Abstract
SiO2 is a versatile inorganic substance with a wide spectrum of applications in areas such as fuel cells. In this study, pristine (p-SiO2) and sulfonated silica (s-SiO2) particles were synthesised using the sol–gel and Stober methods. Furthermore, this study investigated the impact of calcination time and surface changes on the morphology, and hence functionality, of silica particles synthesised as potential fuel cell membrane additives. Tetraethyl orthosilicate (TEOS) was used as a silica precursor dissolved in water, with sulfuric acid serving as the sulfonation agent. Parametric data on particle morphology, such as particle size, porosity, total surface area, and agglomeration, were measured and evaluated using BET, Fourier-transform infrared (FTIR), X-ray diffraction (XRD), and scanning electron microscopy (SEM). The amorphous nature of silica particles was confirmed by XRD analysis. The BET outcome data acquired for the synthesised silica particles were a surface area ranging from 271 to 487 m2/g, a pore diameter of 12.10–21.02 nm, and a total pore volume of 0.76–1.58 cm3/g. These data give crucial characteristics for designing appropriate silica nanofillers for hybrid fuel cell membranes. As a result, the data gathered can be used to make future decisions about silica synthesis methods for various specific applications, such as fuel cell applications.
1. Introduction
Silica exists ubiquitously in the environment in different forms. Apart from not being present in nascent form, it is always present in compounds such as hydroxides, silicic acid, or oxides, as in silica [,]. The Earth’s crust is made up of 78% silicon and oxygen compounds []. Silica particles are prominent in scientific study due to their ease of preparation and vast range of industrial applications including in catalysis, pigments, pharmaceuticals, electronic and thin-film substrates, electronics, thermal insulators, and humidity sensors. Recently, silica has attracted strong interest as a candidate for fuel cell membrane applications. Such fuel cell membranes include sulfonated membranes (SPEEK, SPS, SPAES, SPI), fluorinated membranes (Nafion), and various organic polymer matrixes [,]. In fuel cells, silica is the most used inorganic filler, particularly in proton exchange membrane fuel cells and direct alcohol fuel cells []. This is owing to the amenable properties of silica such as its high stability, excellent conductivity, and ubiquitous nature (lower costs). Furthermore, silica particles can be modified to improve the electrical and morphological properties of membranes [,]. For example, sulfonic-group-functionalised silica particles show improved qualities, which makes them more suitable as an additive (guest) in polymer membranes. The introduction of sulfonated silica particles in chitosan polymers, for example, increases the tensile strength, thermal stability, and proton conductivity [,,]. Subsequently, these changes have the overall effect of enhancing fuel cell performance [,].
According to the literature, traditional processes such as flame synthesis, sol–gel procedures, the Stober process, and microemulsions have been used to produce silica [,]. The sol–gel and Stober processes, for example, involve the simultaneous condensation and hydrolysis of an alkoxide [,] in which the rate of condensation depends on the specific reaction conditions. Depending on the specific conditions employed, the formation of a three-dimensional network or a single monodisperse particle is possible [].
Although it follows the sol–gel principles, the Stober method, unlike the simple sol–gel method, is a special sol–gel process of silica synthesis with a specific outlined route. This method is initiated by a hydrolysis reaction involving the formation of silanol groups (Si-OH) from the reaction of TEOS with water (Equation (1)). The Stober method differs from the simple sol–gel method in that there is the controlled (dropwise) addition of an ammonia catalyst. This is critical with respect to the resultant properties of the silica produced. This step is followed by a condensation reaction in which the formed silanol groups react with either TEOS or with each other to form siloxane (Si-O-Si) groups, as shown in Equations (2) and (3), respectively. After condensation, the formed silica nuclei undergo nucleation and growth to form silica particles. At this stage, tailoring of the silica surface is usually conducted by introducing different functional groups. It is noteworthy that factors such as the temperature, NH3 concentration, TEOS concentration, and water-to-TEOS ratio can be optimised to infer different characteristic variables on the final silica particles.
Si(OC2H5)4 + H2O → Si(OH)(OC2H5)3 + C2H5OH
Si-OH + Si(OC2H5)4 → Si-O-Si + C2H5OH
Si-OH + Si-OH → Si-O-Si + H2O
The sol–gel technique is commonly employed to produce silica particles because it is a simple and effective method. Unlike the Stober method, the NH3 catalyst is pre-added to the water prior to the addition of TEOS in the sol–gel process. Therefore, there is less control of the catalyst concentration, resulting in the formation of silica with different physicochemical properties. The merits of this method include its effectiveness at lower temperatures, its simplicity, the fast reaction, and the production of relatively pure silica. In addition, the reaction kinetics can be regulated by changing the composition of the reaction mixture []. The quality of the silica produced is greatly influenced by parameters such as temperature, the time taken during condensation and hydrolysis, the solvent concentration, and the techniques employed for washing and drying. Typical silica characteristics affected by these parameters include the particles’ size and their size distribution [,]. The disadvantage of this method is that at higher (excess) TEOS concentrations, i.e., when TEOS surpasses a critical value, another population of particles appears, with completely different morphology and functionality properties.
