Correlation Between the Severity of Flatfoot and Risk Factors in Children and Adolescents: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Study Selection and Data Extraction
2.3. Risk of Bias Assessment
2.4. Data Synthesis and Statistical Analysis
3. Results
3.1. Study Characteristics
- Flatfoot Diagnostic Methods:
- Risk Factors:
- ○
- BMI/Obesity: The most commonly assessed, with varying definitions of “overweight” and “obesity” (e.g., WHO cutoffs vs. local pediatric growth charts).
- ○
- Ligamentous Laxity: Tested via the Beighton scale or clinical hypermobility assessments [21].
- ○
- Footwear Habits: Some studies focused on footwear type (boots, sandals, or supportive shoes), while others examined usage frequency.
- ○
- Physical Activity: Measures such as hours per week of sporting activities or daily step counts.
- ○
- Other Biomechanical Factors: Heel valgus, subtalar fusion status, Achilles tendon morphometry.
| Author, Year | Design | N (Male/Female) | Mean Age (Range) | Risk Factor Assessed | Flatfoot Severity Metric | Prevalence (Mild/Moderate/Severe) | Correlation (r/OR/RR) | Risk of Bias | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | J.M. Morales Asencio et al., 2019 [22] | Case–Control | 104 (47/57) | 7.55 (6–9 years) | BMI, late onset of walking | Valgus index (pedigraphy) | 54.5% valgus deformity (right foot) | Obesity (OR: 9.08, p < 0.0001) | Low |
| 2 | Sadeghi-Demneh et al., 2016 [23] | Cross-Sectional | 667 (340/327) | 7–14 years | Heel valgus, dorsiflexion range | Pathological flatfoot (clinical/radiographic) | 10.3% total | High BMI (p < 0.01) | Moderate |
| 3 | Medina-Alcantara et al., 2019 [5] | Cross-Sectional | 132 (61/71) | 7.53 (6–8 years) | Footwear type, frequency | Valgus prevalence (unspecified method) | 45.5% valgus foot | Boots reduce valgus (p = 0.009) | Moderate |
| 4 | Kadhim et al., 2013 [24] | Retrospective | 24 patients (43 feet) | 11 (4.7–18.3 years) | Subtalar fusion vs. calcaneal lengthening | Coronal plane pressure index (CPPI) | Severe deformities | Subtalar fusion reduced pain | High |
| 5 | He et al., 2023 [25] | Cross-Sectional | 74 (40/34) | Adolescents (not specified) | BMI, ligamentous laxity | Meary’s angle, calcaneal valgus | Not specified | Ligamentous laxity (r = 0.413, p < 0.01) | Moderate |
| 6 | Abich et al., 2020 [26] | Cross-Sectional | 823 (Unspecified) | 11–15 years | BMI, footwear, physical activity | Staheli arch index | 17.6% total | Obesity (OR: 4.2, p < 0.001) | Low |
| 7 | Sadeghi-Demneh et al., 2015 [27] | Cross-Sectional | 667 (340/327) | 7–14 years | BMI, foot mechanics | Footprint method | 46% pathological | BMI: OR = 2.1, p < 0.01 | Moderate |
| 8 | Vergara-Amador et al., 2012 [28] | Cross-Sectional | 940 (Unspecified) | 3–10 years | Age, BMI, footwear habits | Clinical exam | 15.7% | Flatfoot associated with BMI, p < 0.05 | Moderate |
| 9 | Chen et al., 2009 [29] | Comparative Case Series | 1024 (549/475) | 5–13 years | Obesity, foot dimensions | 3D foot dimensions | 28% | Obesity significantly correlated (p < 0.01) | Low |
| 10 | Chen et al., 2014 [30] | Cross-Sectional | 605 (405/200) | 3–6 years | Motor development delay, obesity | Footprint measurement | 58.7% (decreasing with age) | Obese children: OR = 3.6, p < 0.001 | Low |
| 11 | Gonul et al., 2016 [31] | Case–Control | 59 (Unspecified) | 11.96 ± 2.44 years | Achilles tendon morphometry | Ultrasound cross-sectional area | Negative correlation with Achilles size | Negative with age (Beta = 1.96, p = 0.04) | Moderate |
| 12 | Birhanu et al., 2023 [32] | Cross-Sectional | 1022 (454/568) | 11–18 years | BMI, footwear, physical activity | Plantar arch index | 10.27% | Urban living (aOR = 2.42, p < 0.05) | Moderate |
