Abstract
Background/Objectives: Rotator cuff tears are a prevalent cause of shoulder pain and functional impairment. Full-thickness tears often require surgical intervention, but managing such injuries can be challenging, particularly when complete anatomical repair is unattainable. Bioinductive implants have emerged as an innovative adjunct to enhance tendon healing and regeneration. Methods: This case report details the partial repair of a full-thickness rotator cuff tear in a 66-year-old woman, augmented with a bioinductive implant. Postoperative recovery was monitored through clinical examinations, MRI, and kinematic analysis at 3 and 6 months. Results: The findings suggest that bioinductive implants may offer a promising strategy for managing complex rotator cuff tears, particularly when complete repair is not feasible. The patient reported improvement in function and pain reduction. Conclusions: The use of bioinductive implants showed promising results, promoting tendon regeneration and improving functional outcomes. Future research should explore patient selection criteria and the long-term effectiveness of this strategy.
1. Introduction
The rotator cuff, a sophisticated musculotendinous structure, is essential for shoulder stability and motion. Its muscles work synergistically to maintain joint centration, enable a broad range of movements, and provide strength for various upper limb activities.
Rotator cuff tears are a relevant cause of musculoskeletal disease, profoundly impacting quality of life and functional autonomy. The prevalence of this condition in the general population ranges from 5% to 40%. Massive rotator cuff tears, characterized as being 5 cm or greater in size or involving two or more tendons, account for 20% of all such injuries. Alarmingly, the retear rates following the primary repair of massive tears range between 40% and 90%, highlighting the significant clinical challenge these injuries pose and the complexity of their management [1].
The incidence of rotator cuff pathology increases notably with age, largely due to degenerative changes that progressively impair tendon integrity. Beyond aging, additional risk factors include occupations requiring repetitive overhead movements and certain athletic activities, both of which can lead to chronic microtrauma and tendon overload. Degenerative alterations in rotator cuff tendons and their attachment site, or footprint, manifest as tensile overload, collagen fiber disorganization, myxoid degeneration, chondroid metaplasia, and fatty infiltration. Consequently, rotator cuff disorders encompass a spectrum ranging from simple inflammatory tendinitis to fibrosis, delamination, partial-thickness tears, and the complete detachment of the rotator cuff footprint. This continuum may be further influenced by acute trauma, anatomical predispositions, or concomitant systemic diseases [2].
Full-thickness rotator cuff tears (FTRCTs) pose a formidable challenge in the domain of shoulder surgery, affecting a considerable segment of the population. Clinically, these tears are associated with marked shoulder pain, often radiating along the arm, along with pronounced weakness and significant difficulty in elevating or abducting the arm [1,3].
The management of FTRCTs is tailored based on various factors, including the patient’s age, activity level, overall health status, and specific tear characteristics. Surgical intervention is typically pursued for larger or symptomatic tears, particularly in patients with active lifestyles. The choice of surgical technique is determined by the tear’s location and degree of retraction, alongside considerations such as the tear size, chronicity, tendon quality, and patient-specific factors [1,4,5].
The success of FTRCT repair hinges on numerous variables, including the selected therapeutic approach. Postoperative healing and symptom resolution are believed to depend, in part, on the underlying physiological contributors to tendon degeneration, such as the vascularity, tissue quality, and footprint pathology. Consequently, there has been a burgeoning interest in biologically augmenting the repair environment to enhance outcomes [2]. Bioinductive implants offer several potential advantages over traditional repair methods. They promote biological healing and regeneration by providing a scaffold for cell infiltration and tissue growth, potentially leading to stronger and more durable repairs. This is particularly beneficial in cases of poor tissue quality or large tears where traditional repair techniques may be insufficient. Furthermore, bioinductive implants may reduce the risk of re-tear by enhancing the integration of the repaired tendon with the bone. Several studies have demonstrated the efficacy of bioinductive implants in improving clinical outcomes and reducing re-tear rates compared to traditional repair methods alone [5,6,7,8,9,10]. Bioinductive implants, comprising collagen-based scaffolds, have emerged as a novel strategy to promote tendon regeneration by leveraging the host tissue’s intrinsic healing mechanisms. These implants facilitate cellular infiltration and matrix remodeling, thereby creating an environment conducive to tissue repair [6].
This case report explores the application of a bioinductive implant to enhance a partial repair of a full-thickness rotator cuff tear. The findings underscore the potential of this approach to transform the management paradigm for irreparable tears, offering a biologically enhanced solution that complements traditional mechanical repair methods.
2. Case Presentation
A 66-year-old right-handed female presented with a progressive history of right-shoulder pain and functional limitations persisting over the course of one year. The discomfort, initially manifesting as a dull ache, progressively intensified, particularly during activities requiring overhead reaching or lifting. She reported significant weakness in the right shoulder, severely impairing her ability to perform routine activities such as combing her hair or putting on a shirt. Her past medical history was unremarkable, with no notable medical conditions, prior surgeries, or major injuries affecting either shoulder.
On physical examination, clinical findings included positive impingement signs, weakness in shoulder abduction and external rotation, restricted range of motion, and tenderness to palpation over the lateral aspect of the right shoulder, corresponding to the anatomical location of the rotator cuff tendons. Magnetic resonance imaging (MRI) corroborated these findings, revealing a full-thickness tear involving the supraspinatus and infraspinatus tendons (Figure 1).
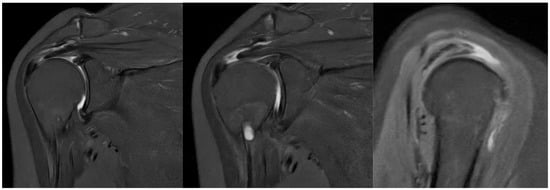
Figure 1.