A recent study by Therese and co-authors [] revealed enhancing effects due to the presence of S-SiO2 in a composite polymer made from sulfonated poly (ether ketone) (SPEEK) and poly (amide imide) (PAI) in fuel cells. There was a marked improvement in current density, power density, conductivity, and chemical stability with moderate swelling. These findings agree with those of Su et al. [], where the enhancing effects of S-SiO2 in a sulfonated poly (phthalazinone ether ketone) composite membrane were reported in a proton exchange fuel cell membrane (PEMFC). Notable increases in conductivity and thermal stability were highlighted.
Notwithstanding the reported advances in silica-bearing nanocomposite membranes, no study has yet been conducted to thoroughly explore the individual influence of silica as a function of its synthesis method, sulfonation, or its calcination parameters in the overall composite membrane. Based on these fundamentals, this study therefore seeks to explore the threefold effects of the sulfonation of silica, synthesis method of silica, and, finally, calcination time on silica particles as viable membrane nanofillers for fuel cell applications.
2. Materials and Methods
2.1. Reagents
Tetraethyl orthosilicate, Si(OC2H5)4, 98% (Merck, Darmstadt, Germany); ammonia, NH3, 25% (Merck); ethanol, C2H5OH, 99.9% (Merck); methanol, CH3OH, 99.9% (Merck); sulfuric acid, H2SO4, 99% (Merck).
2.2. Characterisation
The surface morphology, particle size, and form of the silica nanoparticles were examined using a scanning electron microscope (EVO-18, Carl Zeiss, Oberkochen, Germany). The XRD patterns of these particles were acquired using a GBC Emma X-ray Diffractometer, which was outfitted with a monochromatic Cu k X-ray detector (=1.5206 at 35.5 KV and 28 mA). Fourier-transform infrared (FTIR) spectroscopy (IR Tracer-100, Shimadzu, Nakagyō-ku, Japan) was used to identify chemical bonds at frequencies ranging from 500 to 4000 cm−1. Physical properties such as surface area, pore volume, and diameter were characterised using the Brunauer–Emmett–Teller (BET) method. The electrochemical properties of the prepared SiO2 particles were characterised by cyclic voltammetry in 0.1 M KCl in the presence of 5 mM ferri/ferricyanide at 50 cm·s−1 using FTO as the working electrode. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were performed on an Ivium pocketstat2 potentiostat. A glassy carbon electrode (GCE) was used as a substrate that was connected to the potentiostat (Ivium, Eindhoven, The Netherlands) along with a Ag/AgCl (3 M) reference electrode and platinum wire as the counter electrode.
2.3. Silica Nanoparticle Synthesis
2.3.1. Synthesis of Pristine Silica Particles by Sol–Gel Method
Pure silica particles were synthesised using tetraethyl orthosilicate (TEOS) as a precursor. This process includes the hydrolysis and condensation of TEOS. To a volume of 80 mL TEOS in a 400 mL beaker, an ammonia solution was added to make an NH3:TEOS molar ratio of 1:2. This was followed by the addition of 200 mL ethanol at room temperature, and the resulting solution was agitated for 30 min. Thereafter, the mixture was agitated for another 1 h at 70 °C, resulting in the formation of a gel. The obtained gel-like product (silica) was dried at 100 °C for 24 h to form silica crystals. These silica particles were then calcinated at 600 °C for 2 h and 24 h to remove volatile contaminants.
2.3.2. Synthesis of Unmodified Silica Particles by Stober Method
TEOS was used as the precursor for silica during the Stober process. An 80 mL mixture of TEOS and ethanol was stirred for 30 min at room temperature in a 400 mL beaker. This was followed by the dropwise addition of ammonia solution (2.75 mL) whilst stirring. The ammonia–TEOS molar ratio was 5:1. The mixture was further agitated for another hour at room temperature. After that, the obtained mixture was centrifuged for 31 min 44 s at 3700 rpm. The precipitate (white solid) silica was oven-dried at 100 °C for 24 h and calcinated at 600 °C for a duration of either 2 h or 24 h. The obtained crystals were ground to powdered silica particles using a mortar and pestle and stored at room temperature.