| 13 | Yam et al., 2022 [33] | Case–Control | 121 (59/62) | 8.07 ± 1.10 years | Fat percentage, developmental coordination disorder | Foot Posture Index (FPI-6) | Higher in DCD group | FPI-6 related to fat percentage | Moderate |
| 14 | Alfageme-García et al., 2021 [34] | Longitudinal Cohort | 165 (89/76) | 5–11 years | Backpack use, pronated foot posture | Foot Posture Index (FPI) | 76 developed neutral foot posture | Backpack use (aOR = 1.94, p < 0.05) | Low |
| 15 | Puszczalowska-Lizis et al., 2022 [35] | Regression Analysis | 200 (100/100) | 6 years | Foot arch impact on toe position | Podoscope analysis | Sex differences in arching effects | Transverse arch impacts hallux valgus | Low |
| 16 | Villarroya et al., 2008 [17] | Cross-Sectional | 245 (130/115) | 13.2 ± 1.8 years | BMI, foot structure | Chippaux–Smirak index | Lower MLA in obese group | BMI and MLA (p < 0.01) | Low |
| 17 | García-Rodríguez et al., 1999 [36] | Cross-Sectional | 1181 (Unspecified) | 4–13 years | Over-treatment of flexible flatfoot | Denis classification grades | 2.7% true prevalence | Treatment mismatch (28.1%) | Moderate |
| 18 | El et al., 2006 [4] | Screening Study | 579 (299/280) | 9.23 ± 1.66 years | Hypermobility, hindfoot alignment | Dynamic weight-bearing arch assessment | Moderate–severe: 17.2% | Hypermobility increases risk (p < 0.05) | High |
| 19 | Chen et al., 2010 [37] | Cross-Sectional | 1598 (833/765) | 3–6 years | Age, sex, obesity, W-sitting | Weight-bearing medial arch assessment | 54.5% at age 3, 21% at age 6 | W-sitting increases risk (OR > 1) | Moderate |
| 20 | Halabchi et al., 2013 [21] | Cross-Sectional | 120 (Unspecified) | 6–10 years | Ligamentous laxity, footwear habits | Foot arch grading | 50% flexible flatfoot | Ligamentous laxity (p<0.01) | Moderate |
| 21 | Yan et al., 2013 [19] | Retrospective | 100 (54/46) | 8–13 years | Radiographic talonavicular angle | Talonavicular coverage angle | 10.3% symptomatic | Navicular angle OR = 1.89 | Moderate |
| 22 | Troiano et al., 2017 [38] | Cross-Sectional | 281 (139/142) | 4–20 years) | Age and sex effects on prevalence | Baropodometric analysis | Flatfoot: 31.7%, Hollow foot: 68% | Flatfoot risk (OR = 2.23) | Low |
| 23 | Shapouri et al., 2019 [39] | Cross-Sectional | 194 (112/82) | 6–7 years | Obesity and lower extremity deformities | Clinical observation | 13.38% | BMI linked to prevalence (OR = 1.89) | Moderate |
| 24 | Han et al., 2017 [40] | Cross-Sectional | 72 (32/40) | 15.4 ± 4.0 years | Heel valgus, arch index, Q-angle | Arch index, valgus measurement | Moderate–severe in adolescents | Heel valgus correlated with Q-angle (r = 0.81) | Moderate |
| 25 | Abolarin et al., 2011 [41] | Cross-Sectional | 560 (Mixed) | 6–12 years | Footwear habits and BMI | Footprint analysis | Significant in urban population | Urban living OR = 1.5 | Moderate |
| 26 | Alsuhaymi et al., 2019 [42] | Cross-Sectional | 403 (193/210) | 7–14 years | Age, sex, BMI | Staheli’s plantar index | 29.5% | BMI a significant predictor | Moderate |
| 27 | Chen et al., 2022 [43] | Retrospective Cohort | 69 patients (107 feet) | 7–14 years | Surgical success factors | Radiographic measures (Meary’s angle) | Significant improvement post-surgery | Meary’s angle improvement (p<0.001) | Moderate |
| 28 | Pfeiffer et al., 2006 [2] | Cross-Sectional | 835 (424/411) | 3–6 years | Age, sex, BMI (weight categories) | Rearfoot angle via laser scanner | 44% flexible flatfoot, <1% pathological | Higher BMI strongly linked to flatfoot prevalence (OR > 2) | Low |
| 29 | Evans & Karimi, 2015 [18] | Retrospective Analysis | 728 (Mixed) | 3–15 years | BMI, sex, Foot Posture Index | Foot Posture Index (FPI) | FPI ≥ +6 in 40%, ≥ +8 in 20% | Weak correlation BMI and FPI (r = −0.077) | Moderate |