Preoperative MRI showing full-thickness rotator cuff tear.
The patient had previously undergone a one-year course of conservative management, including physiotherapy, which failed to yield significant improvement. Given the size and anatomical location of the rotator cuff tear, non-surgical treatment options were deemed unlikely to provide sufficient or sustainable relief. Considering the patient’s expressed desire to return to an active lifestyle and the potential for enhanced healing outcomes with the use of the Regeneten bioinductive implant, surgical repair augmented by this implant was discussed and ultimately selected as the optimal treatment strategy.
The decision to use a bioinductive implant in this case was influenced by several factors, including the partial nature of the planned repair, the patient’s age and activity level, and the desire to optimize healing and reduce the risk of re-tear. Specifically, the presence of a large, full-thickness tear with significant retraction made complete anatomical repair challenging. The decision was primarily driven by the partial nature of the planned repair due to the extent of the tear and the desire to optimize healing and reduce the risk of re-tear. While other augmentation techniques exist, the Regeneten implant was chosen for its established safety profile and ease of use in arthroscopic procedures. The patient’s age and activity level were also considered, as bioinductive implants have shown promise in promoting healing in older patients and those with active lifestyles. The patient was deemed a suitable candidate for the Regeneten implant based on the following criteria: the presence of a partial or full-thickness rotator cuff tear deemed amenable to augmentation, adequate bone quality for anchor placement, patient’s desire to return to a previous activity level, and an absence of contraindications to the implant. It is important to note that this is a single case report, and the decision-making process should be individualized based on patient-specific factors and surgeon experience. Future studies with larger cohorts are needed to validate these findings and establish definitive guidelines for the use of bioinductive implants in rotator cuff surgery.
2.1. Kinematic Data Acquisition and Processing
Kinematic analysis was performed before surgery, after 3 months, and after 6 months after surgery. The Qualisys™ stereophotogrammetric system (Qualisys AB, Gothenburg, Sweden) was used for kinematic data acquisition. All markers’ trajectories were recorded by ten Miqus M3 cameras (sampling frequency of 100 Hz). Retroreflective markers (8 mm) were placed on anatomical reference points following the International Society of Biomechanics (ISB) [11]. The markers on the thorax were placed on the following landmarks: the Incisura Jugularis (IJ), Processus Xiphoideus (PX), processus spinosus of the seventh cervical vertebra (C7), and processus spinosus of the eighth thoracic vertebra (T8). On the left and right scapulae, the Angulus Acromialis (AA), the Trigonum Scapulae (TS), the Angulus Inferior (AI), and the Coracoid Process (PC) were identified. On the upper arms and the forearms, the right and left Acromioclavicular joint (AC), Lateral Epicondyle (LE), Medial Epicondyle (ME), Radial Styloid (RS), and Ulnar Styloid (US) were identified. Moreover, five square-shaped clusters, with four markers each, were placed on the thorax and bilaterally on the upper arms and on the forearms.
First, the patient was asked to maintain a static pose for three seconds for the kinematic model definition. Then, the patient was asked to perform elevation in the sagittal plane (Task 1), in the scapular plane (Task 2), and in the frontal plane (Task 3). Moreover, the patient was asked to perform the following exercises: hand-to-the-nape (Task 4), hand-to-the-back (Task 5), and external rotation (Task 6). Each task included three repetitions and was executed at a self-selected speed, avoiding pain conditions (Figure 2).
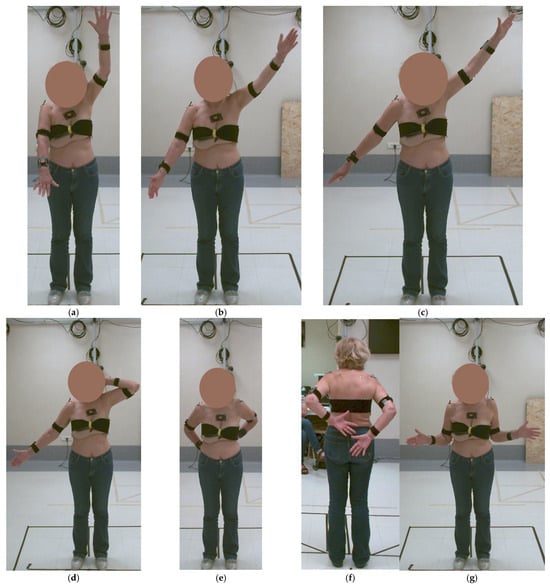
Figure 2.
Preoperative kinematic analysis: (a) elevation in the sagittal plane—Task 1, (b) in the scapular plane—Task 2, (c) in the frontal plane—Task 3, (d) hand-to-the-nape—Task 4, (e,f) hand-to-the-back—Task 5, and (g) external rotation—Task 6.
Data acquisitions were executed using the Qualysis Track Manager (QTM) software v2023.1 (Qualisys AB, Gothenburg, Sweden), also used for markers’ trajectories pre-processing (labeling and gap filling). Then, pre-processed data were imported into Visual 3D (v2023.09.3) for kinematic analysis. In Visual 3D, the shoulder elevation angles and axial rotations were extracted as the motion of the humerus relative to the thorax (Humerothoracic—HT—joint angles) [11]. Kinematic data were then exported into a .mat file format for further analysis in MATLAB R2023b. The maximum values of the shoulder elevation angle and axial rotations were identified for the three repetitions of each task performed at all follow-up assessments and expressed as the mean and standard deviation.