2.3.3. Sulfonation of Silica Particles
The prepared silica particles were sulfonated using sulfuric acid to fabricate their surfaces for enhanced proton conductivity. The sulfonation process for particles produced by the sol–gel and Stober processes was the same. For this process, 10 g of silica particles was mixed with 5ml of sulfuric acid and 200 mL of methanol. The mixture was stirred vigorously at 1500 rpm for 4 h, followed by the centrifugation of the sulfonated silica particles for 31 min 44 s.
3. Results for Nanoparticles Calcinated for 2 h
3.1. FTIR Results
The FTIR spectra of the synthesised silica particles (Stober and sol–gel methods) are shown in Figure 1. The stretch at 3400 cm−1 of Figure 1a,c,d corresponds to the OH− band due to the adsorbed water molecules. It can also be ascribed to the OH− stretching vibration of the silanol (Si-OH) groups in silica. It can also be attributed to the sulfonic acid’s OH− groups present on the surface of the sulfonated silica particles. This is the reason why this peak is more prominent in Figure 1a,d. This band (3400 cm−1) is missing in Figure 1b,c. This suggest that this frequency band is predominantly due to the stretching of the OH− groups of sulfonic acid introduced by the sulfonation process, since only the silica in (a) and (d) was functionalised with the sulfonated groups. This serves as a confirmation of the successful sulfonation of the s-SiO2 in Figure 1a,d []. It is also important to mention the possible role of adsorbed water molecules in the sulfonated silica particles. This is due to the fact that sulfonated particles have a higher affinity for water adsorption and retention than pristine silica. This, therefore, means that the OH stretch from the adsorbed water could have aided the silanol OH groups in producing a more pronounced overall stretch at 3400 cm−1 [,].
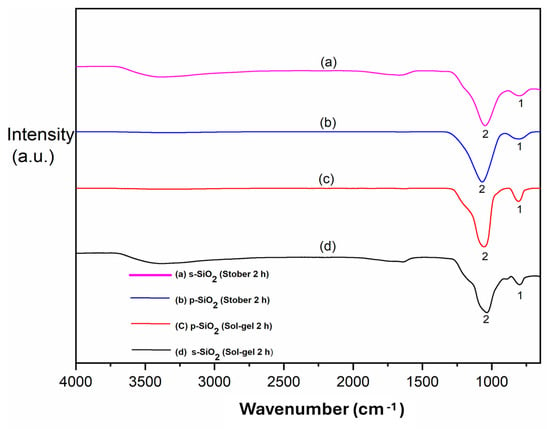
Figure 1.
FTIR results for different silica nanoparticles after calcination for 2 h: (a) s-SiO2—Stober; (b) SiO2—Stober; (c) s-SiO2—sol–gel; (d) and s-SiO2—sol–gel.
The siloxane bond (Si-OH) exhibits a stretching vibration, which correlates to the strong band at 804 cm−1, as shown in Figure 1a–d. The silica matrix’s structural support is provided by this band []. However, it is interesting to note that the intensities of this band are reduced in the corresponding SiO2 (a) and s-SiO2 (b) spectra due to the elimination of volatile components during the calcination of silica. Finally, the main peaks located at 1038, 1072, 1052, and 1047 cm−1 in Figure 1a–d, respectively, are caused by the bending vibrations of Si-O-Si. Based on these observations made from the FTIR spectra in Figure 1, it can be concluded that silica particles were successfully synthesised and that the sulfonation of these particles was successful, as shown in Figure 1a,d.
3.2. XRD of Pure and Sulfonated Silica
Figure 2 indicates the X-ray diffraction (XRD) patterns of silica particles (SiO2 and s-SiO2) synthesised by the sol–gel and Stober processes. As seen in Figure 2a–d, the silica particles’ XRD patterns display standard diffraction peaks that correspond to amorphous nano-silica at a Bragg’s angle (2θ) of 22° for both sulfonated and pristine silica particles synthesised using both the sol–gel and Stober methods and calcinated for 2 h at 600 °C. All four spectra of silica in Figure 2a–d show a broad humped peak, which confirms the short-range ordering of amorphous silica. This implies that the synthetic particles are made of amorphous silica, as reported in the literature [,]. The amorphous nature of silica is also confirmed by the broadened FTIR peaks observed at ≈1050 cm−1 in Figure 1. In contrast, the crystalline substance produces a sharp peak.
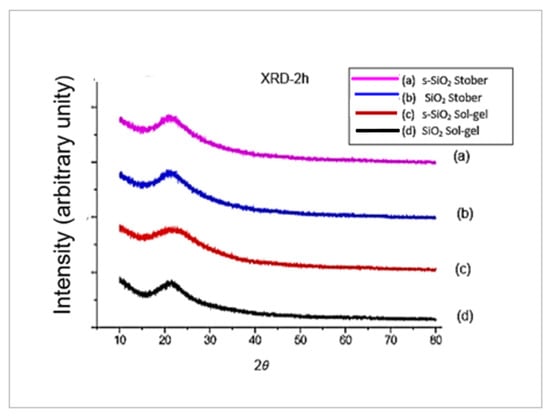
Figure 2.