| 30 | Drefus et al., 2017 [44] | Reliability Study | 30 (Mixed, 60 feet) | 9.61 ± 1.96 years | AHI in sitting/standing postures | Arch height index (AHI) | Moderate to severe: 21–24% | AHI ICC ≥ 0.76 | Low |
| 31 | Twomey et al., 2010 [45] | Comparative Study | 50 (25/25 Normal/Low Arch) | 11.1 ± 1.2 years | Kinematic differences during gait | Heidelberg foot measurement method | Low arch differences noted in gait | Forefoot supination significant (p < 0.03) | Low |
| 32 | Stavlas et al., 2005 [46] | Cross-Sectional | 5866 (Mixed) | 6–17 years | Foot morphology and growth | Dynamic footprints | Growth-related changes in prevalence | Significant growth-related differences (p < 0.05) | Moderate |
| 33 | Yin et al., 2018 [47] | Cross-Sectional | 1059 (Mixed) | 6–13 years | BMI, age, foot size | FootScan SAI ratio | FFF 39.5% at age 6 to 11.8% at age 12 | BMI positively correlates (OR 2.43 obese) | Moderate |
| 34 | Boryczka-Trefler et al., 2021 [48] | Prospective Cohort | 50 patients (100 feet) | 5–9 years | Static vs. Dynamic Flatfoot | Static and dynamic pedobarography | Static 87%, Dynamic 56% | Static vs. Dynamic metrics inconsistent | Low |
| 35 | Chang et al., 2014 [49] | Cross-Sectional | 1228 (Mixed) | 6–12 years | Bimodal footprint index distribution | Staheli’s and Chippaux–Smirak indices | Bimodal arch distribution noted | Arch indices stable across groups | Low |
| 36 | Tashiro et al., 2015 [50] | Cross-Sectional | 619 (311/308) | 11.3 ± 0.7 years | Toe grip strength | Staheli’s arch index | 17.8% flatfoot | Toe grip significantly lower in flatfoot | Moderate |
| 37 | Pauk et al., 2014 [51] | Cross-Sectional | 93 (60/33) | 9–16 years | Plantar pressure and Clarke’s angle | Clarke’s angle | Significant medial pressure in flatfoot | Clarke’s angle correlates with plantar pressure (r > 0.9) | Moderate |
3.2. Risk of Bias in Included Studies
3.3. Qualitative Synthesis of Key Findings
- BMI as a Risk Factor: Across the majority of included studies, elevated BMI was strongly associated with higher flatfoot prevalence or severity. In a study by Pfeiffer et al. (2006) [2], preschool children classified as overweight had a significantly higher chance of flexible flatfoot (OR > 2). Similarly, Leung et al. (2018) [16] found a 39.5% flatfoot prevalence at age 6, progressively declining with age, yet children with obesity exhibited persistently higher flattening rates.
- Ligamentous Laxity: Although fewer studies formally assessed ligamentous laxity, those that did (e.g., He et al. 2023 [25]) reported moderate correlations (r ≈ 0.4) with valgus deformity or reduced arch angles [20]. However, methodological inconsistencies, like different definitions of hypermobility, limit pooling.
- Footwear Habits: A few authors proposed that supportive footwear reduces hindfoot valgus, whereas minimalist or poorly fitted shoes can exacerbate pronation in overweight children [5]. Nonetheless, footwear influences were inconsistent, as some cross-sectional data showed negligible differences once BMI was controlled. Cultural norms (e.g., walking barefoot vs. wearing shoes indoors) may also modulate these findings, but data were insufficient for formal subgroup analysis.
- Physical Activity: Paradoxical findings arose, with some studies showing that sedentary children had weaker foot musculature and more pronounced flatfoot [52], while others suggested that intense sports might overload the immature foot structure, leading to arch strain in overweight individuals. Overall, the net effect of physical activity likely depends on weight status, foot muscle conditioning, and biomechanical alignment.
