy_of_the_Enough.pdf?__blob=publicationFile&v=2 (accessed on 25 May 2025).
- Mederake, L. Without a Debate on Sufficiency, a Circular Plastics Economy will Remain an Illusion. Circ. Econ. Sustain. 2023, 3, 1425–1439. [Google Scholar] [CrossRef]
- Moser, F.; Karavezyris, V.; Blum, C. Chemical Leasing in the context of Sustainable Chemistry. Environ. Sci. Pollut. Res. 2015, 21, 6968–6988. [Google Scholar] [CrossRef]
- German Environment Agency (UBA). Towards Sustainable Chemical Intensity—A glo6al, Inclusive Goal for the Sustainable Use of Chemicals is Needed to Safeguard Both Societal Wellbeing and Earth’s Life-Sustaining Capacity, Thought Starter, Dessau-Roßlau, Germany. 2021. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/1/publikationen/2021-11-24-uba-fact-sheet-thought-starter_towards-chemical-intensity.pdf (accessed on 25 May 2025).
- Stolzenberg, H.-C.; Blum, C.; Klauk, A. How chemicals can serve people sustainably without polluting the planet: Through common objectives, integration, and more effective cooperation. J. Bus. Chem. 2022, 19, 100–122. [Google Scholar] [CrossRef]
- BUND (FoE Germany). Sustainable Chemicals and Materials Policy for Protecting the Climate and Biodiversity; BUND-Background, BUND: Berlin, Germany, 2021; Available online: https://www.bund.net/fileadmin/user_upload_bund/publikationen/chemie/chemie_Hintergrund_Nachhaltige_Stoffpolitik_EN.pdf (accessed on 25 May 2025).
- Okereke, C. Degrowth, green growth, and climate justice for Africa. Rev. Int. Stud. 2024, 50, 910–920. [Google Scholar] [CrossRef]
- United Nations. Agenda 21. In Proceedings of the United Nations Conference on Environment & Development, Rio de Janeiro, Brazil, 3–14 June 1992; Available online: https://sustainabledevelopment.un.org/content/documents/Agenda21.pdf (accessed on 25 May 2025).
- OSPAR (1992). Convention for the Protection of the Marine Environment of the North-East Atlantic. In Proceedings of the OSPAR Convention (OSPAR 2007), OSPAR CommissionOstend, Belgium, 25–29 June 2007; Available online: https://www.ospar.org/site/assets/files/1169/ospar_convention.pdf (accessed on 27 February 2025).
- Cannon, A.; Edwards, S.; Jacobs, M.; Moir, J.W.; Roy, M.A.; Tickner, J.A. An actionable definition and criteria for “sustainable chemistry” based on literature review and a global multisectoral stakeholder working group. RSC Sustain. 2023, 1, 2092. [Google Scholar] [CrossRef]
- UNEP. Green and Sustainable Chemistry: Framework Manual–Executive Summary; United Nations Environment Programme; Châtelaine: Geneva, Switzerland, 2020; ISBN 978-92-807-3839-1. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/35312/GSCF_ES.pdf?sequence=1&isAllowed=y (accessed on 25 May 2025).
- ISC3 (International Sustainable Chemistry Collaborative Centre) (Ed.) Key Characteristics of Sustainable Chemistry—Towards a Common Understanding of Sustainable Chemistry; ISC3: Bonn, Germany, 2020; Available online: https://www.isc3.org/cms/wp-content/uploads/2022/06/ISC3_Sustainable_Chemistry_key_characteristics_20210113.pdf (accessed on 25 May 2025).
- Blum, C.; Bunke, D.; Hungsberg, M.; Roelofs, E.; Joas, A.; Joas, R.; Blepp, M.; Stolzenberg, H.-C. The concept of sustainable chemistry: Key drivers for the transition towards sustainable development. Sustain. Chem. Pharm. 2017, 5, 94–104. [Google Scholar] [CrossRef]
- German Environment Agency (UBA). Chemicals: Better Protection of Environment and Health-Steady Increase of Chemicals Use Worldwide; Umweltbundesamt: Dessau-Roßlau, Germany; Available online: https://www.umweltbundesamt.de/en/press/pressinformation/chemicals-better-protection-of-environment-health (accessed on 23 January 2025).
- Friege, H.; Heidbüchel, E.; Zeschmar-Lahl, B. Indicators for Sustainable Management of Chemicals Contributions to Upcoming Development Work under the New Global Framework for Chemicals UBA-Texte 79/2024, Umweltbundesamt Dessau-Roßlau. 2024. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/11850/publikationen/79_2024_texte_sustainable_management_of_chemicals_0.pdf (accessed on 25 May 2025).
- Schäffer, A.; Groh, K.J.; Sigmund, G.; Azoulay, D.; Backhaus, T.; Bertram, M.G.; Carney Almroth, B.; Cousins, I.T.; Ford, A.T.; Grimalt, J.O.; et al. Conflicts of Interest in the Assessment of Chemicals, Waste, and Pollution. Environ. Sci. Technol. 2023, 57, 19065–20452. [Google Scholar] [CrossRef]
- Schäffer, A.; Mueller, L. Geballtes Wissen für die Politik. In Politische Ökologie; oekom-Verlag: München, Germany, 2022; Volume 171, pp. 74–79. [Google Scholar]
- Antwerp Declaration. The Antwerp Declaration for a European Industrial Deal the Call for a Business Case for Investments in Europe. 2024. Available online: https://antwerp-declaration.eu/pdf/declaration.pdf (accessed on 24 February 2025).
- Cole, M.A.; Elliott, R.J.R.; Zhang, L. Foreign Direct Investment and the Environment. Annu. Rev. Environ. Resour. 2017, 42, 465–487. [Google Scholar] [CrossRef]
- Vital Caetano, R.; ·Cardoso Marques, A.; Lopes Afonso, T. Can Sustainable Development Induce Foreign Direct Investment? Analysis of the Complex Inward Outward Flows of Investment in European Union Countries. J. Knowl. Econ. 2024, 15, 9756–9783. [Google Scholar] [CrossRef]
- CIEL; IPEN. Financing the Sound Management of Chemicals Beyond 2020: Options for a Coordinated Tax, Brussels, Belgium. 2020. Available online: https://www.ciel.org/wp-content/uploads/2020/09/ipen-ciel-producer-responsibility-vf1_9e-web-en.pdf (accessed on 25 May 2025).
- OECD. Extended Producer Responsibility, Basic Facts and Key Principles, OECD Environment Policy Paper No. 41, Paris, France. 2024. Available online: https://www.oecd.org/en/publications/extended-producer-responsibility_67587b0b-en.html (accessed on 25 May 2025).
- Tickner, J.A.; Geiser, K.; Baima, S. Transitioning the Chemical Industry: The Case for Addressing the Climate, Toxics, and Plastics Crises. Environ. Sci. Policy Sustain. Dev. 2021, 63, 4–15. [Google Scholar] [CrossRef]
- Bazzanella, A.M.; Ausfelder, F. Low Carbon Energy and Feedstock for the European Chemical Industry: Technology Study, DECHEMA, Frankfurt am Main, Germany. 2017. Available online: https://dechema.de/dechema_media/Downloads/Positionspapiere/Technology_study_Low_carbon_energy_and_feedstock_for_the_European_chemical_industry.pdf (accessed on 25 May 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