XRD patterns after calcination for 2 h: (a) s-SiO2—Stober; (b) SiO2—Stober; (c) s-SiO2—sol–gel; and (d) SiO2—sol–gel.
3.3. SEM Results for Pure and Sulfonated Silica
Figure 3 demonstrates the SEM results for silica particles produced by the sol–gel and Stober methods, calcinated for 2 h. The SEM images for the sol–gel method in Figure 3a,b indicate an uneven distribution of the amorphous silica particles. The amorphous nature of the particles can be verified by the irregular morphology and the smooth particles, as opposed to crystalline particles which have more regular shapes with sharp edges. As seen in Figure 3, the general morphology of both sulfonated and pristine silica particles shows rounded/spherical discrete particles and clusters of agglomerated particles. It can also be observed that the particles synthesised using the sol–gel method (Figure 3a,b) exhibit a slightly larger particle size than the ones synthesised using the Stober method (Figure 3c,d). This observation agrees with the particle size distribution in Figure 4. The numerical data of the average silica particle sizes of these particles were determined using ImageJ2 software and the particle size distributions presented in Figure 4. The average particle size increased in the order p-SiO2 (Stober), s-SiO2 (Stober), s-SiO2 (sol–gel), and finally p-SiO2 (sol–gel), showing averages of 1.0 µm, 1.2 µm, 2.1 µm, and 2.3 µm, respectively. These results agree with the literature, which highlights that the Stober mechanism is the most efficient method for controlling silica particle size, compared to the sol–gel method [,]. Consequently, the smaller-sized silica particles synthesised by the Stober mechanism have a higher surface area compared to the ones synthesised using the sol–gel method, since it is generally accepted that the smaller the particles, the larger the surface area [,]. The larger surface area improves the catalytic activity and the ultimate fuel cell membrane performance of these particles, thereby making silica particles more potent. On the other hand, however, the smaller-sized Stober-synthesised silica particles show a higher tendency for particle agglomeration, which leads to a reduced surface area. The trade-off between an improved surface area with decreasing particle size and the adverse effect of agglomeration is usually inevitable. It is noteworthy that the improved surface area effects predominated over the agglomeration effects in this study, since there is a marked improvement in surface area at a lower particle size.
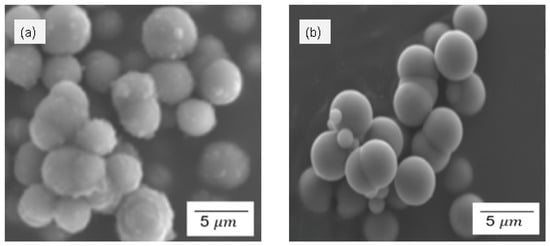
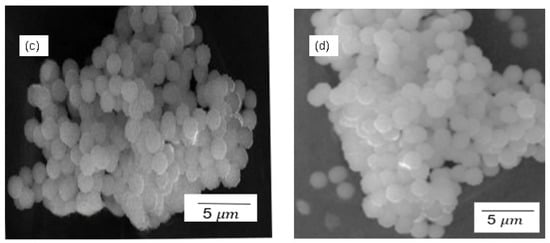
Figure 3.
SEM for (a) SiO2—sol–gel; (b) s-SiO2—sol–gel; (c) SiO2—Stober; and (d) s-SiO2—Stober—all calcinated for 2 h.
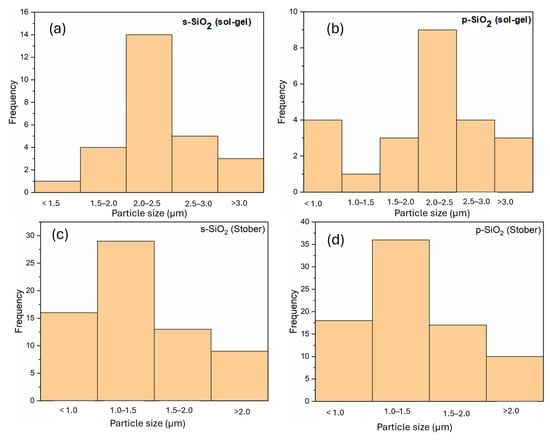
Figure 4.
Size distribution of silica particles calcinated for 2 h.