- Diagnostic Heterogeneity: The key limitations stem from the array of diagnostic criteria. Studies using the Foot Posture Index (FPI) often classified a broader range of mild pronation as “flatfoot”. Notably, pedobarography measurements tended to yield higher prevalence estimates, potentially reflecting increased sensitivity to weight-bearing factors (i.e., body mass). Radiographic definitions typically identified more moderate-to-severe deformities. This methodological discrepancy likely contributed to the wide prevalence range (10–54%) and influenced how BMI and other factors correlated with severity.
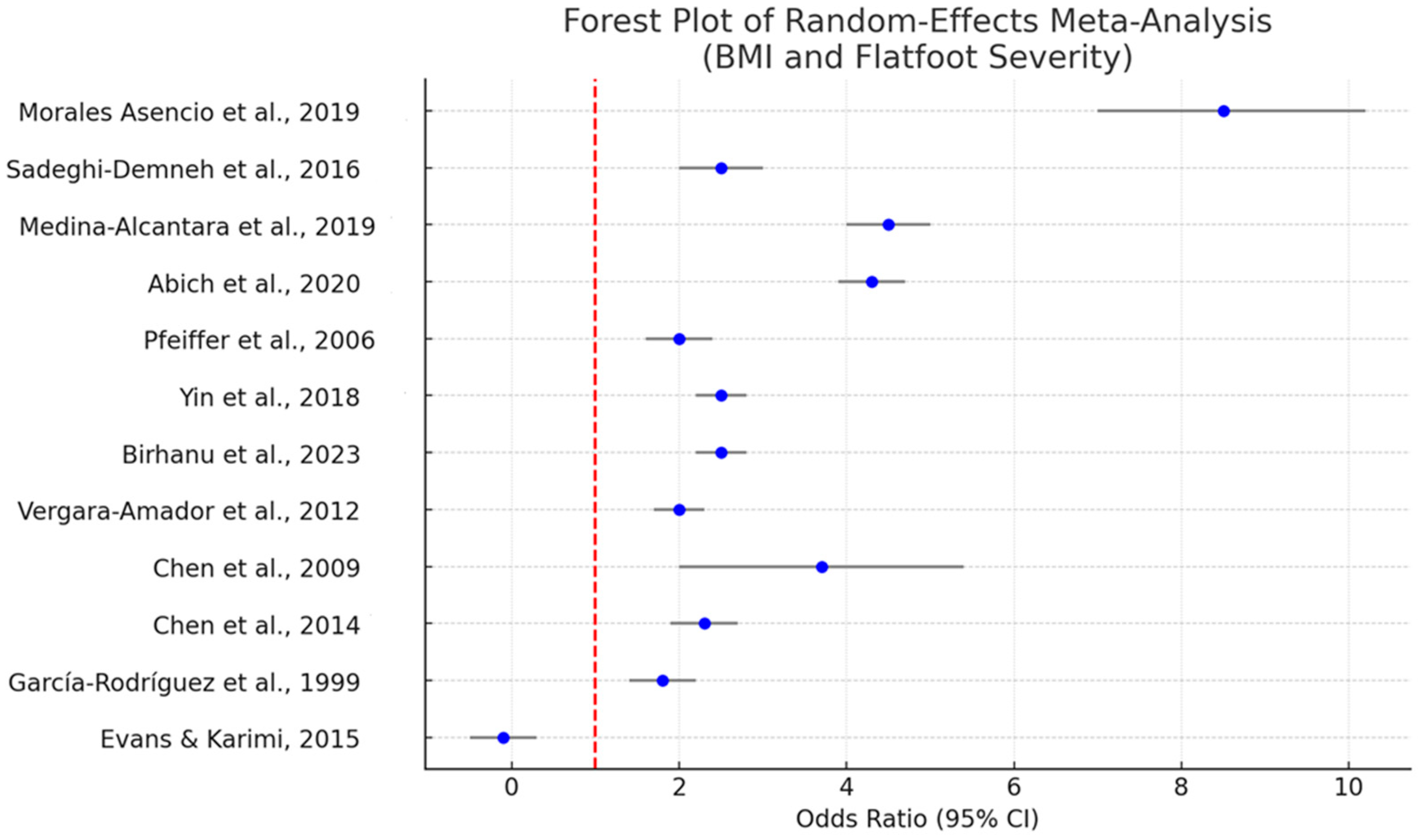
3.4. Quantitative Synthesis: Meta-Analysis of BMI
- Pooled OR for Overweight/Obesity: 2.3 (95% CI: 1.6–3.1), indicative of a significant association between elevated BMI and flatfoot (p < 0.001). Individual studies displayed ORs ranging from 1.7 to 9.08 [27]. One outlier study reported an OR of 9.08 for obese children; we included this estimate in our narrative synthesis, noting that it focused on a highly specific population sample.