2.2. Surgical Technique
In the preoperative holding area, a regional nerve block is administered. The patient is positioned in the beach-chair configuration under mild sedation. Following standard sterile preparation, draping, and the identification of pertinent anatomical landmarks, a posterior viewing portal is created to conduct a diagnostic arthroscopy of the glenohumeral joint. An anterior portal is established using an outside-in technique with a spinal needle, allowing for the management of any intra-articular pathologies, such as biceps tenosynovitis. The arthroscope is subsequently advanced into the subacromial space, and two additional portals are created: a lateral viewing portal positioned 3 cm lateral to the acromial edge along the posterior clavicular border, and an anterolateral portal, into which a screw-in cannula is inserted. A subacromial bursectomy and acromioplasty are performed to enhance visualization and facilitate access to the rotator cuff.
Arthroscopic visualization revealed multiple layers of the rotator cuff. Sheets 2 and 3 of the infraspinatus tendons were identifiable, while only sheet 3 of the supraspinatus tendon was visible, with sheet 2 absent (Figure 3). The mobilization of the rotator cuff confirmed that restoration to the footprint could be achieved without excessive tension (Figure 4).
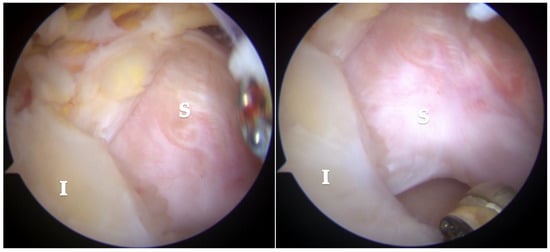
Figure 3.
Subacromial view demonstrating full-thickness rotator cuff tear involving the supraspinatus (S) and infraspinatus (I) tendons. Only sheet 2 of the supraspinatus tendon is visible, while both sheets 2 and 3 of the infraspinatus tendon are present.
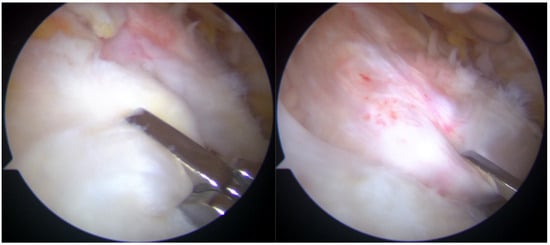
Figure 4.
Reduction maneuvers demonstrated the ability to reposition sheet 3 of the supraspinatus tendon (right image) and sheets 2 and 3 of the infraspinatus tendon (left image) back to their native footprint.
The lesion involved a full-thickness tear affecting both the supraspinatus and infraspinatus tendons, with separation into superficial bursal and deep articular layers. The layers exhibited differential retraction and elasticity; the superficial layers were more retracted compared to the deep layers. The complete reduction of the infraspinatus tendon was achieved, whereas only the deep layer of the supraspinatus tendon could be restored to the footprint due to the excessive retraction and stiffness of the superficial layer. Given the chronicity and severity of the tear, a complete anatomical repair was deemed unattainable. The frayed edges of the tendon were meticulously debrided to prepare for repair. Two suture anchors (Q-FIX 2.8 mm, Smith and Nephew, London, UK) were employed to secure the torn tendon back to its native humeral attachment using a single-row repair technique.
Post-repair arthroscopic visualization revealed a partial repair of the supraspinatus tendon, leaving a noticeable gap in the rotator cuff tissue (Figure 5).
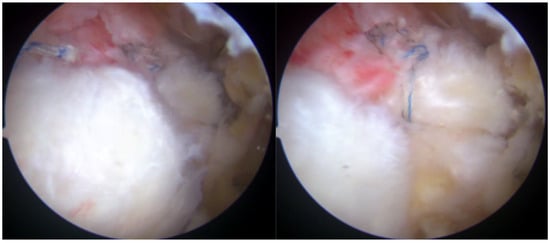
Figure 5.
Rotator cuff repair has been achieved with two suture anchors, but a defect remained due to the absence of sheet 2 of the supraspinatus tendon.
To address this residual defect, a bioinductive collagen membrane (Regeneten, Smith and Nephew) was implanted to augment the repair and enhance healing. The Regeneten patch is available in two sizes, with the appropriate size selected based on the tear’s dimensions and morphology. The bursal surface of the patch is distinctively marked to differentiate it from the interlaced articular surface.
A thorough bursectomy was performed to create adequate space for patch augmentation. Soft tissue lateral to the tendon insertion was ablated to ensure direct contact between the lateral edge of the implant and the bone. The patch was loaded onto an arthroscopic delivery device and introduced into the subacromial space via a lateral cannula. After achieving the desired medial-to-lateral positioning over the repair construct, the delivery device was engaged, allowing the implant to unfurl. Two additional accessory portals were created for optimal anchor placement. Anchors were deployed with precision, using a quick motion to secure the implant to the tendon construct. Eight tendon anchors were inserted to affix the patch securely along its edges, ensuring stabilization (Figure 6). Although bone anchors could be used to enhance the contact between the lateral portion of the implant and the bone, they are not commonly utilized by the senior Author.
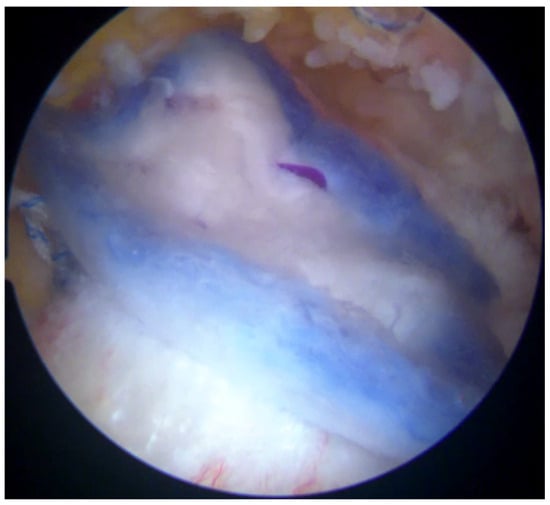
Figure 6.