3.4. BET Results for Pristine and Sulfonated Silica Calcinated for 2 h
The results for particle parameters, such as surface area, total pore volume, and average pore diameter, of the synthesised silica particles (calcinated for 2 h) are shown in Table 1. This data represents averages calculated from triplicate measurements. Their average pore diameters ranged in size from 10.67 nm to 21.00 nm, agreeing with the literature [,,]. The average pore size information suggests that silica is a mesoporous substance. A mesoporous material is one whose pores are smaller than 50 nm in diameter, according to the International Union of Pure and Applied Chemistry []. It is noteworthy that the sulfonated silica particles show smaller average surface areas and pore volumes compared to pristine SiO2 particles, with SiO2 having an average surface area of 487 m2/g and the corresponding surface area of sulfonated silica being 271 m2/g. The two also have average pore volumes of 1.56 and 0.76 cm3/g, respectively. On the same note, it can also be observed generally that silica particles with higher average surface areas have a higher average pore volume and a small pore diameter in turn. Similar results were reported in the literature [,]. The high average surface area of SiO2 is likely due to the inductive effect of the preformed silica network in SiO2 []. Silica-based mesoporous particles are characterised by high specific surface areas, facile and varied surface chemistry modifications, a narrow pore size distribution, customisable properties of the pore network, and great biocompatibility with a low incidence of non-specific or negative effects []. Another trend is also shown in the average pore volumes of the sulfonated silica particles (both sol–gel- and Stober-synthesised) versus the pristine silica particles. The sulfonated silica particles show a lower average pore volume and surface area compared to the non-sulfonated ones. This is because crosslinking sulfonated groups may occupy some of the pore spaces of the fabricated silica particles, thereby reducing the average pore volumes [,]. Due to these reported average pore volumes, smaller particle sizes, and large surface area of the synthesised silica particles, they are promising performance-enhancing candidates for application in fuel cells.

Table 1.
BET results for the comparison of the physical parameters of silica particles synthesised through two different methods after 2 h of calcination.
3.5. Results for Silica Calcinated for 24 h
FTIR Results for Pure and Sulfonated Silica
Figure 4 shows the FTIR spectra of pristine (5a) and sulfonated (5c) silica nanoparticles synthesised using the sol–gel and Stober ((c) and (d)) methods. The notable peak at 3410 cm−1 in the Figure 5b spectrum corresponds to OH vibrations, possibly due to adsorbed moisture on the nanoparticles. This is the sol–gel-synthesised silica with sulfonated surfaces. This is supported by the fact that the sulfonation of the silica makes the nanoparticles more hygroscopic. Alternatively, this peak may be the result of the hydrolysis of TEOS, which varies with the concentration of H2O and NH3 in the solution. As the concentration of NH3 increases, H2O dissociates, releasing more OH− ions, which attack the Si atoms and accelerate hydrolysis []. The asymmetric Si-O (1001 cm−1), asymmetric Si-OH (996 cm−1), and symmetric Si-O (778 cm−1) vibrations are more visible in the amorphous silica particles in Figure 5b, while the corresponding spectra (a, c, and d) are not visible, except that of the symmetric Si-O band of a, b, c, and d, which is around 778 cm−1. The superimposition of distinct SiO2 peaks and Si-OH bonding peaks, caused by leftover organic groups, is recognised as the origin of the absorption bands between 800 and 1270 cm−1 in Figure 5a–d.
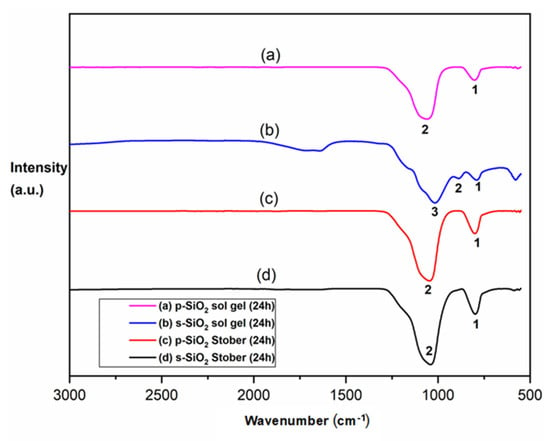
Figure 5.
FTIR spectra of silica particles after calcination for 24 h: (a) p-SiO2—sol–gel; (b) s-SiO2—sol–gel; (c) p-SiO2—Stober; and (d) s-SiO2—Stober.
3.6. XRD of Pure and Sulfonated Silica Calcinated for 24 h
Diffraction patterns of silica nanoparticles produced using the sol–gel and Stober methods and calcinated for 24 h are demonstrated in Figure 6. The pure and sulfonated silica particles shown in Figure 6 bear an amorphous structure due to a continuum random network, which maintains a crystalline connection and distributes the geometric parameters throughout a range []. The amorphous peak of these particles is situated at 2θ = 20° []. These findings are consistent with the results obtained in the literature [,], which reported that the amorphous silica has a 2θ hump between 15° and 35°. Also, the outcome is consistent with the silica standard pattern established by the Joint Committee on Powder Diffraction Standards (JCPDS). Amorphous nano-silica particles can function as a nucleus for silicic acid when the right amount of H2O is added to NH3 at the right temperature, resulting in microspheres with rounded and smooth surfaces. When nano-silica is formed, silicic acid acts as a nucleating agent, creating a substrate on which symmetric particles can grow [].