- Heterogeneity (I2): 68.2%, suggesting substantial between-study variability. Potential drivers include differences in diagnostic methodologies, cutoffs for BMI/obesity, and population demographics.
- Subgroup Analysis:
- ○
- By Age (<10 years vs. ≥10 years): Studies focusing on younger children (<10 years) reported higher baseline prevalence of “flexible” flatfoot, possibly reflecting normal developmental stages. In contrast, studies including older children and adolescents (≥10 years) indicated that elevated BMI was more strongly linked to persistent or severe flatfoot deformities (ORs ranging 2.0–4.2).
- ○
- By Diagnostic Method (FPI vs. Footprint/Radiograph): Studies using the FPI reported lower effect sizes (OR ≈ 2.0) than those using footprints or radiographs (OR ≈ 3.1), hinting that certain metrics might capture more clinically significant deformities.
- Publication Bias: Egger’s test (p = 0.061) suggested a mild possibility of small-study effects, but the funnel plot did not reveal overt asymmetry. Sensitivity analyses, excluding high risk of bias studies, slightly reduced the heterogeneity (I2 = 52.5%) but did not change the direction of the main effect.
4. Discussion
4.1. Strengths and Limitations
4.2. Clinical and Research Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, A.M. The Flat-Footed Child—To Treat or Not to Treat. J. Am. Podiatr. Med. Assoc. 2008, 98, 386–393. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Kotz, R.; Ledl, T.; Hauser, G.; Sluga, M. Prevalence of Flat Foot in Preschool-Aged Children. Pediatrics 2006, 118, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.C.; Durward, B.R.; Watt, G.F.; Donaldson, M.D.C. Anthropometric Foot Structure of Peripubescent Children with Excessive versus Normal Body Mass. J. Am. Podiatr. Med. Assoc. 2007, 97, 366–370. [Google Scholar] [CrossRef]
- El, O.; Akcali, O.; Kosay, C.; Kaner, B.; Arslan, Y.; Sagol, E.; Soylev, S.; Iyidogan, D.; Cinar, N.; Peker, O. Flexible flatfoot and related factors in primary school children: A report of a screening study. Rheumatol. Int. 2006, 26, 1050–1053. [Google Scholar] [CrossRef]
- Medina-Alcantara, M.; Morales-Asencio, J.M.; Jimenez-Cebrian, A.M.; Paez-Moguer, J.; Cervera-Marin, J.A.; Gijon-Nogueron, G.; Ortega-Avila, A.B. Influence of Shoe Characteristics on the Development of Valgus Foot in Children. J. Clin. Med. 2019, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Shin, H.S.; Ko, J.H.; Cha, Y.H.; Ahn, J.H.; Hwang, J.Y. Gait Analysis of Symptomatic Flatfoot in Children: An Observational Study. Clin. Orthop. Surg. 2017, 9, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Catan, L.; Amaricai, E.; Onofrei, R.R.; Popoiu, C.M.; Iacob, E.R.; Stanciulescu, C.M.; Cerbu, S.; Horhat, D.I.; Suciu, O. The Impact of Overweight and Obesity on Plantar Pressure in Children and Adolescents: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 6600. [Google Scholar] [CrossRef]
- Chen, K.-C.; Tung, L.-C.; Yeh, C.-J.; Yang, J.-F.; Kuo, J.-F.; Wang, C.-H. Change in flatfoot of preschool-aged children: A 1-year follow-up study. Eur. J. Pediatr. 2013, 172, 255–260. [Google Scholar] [CrossRef]
- Evans, A.M.; Rome, K.; Peet, L. The foot posture index, ankle lunge test, Beighton scale and the lower limb assessment score in healthy children: A reliability study. J. Foot Ankle Res. 2012, 5, 1–5. [Google Scholar] [CrossRef]