The bioinductive implant (Regeneten) was positioned carefully over the repaired tendon site. The implant served as a scaffold to encourage the growth of new tendon-like tissue and enhance the strength of the repair.
The stability and positioning of the implant were confirmed with a probe. Following the verification of the repair, all instruments were removed, and the surgical wounds were closed in the standard fashion.
2.3. Postoperative Rehabilitation
The patient demonstrated excellent tolerance to the surgical procedure and experienced an uneventful recovery during her overnight hospitalization. A sling was utilized for initial shoulder immobilization to facilitate optimal healing. Pain management was effectively addressed using a combination of pharmacological interventions and physical therapy techniques, such as ice application and gentle passive range of motion exercises.
Upon discharge, the patient commenced a structured physical therapy regimen, that was not modified due to the use of the bioinductive implant. During the first month, immobilization was prioritized, with the patient performing only pendulum exercises and elbow mobilization. In the subsequent month, the rehabilitation plan progressed to focus on restoring the passive range of motion and alleviating pain. By the two-month mark, the program was gradually expanded to incorporate gentle active-assisted exercises aimed at strengthening the repaired rotator cuff and surrounding musculature. This carefully calibrated rehabilitation protocol was designed to enhance tendon healing while avoiding undue strain, ensuring the development of strength and stability.
2.4. Follow-Up
The patient was closely monitored throughout the rehabilitation process, attending regular follow-up visits with both the surgeon and physical therapist. By the three-month postoperative visit, she reported substantial pain relief and a marked improvement in her shoulder strength and range of motion. Her confidence in utilizing the affected arm continued to grow as her rehabilitation advanced.
At three months and six months post-surgery, the patient repeated kinematic analysis, which confirmed excellent progress in regaining the range of motion, reflecting a highly favorable surgical outcome (Figure 7 and Figure 8).
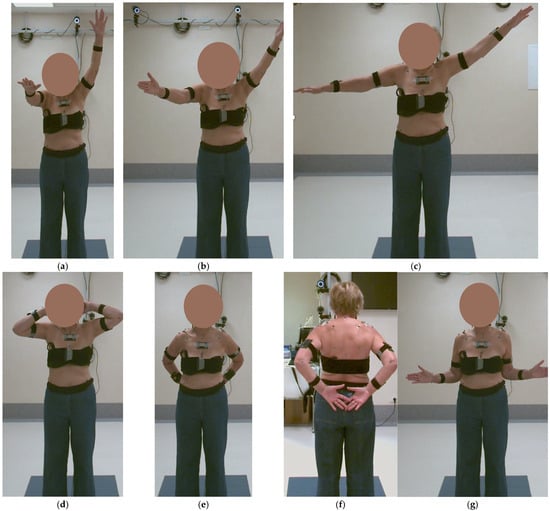
Figure 7.
Kinematic analysis at 3 months post-surgery: (a) elevation in the sagittal plane—Task 1, (b) in the scapular plane—Task 2, (c) in the frontal plane—Task 3, (d) hand-to-the-nape—Task 4, (e,f) hand-to-the-back—Task 5, and (g) external rotation—Task 6.
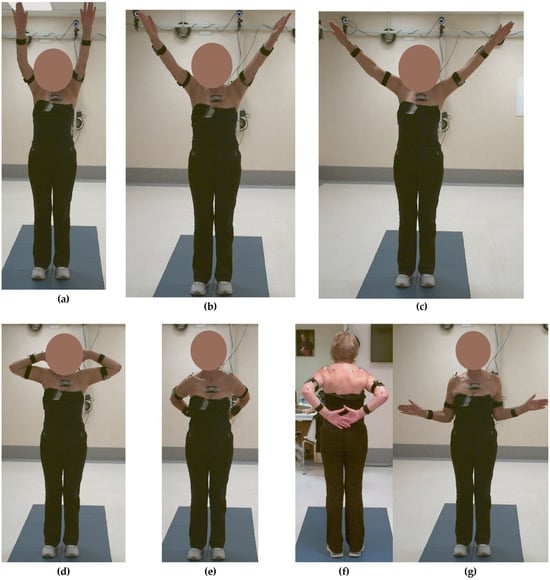
Figure 8.
Kinematic analysis at 6 months post-surgery: (a) elevation in the sagittal plane—Task 1, (b) in the scapular plane—Task 2, (c) in the frontal plane—Task 3, (d) hand-to-the-nape—Task 4, (e,f) hand-to-the-back—Task 5, and (g) external rotation—Task 6.
In addition to the objective improvements in her range of motion and tendon healing, the patient also reported significant subjective improvements in pain, function, and overall satisfaction with the procedure. Specifically, she reported an improvement in her ability to perform activities of daily living. These patient-reported outcomes are consistent with the objective findings and further support the potential benefits of bioinductive implants in improving patient well-being.
At six months after the surgery, a new MRI was performed, showing the reaction induced by the implant, with the progressive formation of tendon-like tissue (Figure 9).