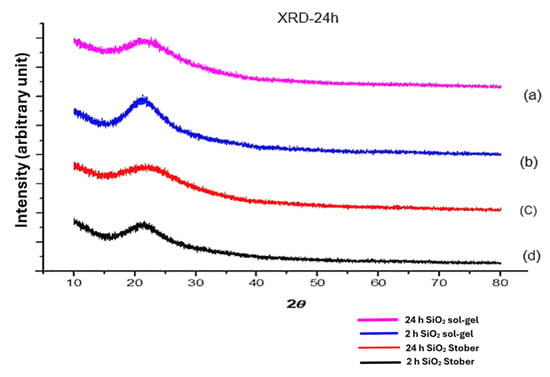
Figure 6.
X-ray diffraction patterns for silica particles calcinated for 2 h (b,d) versus 24 h (a,c): (a) p-SiO2—sol–gel; (b) s-SiO2—sol–gel; (c) p-SiO2—Stober; and (d) s-SiO2—Stober.
3.7. SEM of Pure and Sulfonated Silica Calcinated for 24 h
The surface morphology of the sol–gel- and Stober-synthesised silica particles is shown in Figure 7 The same SEM images can be used for the comparison of the morphology of the pristine versus sulfonated silica particles, calcinated for 24 h. Since replica silica particles calcinated for 2 h have been previously reported, by extension, comparative studies can therefore be conducted on the influence of the calcination time on the morphology of silica particles.
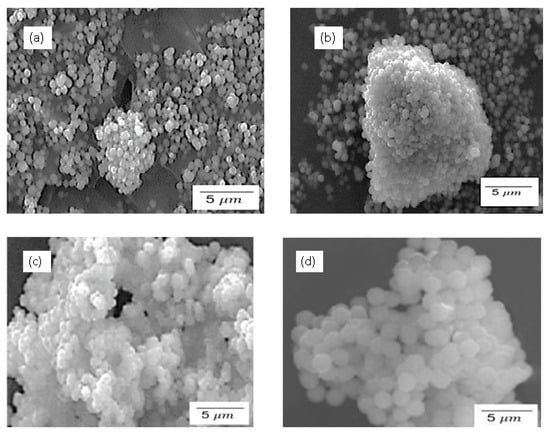
Figure 7.
SEM images of different forms of silica showing particles calcinated for 24 h, synthesised using different methods: (a) s-SiO2—sol–gel; (b) p-SiO2—sol–gel; (c) p-SiO2—Stober; and (d) s-SiO2—Stober.
Approximately equal particle sizes of silica synthesised using the sol–gel method were observed for both the pristine silica and the sulfonated silica (Figure 7a,b). Compared to the silica particles synthesised using the Stober method (Figure 7c,d), these particles have relatively smaller sizes. This can be explained by the fact that the Stober method is a more efficient way of synthesising silica with regulated particle sizes []. This can be achieved by altering the ratios of ethanol, water, ammonia, and tetraethyl orthosilicate during synthesis.
The surface morphology of sulfonated silica shows particles that are more spherical (rounded) and smooth than the pristine silica particles. Compared to the pristine silica particles (p-SiO2), the sulfonated particles (s-SiO2) show an almost uniform particle size. Furthermore, there is a lower degree of agglomeration on the SEM images of the sulfonated silica compared to the pure silica counterparts. The pure (pristine) silica particles in Figure 7b,c synthesised by the sol–gel and Stober processes, respectively, show variety in the size and shape of the particles; thus, both of the synthesis methods were unable to create silica particles that have a uniform size distribution. Previous studies [] show the same behaviour of silica. These silica particles in (b) and (c) tend to agglomerate, and this is attributed to silica having high energy and surface tension. The silica particles in Figure 7a–d indicate a smooth surface morphology.