- Staheli, L.T.; Chew, D.E.; Corbett, M. The longitudinal arch. A survey of eight hundred and eighty-two feet in normal children and adults. J. Bone Joint Surg. Am. 1987, 69, 426–428. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Leung, A.K.L.; Cheng, J.C.Y.; Mak, A.F.T. A cross-sectional study on the development of foot arch function of 2715 Chinese children. Prosthet. Orthot. Int. 2005, 29, 241–253. [Google Scholar] [CrossRef]
- Villarroya, M.A.; Esquivel, J.M.; Tomás, C.; Buenafé, A.; Moreno, L. Foot structure in overweight and obese children. Pediatr. Obes. 2008, 3, 39–45. [Google Scholar] [CrossRef]
- Evans, A.M.; Karimi, L. The relationship between paediatric foot posture and body mass index: Do heavier children really have flatter feet? J. Foot Ankle Res. 2015, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.-S.; Yang, Z.; Lu, M.; Zhang, J.-L.; Zhu, Z.-H.; Guo, Y. Relationship between symptoms and weight-bearing radiographic parameters of idiopathic flexible flatfoot in children. Chin. Med. J. 2013, 126, 2029–2033. [Google Scholar] [CrossRef]
- Kim, M.H.; Cha, S.; Choi, J.E.; Jeon, M.; Choi, J.Y.; Yang, S.-S. Relation of Flatfoot Severity with Flexibility and Isometric Strength of the Foot and Trunk Extensors in Children. Children 2022, 10, 19. [Google Scholar] [CrossRef]
- Halabchi, F.; Mazaheri, R.; Mirshahi, M.; Abbasian, L. Pediatric Flexible Flatfoot; Clinical Aspects and Algorithmic Approach. Iran. J. Pediatr. 2013, 23, 247–260. [Google Scholar] [PubMed]
- Asencio, J.M.M.; Medina-Alcántara, M.F.; Ortega-Avila, A.B.; Jimenez-Cebrian, A.M.; Moguer, J.P.; Cervera-Marin, J.A.; Gijon-Nogueron, G. Anthropometric and Psychomotor Development Factors Linked to Foot Valgus in Children Aged 6 to 9 Years. J. Am. Podiatr. Med. Assoc. 2019, 109, 30–35. [Google Scholar] [CrossRef]
- Sadeghi-Demneh, E.; Azadinia, F.; Jafarian, F.; Shamsi, F.; Melvin, J.M.A.; Jafarpishe, M.; Rezaeian, Z. Flatfoot and obesity in school-age children: A cross-sectional study. Clin. Obes. 2016, 6, 42–50. [Google Scholar] [CrossRef]
- Kadhim, M.; Holmes, L.; Miller, F. Long-term Outcome of Planovalgus Foot Surgical Correction in Children with Cerebral Palsy. J. Foot Ankle Surg. 2013, 52, 697–703. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, W.; Teraili, A.; Wang, X.; Wang, C. Correlation between flat foot and patellar instability in adolescents and analysis of related risk factors. J. Orthop. Surg. 2023, 31. [Google Scholar] [CrossRef] [PubMed]
- Abich, Y.; Mihiret, T.; Akalu, T.Y.; Gashaw, M.; Janakiraman, B. Flatfoot and associated factors among Ethiopian school children aged 11 to 15 years: A school-based study. PLoS ONE 2020, 15, e0238001. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Demneh, E.; Jafarian, F.; Melvin, J.M.A.; Azadinia, F.; Shamsi, F.; Jafarpishe, M. Flatfoot in School-Age Children. Foot Ankle Spec. 2015, 8, 186–193. [Google Scholar] [CrossRef]
- Amador, E.V.; Sánchez, R.F.S.; Posada, J.R.C.; Molano, A.C.; Guevara, O.A. Prevalence of flatfoot in school between 3 and 10 years. Study of two different populations geographically and socially. Colomb. Medica 2012, 43, 141–146. [Google Scholar] [CrossRef]
- Chen, J.-P.; Chung, M.-J.; Wang, M.-J. Flatfoot Prevalence and Foot Dimensions of 5– to 13-Year-Old Children in Taiwan. Foot Ankle Int. 2009, 30, 326–332. [Google Scholar] [CrossRef]