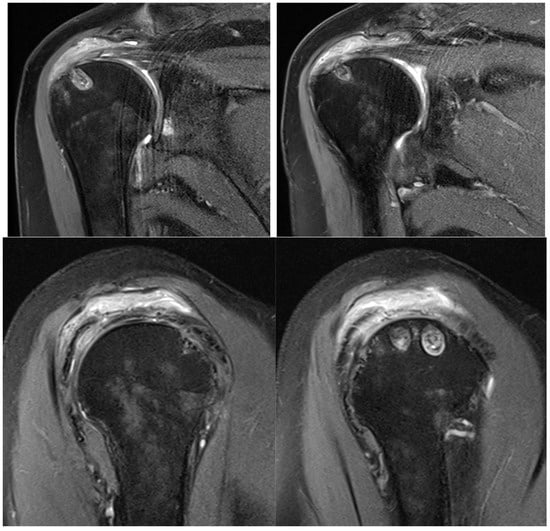
Figure 9.
Postoperative MRI at 6 months after surgery, showing progressive formation of new tendon-like tissue with improvement of tendon thickness.
For Task 1, the results showed shoulder elevation angles equal to 34.6 ± 4.3°, 63.6 ± 0.1°, and 114.4 ± 1.5° before surgery, after 3 months, and after 6 months, respectively (Figure 10a). For Task 2, the results showed shoulder elevation angles equal to 31.9 ± 3.7°, 64.5 ± 2.2°, and 106.2 ± 1.3° before surgery, after 3 months, and after 6 months, respectively (Figure 10b). For Task 3, the results showed shoulder elevation angles equal to 52.4 ± 3.4°, 66.5 ± 2.6°, and 108.0 ± 1.3° before surgery, after 3 months, and after 6 months, respectively (Figure 10c). For Task 4, the results showed shoulder elevation angles equal to 42.0 ± 1.5°, 78.7 ± 2.8°, and 94.0 ± 3.0° before surgery, after 3 months, and after 6 months, respectively (Figure 10d). For Task 5, the results showed shoulder elevation angles equal to 52.6 ± 2.3°, 48.8 ± 3.3°, and 63.6 ± 1.2° before surgery, after 3 months, and after 6 months, respectively (Figure 10e). For Task 6, the results showed that shoulder external rotation was equal to 43.4 ± 3.0°, 40.1 ± 3.3°, and 60.9 ± 2.7° before surgery, after 3 months, and after 6 months, respectively (Figure 10f).
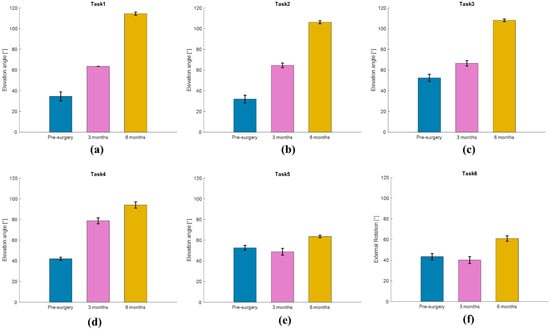
Figure 10.
Kinematic analysis results for (a) elevation in the sagittal plane—Task 1, (b) in the scapular plane—Task 2, (c) in the frontal plane—Task 3, (d) hand-to-the-nape—Task 4, (e) hand-to-the-back—Task 5, and (f) external rotation—Task 6, before surgery and at 3 months and 6 months after surgery.
3. Discussion
The rotator cuff is an intricate, multilayered structure. Histological analyses conducted by Clark and Harryman identified five distinct layers within the tendons of the rotator cuff (Figure 11).
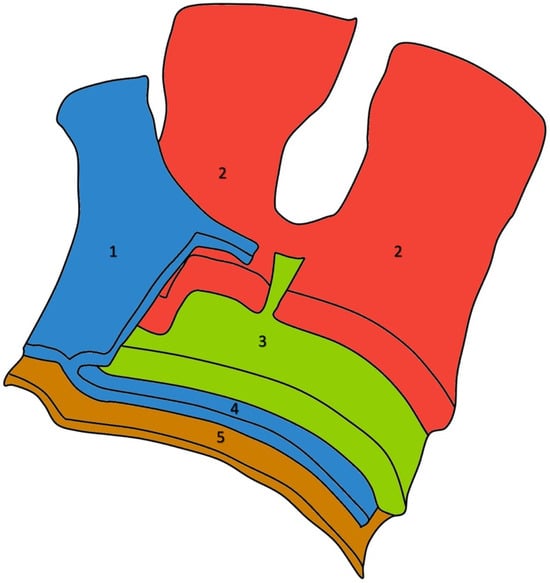
Figure 11.
Five-sheets structure of the rotator cuff in a left shoulder: the coracohumeral ligament fibers (1), the bundles originating from the infraspinatus (on the right side of the image) and supraspinatus (on the left side of the image) tendons (2), smaller tendon fascicles layer (3), loose connective tissue merging with the coracohumeral ligament at the anterior margin of the supraspinatus tendon (4), and the superior joint capsule (5).
The most superficial layer consists of the coracohumeral ligament fibers, which are oriented obliquely to the axis of the muscle and extend across the space between the subscapularis and supraspinatus tendons. The second layer comprises tendon fibers organized into substantial bundles that originate from the supraspinatus tendon and form a covering over the biceps tendon in its groove. The third layer includes smaller tendon fascicles that are less densely packed and display a less uniform orientation compared to the bundles in the second layer. The fourth layer consists of loose connective tissue interspersed with thick collagen fibers, merging with the coracohumeral ligament at the anterior margin of the supraspinatus tendon. The fifth and final layer is a continuous sheet of collagen fibrils that form the superior joint capsule [12].
This complex anatomical configuration is essential for the biomechanical function of the shoulder, but presents significant challenges when it comes to surgical repair following injury.