Using ImageJ2 software, the average particle sizes were found to be 0.5 µm, 0.3 µm, 0.6 µm, and 1.2 µm, respectively, for s-SiO2—sol–gel; p-SiO2—sol–gel; p-SiO2—Stober; and s-SiO2—Stober. The respective silica particle size distributions are shown in Figure 8. For the sol–gel-synthesised silica particles, only a slight difference in the particle sizes is observed between the sulfonated and pristine counterparts. This trend is different for the silica particles synthesised using the Stober method, where the sulfonated particles are significantly larger (1.2 µm) than the pristine silica ones (0.6 µm). This observed difference in particle size in the p- and s-Stober silica may arise because of sulfonation. The additional sulfonic acid layer on the silica particles, and their increased interactions, results in increased particles. However, the same silica samples do not show a big deviation in particle size when calcinated for 2 h due to the presence of impurities, which equally affect both the s- and p-silica. This means that the impurity effects are more dominant than the sulfonation effect with shorter calcination times (2 h). Compared to the silica particles calcinated for 2 h (Section 3.3), there are variations in particle size with the ones calcinated for 24 h for both the sol–gel- and Stober-synthesised silica. There is a trend of a reduction in particle size with increasing calcination time. This trend may be attributed to the effects of the complete removal of impurities with longer calcination times (24 h).
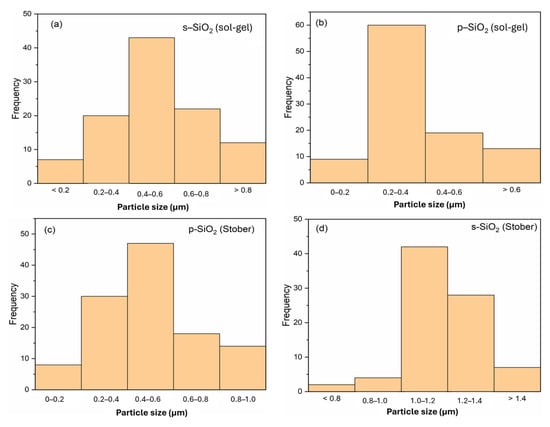
Figure 8.
Size distribution of silica particles calcinated for 24 h.
3.8. BET Results for Pristine and Sulfonated Silica Calcinated for 24 h
BET measurements were applied for the determination of particle parameters such as the surface area and pore volume. Three samples were measured for each class of silica particles, and the average pore volumes, total pore volumes, and surface areas were calculated. From these results, comparisons were then made for the morphology of the sulfonated versus pristine silica and silica synthesised using the sol–gel or the Stober method. The results are summarised in Table 2.

Table 2.
BET results to demonstrate the differences in the sulfonated versus pristine silica and silica synthesised from the sol–gel versus the Stober method. All the nanoparticles were calcinated for 24 h.
The samples’ BET average surface area and the average pore volume of the silica particles calcinated for 24 h are demonstrated in Table 2. High average surface area and volume of sol–gel and Stober silica particles were recorded. Table 2 further indicates that when silica is modified by sulfonation, the particle surface area decreases compared to the pristine silica for both s- and p-SiO2. This may be because the introduction of sulfonic acid groups promotes agglomeration in the particles. Consequently, this leads to a reduced surface area, since the agglomerated particles have a limited surface area. Also observed at this calcination time (24 h) is that there are no significant differences in the surface areas of s-SiO2 and p-SiO2 although the particle sizes are different. This can be explained by considering the role of other particle parameter differences such as the surface roughness, agglomeration, or shape. Another trend is observed between the total pore volumes of p- and s-SiO2 synthesised using both methods. Sulfonated silica has higher pore volumes than pristine silica for both synthesis methods. This may possibly be due to the electrostatic repulsion of the introduced negatively charged sulfonic acid groups, changes in the structure of silica, or the effects of sulfonation in removing some impurities, thereby opening some spaces previously occupied.
The introduction of sulfonic groups is therefore an added advantage for the targeted application of silica in fuel cell membranes. Overall, the obtained large surface areas and pore volumes in this work make silica a suitable membrane filler for fuel cell applications. For example, the large silica filler surface area minimises methanol permeability, as silica tends to absorb methanol on its surface, which will reduce the methanol permeability of the membrane. The surface areas obtained in this work are generally higher than the ones reported in most of the literature. Dhaneswara et al. and Usgodaarachchi et al. [,] reported silica surface areas of 236 m2/g and 222 m2/g, respectively, which are smaller than the ones found in this report. The differences can be attributed to the different synthesis methods employed.
3.9. Calcination Time Effects
Calcination time is a critical parameter in the synthesis of silica particles. It significantly impacts the morphology and, hence, the activity of silica particles. For instance, increasing the calcination time can lead to the production of a smaller particle size due to the complete removal of organic pollutants on the particles. Such findings were observed in titanium oxide particles when calcinated at 500 °C for 1 h compared to 5 h. The particle diameter was reduced by nearly fourfold from 83 ± 5 nm to 23 ± 2 nm. The results in this work (Figure 9) likewise show the parametric effects of calcination time on the morphology of the synthesised silica particles between 2 h and 24 h. A significant decrease in the silica particles was observed. These results be explained using the same model of reasoning, i.e., that the reduction in size upon prolonged calcination is due to complete contaminant removal. On the other hand, research also suggest that increasing the calcination time may cause the production of larger particles. This is because longer calcination times tend to result in particle aggregation (agglomeration), thereby increasing the particle size.