- Chen, K.-C.; Tung, L.-C.; Tung, C.-H.; Yeh, C.-J.; Yang, J.-F.; Wang, C.-H. An investigation of the factors affecting flatfoot in children with delayed motor development. Res. Dev. Disabil. 2014, 35, 639–645. [Google Scholar] [CrossRef]
- Gonul, Y.; Yucel, O.; Eroglu, M.; Senturk, I.; Eroglu, S.; Dikici, O.; Cartilli, O.; Ulasli, M. Ultrasonographic evaluation of Achilles tendon in children with flatfoot: A case-control morphometric study. Diagn. Interv. Imaging 2016, 97, 907–913. [Google Scholar] [CrossRef]
- Birhanu, A.; Nagarchi, K.; Getahun, F.; Gebremichael, M.A.; Wondmagegn, H. Magnitude of flat foot and its associated factors among school-aged children in Southern Ethiopia: An institution-based cross-sectional study. BMC Musculoskelet. Disord. 2023, 24, 966. [Google Scholar] [CrossRef]
- Yam, T.T.T.; Fong, S.S.M.; Tsang, W.W.N. Foot posture index and body composition measures in children with and without developmental coordination disorder. PLoS ONE 2022, 17, e0265280. [Google Scholar] [CrossRef]
- Alfageme-García, P.; Calderón-García, J.F.; Martínez-Nova, A.; Hidalgo-Ruiz, S.; Basilio-Fernández, B.; Rico-Martín, S. Association between the Use of Backpack and Static Foot Posture in Schoolchildren with Static Pronated Foot Posture: A 36-Month Cohort Study. Children 2021, 8, 800. [Google Scholar] [CrossRef] [PubMed]
- Puszczalowska-Lizis, E.; Krawczyk, K.; Omorczyk, J. Effect of Longitudinal and Transverse Foot Arch on the Position of the Hallux and Fifth Toe in Preschool Children in the Light of Regression Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1669. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodriguez, A.; Martin-Jimenez, F.; Carnero-Varo, M.; Gomez-Gracia, E.; Gomez-Aracena, J.; Fernandez-Crehuet, J. Flexible Flat Feet in Children: A Real Problem? Pediatrics 1999, 103, e84. [Google Scholar] [CrossRef]
- Chen, K.-C.; Yeh, C.-J.; Tung, L.-C.; Yang, J.-F.; Yang, S.-F.; Wang, C.-H. Relevant factors influencing flatfoot in preschool-aged children. Eur. J. Pediatr. 2011, 170, 931–936. [Google Scholar] [CrossRef]
- Troiano, G.; Nante, N.; Citarelli, G.L. Pes planus and pes cavus in Southern Italy: A 5 years study. Ann. Ist. Super Sanita 2017, 53, 142–145. [Google Scholar] [CrossRef]
- Shapouri, J.; Aghaali, M.; Aghaei, M.; Iranikhah, A.; Ahmadi, R.; Hovsepian, S. Prevalence of Lower Extremities’ Postural Deformities in Overweight and Normal Weight School Children. Iran. J. Pediatr. 2019, 29, 6. [Google Scholar] [CrossRef]
- Han, Y.; Duan, D.; Zhao, K.; Wang, X.; Ouyang, L.; Liu, G. Investigation of the Relationship Between Flatfoot and Patellar Subluxation in Adolescents. J. Foot Ankle Surg. 2017, 56, 15–18. [Google Scholar] [CrossRef]
- Abolarin, T.; Aiyegbusi, A.; Tella, A.; Akinbo, S. Predictive factors for flatfoot: The role of age and footwear in children in urban and rural communities in South West Nigeria. Foot 2011, 21, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Alsuhaymi, A.; Almohammadi, F.; Alharbi, O.; Alawfi, A.; Olfat, M.; Alhazmi, O.; Khoshhal, K. Flatfoot among school-age children in Almadinah Almunawwarah: Prevalence and risk factors. J. Musculoskelet. Surg. Res. 2019, 3, 204. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, J.; Fu, S.; Wang, C.; Su, Y.; Mei, G.; Xue, J.; Zou, J.; Li, X.; Shi, Z. HyProCure for Pediatric Flexible Flatfoot: What Affects the Outcome. Front. Pediatr. 2022, 10, 857458. [Google Scholar] [CrossRef] [PubMed]