The extent of tendon retraction and the quality of the remaining tissue are highly variable and play a crucial role in determining the feasibility of complete repair. Tears that exhibit significant retraction, fatty infiltration, or the thinning of the tendon layers are particularly difficult to manage. The layers of the rotator cuff retract at different rates due to their unique tensile properties, making it challenging to achieve anatomical restoration and leading to higher tension on repairs and increased failure rates [13].
Taking a closer look at preoperative MR images, it is possible to glimpse the line of the tear, which is oblique, and the two sheets of the tendon, which have different degrees of retraction (Figure 12).
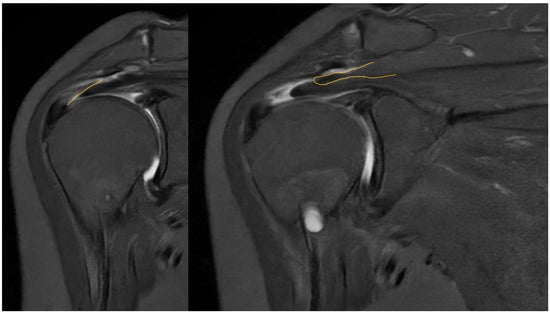
Figure 12.
Preoperative MRI with identification of the line of the tear and the two separated sheets of the supraspinatus tendon, with different retraction.
Preoperative magnetic resonance imaging can reveal the orientation of the tear, and the varying degrees of retraction in the tendon sheets. However, while imaging provides insight into tendon retraction, it does not yield information about the elasticity of the tissue, which can only be accurately assessed during an arthroscopic examination. Arthroscopy in the presented case revealed the separation of sheets 2 and 3 within both the infraspinatus and supraspinatus tendons. Both sheets of the infraspinatus tendon were deemed repairable, while sheet 2 of the supraspinatus tendon could not be adequately repositioned. Consequently, following the rotator cuff repair, a defect remained in the supraspinatus tendon, which the senior Author opted to address with the application of a Regeneten implant.
Various surgical techniques have been developed to address rotator cuff tears, each presenting its own set of advantages and limitations. Single-row repair techniques, which employ anchors to fix the tendon to the bone in a linear pattern, are relatively straightforward and minimally invasive, but may fall short of fully restoring the natural footprint of the rotator cuff. Double-row repairs, on the other hand, increase the contact area and biomechanical strength of the repair, generally resulting in improved outcomes, though they require more technical expertise and can lead to longer surgical times and higher costs. Suture bridge repairs, which combine elements from both single-row and double-row techniques, enhance tendon compression and stability, although they raise concerns about potential vascular compromise due to excessive compressive forces [14,15,16]. Other approaches include partial repairs with or without the medialization of the footprint, rotator cuff debridement and decompression, superior capsular reconstruction, and tendon transfers involving the latissimus dorsi and/or lower trapezius, as well as subacromial balloon spacers, and, ultimately, reverse total shoulder arthroplasty, depending on the extent of the tear’s reparability [1].
When complete anatomic repair is not achievable, partial repairs can help restore shoulder biomechanics and alleviate symptoms by repositioning the tendon to facilitate a functional range of motion. However, such repairs often fail to fully restore tendon integrity, particularly in cases involving substantial tendon degeneration or poor tissue quality, leaving patients vulnerable to re-tear and suboptimal long-term outcomes. These limitations highlight the need for strategies that address not only the mechanical aspects of repair, but also the biological mechanisms involved in tendon healing.
The biological environment significantly impacts the success of surgical intervention in rotator cuff repair. Tendon healing is a complex process involving inflammation, cell proliferation, and extracellular matrix remodeling. In instances of chronic tears or compromised tissue quality, the healing potential is often greatly diminished due to tendon degeneration, reduced vascularity, and insufficient cellular activity. These factors make it challenging for repaired tendons to regain the structural and functional properties necessary to withstand physiological biomechanical loads. Bioinductive implants promote biological healing and regeneration by providing a scaffold for cell infiltration and tissue growth. This creates an environment conducive to tissue repair and may lead to stronger and more durable repairs. Specifically, the collagen scaffold of the Regeneten implant mimics the natural structure of tendon tissue, attracting cells involved in the healing process and promoting the formation of new, healthy tissue [6].
As with any surgical procedure, there is a risk of complications associated with the use of bioinductive implants. These can include infection, inflammation, foreign body reactions, implant migration, and failure of integration. However, these complications are rare and can be mitigated with proper surgical technique, appropriate patient selection, and diligent postoperative follow-up. Specifically, the meticulous debridement of the recipient site, careful implant handling, and secure fixation can minimize the risk of infection and migration. Furthermore, patient education regarding postoperative care and activity restrictions is crucial for promoting healing and preventing complications [9,17].
Considering the limitations inherent in purely mechanical repair strategies, enhancing the biological environment to support tendon regeneration has become increasingly recognized as an essential component of successful rotator cuff surgery.
Bioinductive implants have emerged as an innovative approach to overcoming the biological challenges associated with rotator cuff repairs. Several studies have demonstrated the efficacy of bioinductive implants in improving clinical outcomes and reducing re-tear rates compared to traditional repair methods alone [5,6,7,8,9,10]. These implants, typically composed of collagen-based scaffolds, create a biologically conducive environment that promotes tendon regeneration through cellular infiltration, vascular ingrowth, and enhanced extracellular matrix production. Importantly, if used in partial-thickness tears, these implants do not alter the anatomic footprint of the tendon, thereby preserving the native tendon structure and minimizing strain during the healing phase. By improving the quality and thickness of the tendon, bioinductive implants target the underlying causes of repair failure, particularly in cases of degenerated or avascular tissue [6,7,18].