- Drefus, L.C.; Kedem, P.; Mangan, S.M.; Scher, D.M.; Hillstrom, H.J. Reliability of the Arch Height Index as a Measure of Foot Structure in Children. Pediatr. Phys. Ther. 2017, 29, 83–88. [Google Scholar] [CrossRef]
- Twomey, D.; McIntosh, A.; Simon, J.; Lowe, K.; Wolf, S. Kinematic differences between normal and low arched feet in children using the Heidelberg foot measurement method. Gait Posture 2010, 32, 1–5. [Google Scholar] [CrossRef]
- Stavlas, P.; Grivas, T.B.; Michas, C.; Vasiliadis, E.; Polyzois, V. The Evolution of Foot Morphology in Children Between 6 and 17 Years of Age: A Cross-Sectional Study Based on Footprints in a Mediterranean Population. J. Foot Ankle Surg. 2005, 44, 424–428. [Google Scholar] [CrossRef]
- Yin, J.; Zhao, H.; Zhuang, G.; Liang, X.; Hu, X.; Zhu, Y.; Zhang, R.; Fan, X.; Cao, Y. Flexible flatfoot of 6–13-year-old children: A cross-sectional study. J. Orthop. Sci. 2018, 23, 552–556. [Google Scholar] [CrossRef]
- Boryczka-Trefler, A.; Kalinowska, M.; Szczerbik, E.; Stępowska, J.; Łukaszewska, A.; Syczewska, M. How to Define Pediatric Flatfoot: Comparison of 2 Methods: Foot Posture in Static and Dynamic Conditions in Children 5 to 9 Years Old. Foot Ankle Spec. 2023, 16, 43–49. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chen, Y.-C.; Yang, W.-T.; Ho, P.-C.; Hwang, A.-W.; Chen, C.-H.; Chang, J.-H.; Chang, L.-W. Flatfoot Diagnosis by a Unique Bimodal Distribution of Footprint Index in Children. PLoS ONE 2014, 9, e115808. [Google Scholar] [CrossRef]
- Tashiro, Y.; Fukumoto, T.; Uritani, D.; Matsumoto, D.; Nishiguchi, S.; Fukutani, N.; Adachi, D.; Hotta, T.; Morino, S.; Shirooka, H.; et al. Children with flat feet have weaker toe grip strength than those having a normal arch. J. Phys. Ther. Sci. 2015, 27, 3533–3536. [Google Scholar] [CrossRef]
- Pauk, J.; Ihnatouski, M.; Najafi, B. Assessing Plantar Pressure Distribution in Children with Flatfoot Arch. J. Am. Podiatr. Med. Assoc. 2014, 104, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-F.; Chen, C.-Y.; Chen, W.-Y.; Lin, H.-C. Lower extremity kinematics in children with and without flexible flatfoot: A comparative study. BMC Musculoskelet. Disord. 2012, 13, 31. [Google Scholar] [CrossRef]
- Mosca, V.S. Flexible flatfoot in children and adolescents. J. Child. Orthop. 2010, 4, 107–121. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuca, G.; Marletta, D.A.; Zampogna, B.; Sanzarello, I.; Nanni, M.; Leonetti, D. Correlation Between the Severity of Flatfoot and Risk Factors in Children and Adolescents: A Systematic Review. Osteology 2025, 5, 11. https://doi.org/10.3390/osteology5020011
Giuca G, Marletta DA, Zampogna B, Sanzarello I, Nanni M, Leonetti D. Correlation Between the Severity of Flatfoot and Risk Factors in Children and Adolescents: A Systematic Review. Osteology. 2025; 5(2):11. https://doi.org/10.3390/osteology5020011
Chicago/Turabian StyleGiuca, Gabriele, Daniela Alessia Marletta, Biagio Zampogna, Ilaria Sanzarello, Matteo Nanni, and Danilo Leonetti. 2025. "Correlation Between the Severity of Flatfoot and Risk Factors in Children and Adolescents: A Systematic Review" Osteology 5, no. 2: 11. https://doi.org/10.3390/osteology5020011
APA StyleGiuca, G., Marletta, D. A., Zampogna, B., Sanzarello, I., Nanni, M., & Leonetti, D. (2025). Correlation Between the Severity of Flatfoot and Risk Factors in Children and Adolescents: A Systematic Review. Osteology, 5(2), 11. https://doi.org/10.3390/osteology5020011