Also, bioinductive implants enhance early clinical outcomes. Bushnell et al. showed that in higher-grade partial tears, isolated bioinductive repair with Regeneten yielded significantly superior clinical scores at 2 and 6 weeks when compared with an augmented takedown and repair group (p < 0.05) [8].
The effects of biological augmentation are evident in histologic studies and MRI. After 6 months, there is no histological evidence of the Regeneten implant remaining and the neotendon is indistinguishable from the native tendon on MRI, both in partial tears treated with isolated bioinductive repair and in full-thickness tears managed with complete augmented repair [5]. Schlegel et al. found that 87% of partial tears treated solely with bioinductive repair using Regeneten showed more than a 50% reduction in tear size in MRI after 24 months, along with a significant increase in tendon thickness. Additionally, no significant differences in clinical outcomes, tear healing, or tendon thickness were observed based on the location of the tear [7,19].
At the 12-month follow-up, the augmented repair of full-thickness tears using the Regeneten implant demonstrated a significantly lower re-tear rate, enhanced tendon integrity, and no difference in clinical outcomes and complications compared to a repair alone [9]. Also, Bokor et al. have demonstrated that the use of bioinductive implants leads to facilitated tendon healing, improved tendon integrity, lower re-tear rates, and more sustained functional outcomes [10].
These findings suggest that bioinductive implants could play a pivotal role in shifting the treatment paradigm for irreparable rotator cuff tears, offering renewed hope for patients with complex shoulder pathologies.
The subjective improvements and the improvements in the range of motion and tendon regeneration observed in this case are encouraging and suggest that the bioinductive implant may have contributed to enhanced healing. While a direct comparison with patients undergoing mechanical repair alone is not possible in this case report, the observed outcomes appear comparable to, or potentially better than, those reported in studies evaluating traditional repair techniques. Specifically, the patient achieved a near-full range of motion and a significant reduction in pain at 6 months post-surgery, which is a positive outcome. The observed outcomes in this case are consistent with the manufacturer’s reported data for the Regeneten implant, which indicate that the implant can promote tendon healing and improve clinical outcomes in patients with rotator cuff tears. Specifically, Smith and Nephew reports that Regeneten reduced the size of partial-thickness rotator cuff tears by over 50% in all tear locations [7]. The positive results observed in this case further support the use of Regeneten for the augmentation of rotator cuff repair. However, further research is needed to directly compare the outcomes of bioinductive implants with mechanical repair alone and other augmentation techniques.
This study has several limitations, including the small sample size, the lack of a control group, and the relatively short follow-up period. Additionally, the patient in this case was a 66-year-old female, and the results may not be generalizable to all patients with rotator cuff tears. Despite these limitations, we believe that this case report provides valuable information about the potential benefits of bioinductive implants in the treatment of rotator cuff tears, stimulating further research and large-scale studies to validate our preliminary findings. Future studies with larger cohorts and longer follow-up periods are needed to confirm these results and to investigate the long-term efficacy of bioinductive implants in rotator cuff surgery.
The outcomes observed in this case are consistent with those reported in other studies evaluating the use of bioinductive implants for rotator cuff tears. Compared to mechanical repair alone, bioinductive implants offer the potential for enhanced healing and reduced re-tear rates, particularly in cases of poor tissue quality or large tears. However, further research is needed to directly compare the outcomes of bioinductive implants with other augmentation techniques, such as tendon transfers or patch grafting.
4. Conclusions
The application of bioinductive implants signifies a shift from traditional, purely mechanical repair methods toward biologically oriented approaches, underscoring the increasing emphasis on biological interventions in the treatment of rotator cuff tears. These implants have the potential to mitigate the challenges associated with poor tendon quality and reduced vascularity, thereby enhancing both short- and long-term outcomes. This leads to a decrease in re-tear rates, improved shoulder function, and greater patient satisfaction. In the case presented, the incorporation of a bioinductive implant into a partial repair procedure facilitated substantial tendon regeneration, contributing to superior structural and functional results. This method aligns with the growing trend of incorporating biological augmentation into surgical strategies to optimize long-term outcomes.
Ongoing research should aim to refine the criteria for patient selection, identify reliable predictors of surgical success, and evaluate the long-term effectiveness of bioinductive implants. As surgical techniques advance, a deeper comprehension of the interaction between biological and mechanical factors will be crucial for attaining durable and functionally optimal outcomes for patients suffering from rotator cuff pathology. By addressing the complex challenges inherent in tendon repair, bioinductive implants offer a promising innovation in the management of this intricate condition.
Author Contributions
Conceptualization, A.C. (Arianna Carnevale) and U.G.L.; methodology, G.M. and U.G.L.; software, M.M. and A.C. (Arianna Carnevale); validation, A.C. (Alice Ceccaroli), A.C. (Alessandra Corradini) and L.M.; formal analysis, P.D.; investigation, M.A.R.I.; resources, E.S.; data curation, M.M., A.C. (Alice Ceccaroli) and A.C. (Alessandra Corradini); writing—original draft preparation, A.C. (Arianna Carnevale); writing—review and editing, G.M.; visualization, L.M.; supervision, U.G.L.; project administration, U.G.L.; funding acquisition, U.G.L. and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Fondazione Policlinico Universitario Campus Bio-Medico (protocol code 04.23, date of approval 25 January 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Frazier, L.P.; Quigley, R.A.; Galvin, J.W.; Waterman, B.R.; Brusalis, C.M.; Cole, B.J. Put a Patch on It!: When and How to Perform Soft-Tissue Augmentation in Rotator Cuff Surgery. Oper. Tech. Sports Med. 2023, 31, 150984. [Google Scholar] [CrossRef]
- McIntyre, L.F.; McMillan, S.; Trenhaile, S.W.; Bishai, S.K.; Bushnell, B.D. Full-Thickness Rotator Cuff Tears Can Be Safely Treated With a Resorbable Bioinductive Bovine Collagen Implant: One-Year Results of a Prospective, Multicenter Registry. Arthrosc. Sports Med. Rehabil. 2021, 3, e1473–e1479. [Google Scholar] [CrossRef] [PubMed]
- Thon, S.G.; Belk, J.W.; Bravman, J.T.; McCarty, E.C.; Savoie, F.H., III. Regeneten bio-inductive collagen scaffold for rotator cuff tears: Indications, technique, clinical outcomes, and review of current literature. Ann. Jt. 2020, 5, 41. [Google Scholar]
- Kim, I.B.; Kim, M.W. Risk Factors for Retear After Arthroscopic Repair of Full-Thickness Rotator Cuff Tears Using the Suture Bridge Technique: Classification System. Arthroscopy 2016, 32, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Chacon, J.A.; Cuenca-Espierrez, J.; Roda-Rojo, V.; Martin-Martinez, A.; Calderon-Meza, J.M.; Alvarez-Alegret, R.; Martin-Hernandez, C. Bioinductive collagen implants facilitate tendon regeneration in rotator cuff tears. J. Exp. Orthop. 2022, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Prabhath, A.; Vernekar, V.N.; Sanchez, E.; Laurencin, C.T. Growth factor delivery strategies for rotator cuff repair and regeneration. Int. J. Pharm. 2018, 544, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, T.F.; Abrams, J.S.; Angelo, R.L.; Getelman, M.H.; Ho, C.P.; Bushnell, B.D. Isolated bioinductive repair of partial-thickness rotator cuff tears using a resorbable bovine collagen implant: Two-year radiologic and clinical outcomes from a prospective multicenter study. J. Shoulder Elb. Surg. 2021, 30, 1938–1948. [Google Scholar] [CrossRef]
- Bushnell, B.D.; Bishai, S.K.; Krupp, R.J.; McMillan, S.; Schofield, B.A.; Trenhaile, S.W.; McIntyre, L.F. Treatment of Partial-Thickness Rotator Cuff Tears With a Resorbable Bioinductive Bovine Collagen Implant: 1-Year Results From a Prospective Multicenter Registry. Orthop. J. Sports Med. 2021, 9, 23259671211027850. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Ibán, M.; García Navlet, M.; Moros Marco, S.; Diaz Heredia, J.; Hernando Sánchez, A.; Ruiz Díaz, R.; Comino, C.V.; Ojeda, M.L.R.; Bello, G.d.M.; Lafuente, J.L.Á. Augmentation of a Transosseous-Equivalent Repair in Posterosuperior Nonacute Rotator Cuff Tears With a Bioinductive Collagen Implant Decreases the Retear Rate at 1 Year: A Randomized Controlled Trial. Arthroscopy 2024, 40, 1760–1773. [Google Scholar] [CrossRef] [PubMed]
- Bokor, D.J.; Sonnabend, D.H.; Deady, L.; Cass, B.; Young, A.A.; Van Kampen, C.L.; Arnoczky, S. Healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a highly-porous collagen implant: A 5-year clinical and MRI follow-up. Muscles Ligaments Tendons J. 2019, 9, 338–347. [Google Scholar]
- Wu, G.; van der Helm, F.C.; Veeger, H.E.; Makhsous, M.; Van Roy, P.; Anglin, C.; Nagels, J.; Karduna, A.R.; McQuade, K.; Wang, X.; et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: Shoulder, elbow, wrist and hand. J. Biomech. 2005, 38, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M.; Harryman, D.T. Tendons, ligaments, and capsule of the rotator cuff. Gross and microscopic anatomy. J. Bone Jt. Surg. Am. 1992, 74, 713–725. [Google Scholar]
- Cha, S.W.; Lee, C.K.; Sugaya, H.; Kim, T.; Lee, S.C. Retraction pattern of delaminated rotator cuff tears: Dual-layer rotator cuff repair. J. Orthop. Surg. Res. 2016, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Nakamizo, H.; Horie, R. Comparison of En Masse Versus Dual-Layer Suture Bridge Procedures for Delaminated Rotator Cuff Tears. Arthroscopy 2018, 34, 3150–3156. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, Z.Y.; Ren, Y.M. Separate double-layer repair versus en masse repair for delaminated rotator cuff tears: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2020, 15, 171. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Wu, J.; Liu, Z.; Li, X.; Xiao, Y.; Shu, H.; Zhou, A.; Wang, T.; Nie, M. Outcomes After Double-Layer Repair Versus En Masse Repair for Delaminated Rotator Cuff Injury: A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2023, 11, 23259671231206183. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, N.; Massaroni, C.; Lo Presti, D.; Saccomandi, P.; Di Tomaso, G.; Zollo, L.; Perego, P.; Andreoni, G.; Schena, E. Wearable textile based on silver plated knitted sensor for respiratory rate monitoring. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 2865–2868. [Google Scholar] [CrossRef]
- Maffulli, N.; Longo, U.G. Conservative management for tendinopathy: Is there enough scientific evidence? Rheumatology 2008, 47, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, D.; Carnevale, A.; D’Abbraccio, J.; Massari, L.; Massaroni, C.; Sabbadini, R.; Zaltieri, M.; Di Tocco, J.; Bravi, M.; Miccinilli, S.; et al. A Multi-Parametric Wearable System to Monitor Neck Movements and Respiratory Frequency of Computer Workers. Sensors 2020, 20, 536. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).