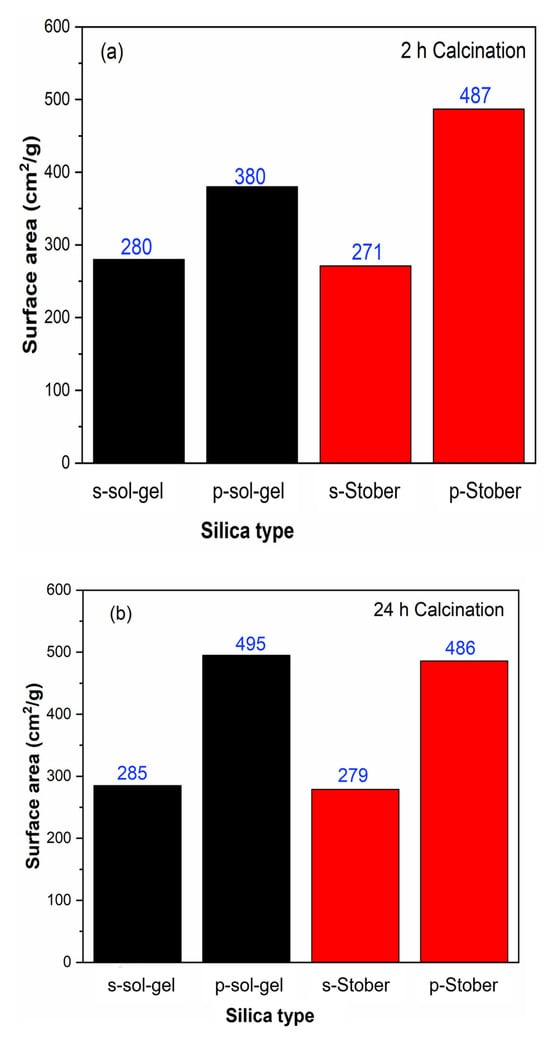
Figure 9.
Effects of calcination time on the surface area of pristine and sulfonated silica particles synthesised using the sol–gel and Stober methods. The duration of calcination at 600 °C was (a) 2 h and (b) 24 h.
Since particle size affects the particle surface area, and by extension the surface reactions occurring on the particles, it follows that surface area is a critical metric for the evaluation of silica particle activity.
In this study, there is a general silica particle surface area increment with increasing calcination time from 2 h to 24 h, observed when comparing Table 1 and Table 2, in addition to the further information in Figure 9. This effect is more apparent on the pristine silica synthesised using the sol–gel method, which improved from 380 to 495 cm2/g. This can be explained in terms of the reduced particle size with increasing calcination time. This has a direct impact on the surface area of the particles, since surface area is inversely proportional to particle size. Notably, the same trend was observed for the overall particle pore volumes. An overall increase in the pore volume was observed with increasing calcination time from 2 h to 24 h. Similarly, this is attributed to the reduced particle size at an increased calcination time. The smaller the particle size, the larger the total pore volume.
It is noteworthy that the Stober-synthesised silica particles show a higher surface area compared to the sol–gel-synthesised silica particles for both the pristine and the sulfonated particles. There is no significant difference between the surface areas of the 2 h and the 24 h calcinated particles synthesised using the Stober mechanism, whilst the sol–gel-synthesised silica particles showed an enhanced surface area with a longer calcination time. This may be explained in terms of the differences in the impurity composition between the sol–gel-synthesised silica and the Stober-synthesised silica. Such impurities can include organic pollutants which form a surface coating on the silica particles. Hence, upon their removal during prolonged calcination, the ‘de-coated’ silica particles then appear smaller and have a larger surface area. To this end, only the pristine silica particles synthesised by the sol–gel method showed a marked improvement in surface area upon increasing the calcination time from 2 h to 24 h.
3.10. Effects of Sulfonation on Silica Nanoparticles
Compared to the pristine silica particles, the sulfonated silica particles showed a lower surface area for both the Stober- and sol–gel-synthesised silica particles. This effect was observed under different calcination times. The sulfonation process introduces a negatively charged layer around the particles. The electrostatic interaction between the negatively charged SO3− groups and the partially positive Si in the SiO2 results in strong adherence of the sulfonation layer around the silica particles. This ultimately results in the overall enlargement of the sulfonated silica particles. This particle enlargement effect is further supported by the crosslinked interactions of the SO3− groups and the neighbouring silica particles, which leads to the clustering of the particles (agglomeration). Consequently, the synergy between these factors leads to a reduction in the silica particle surface area, since surface area is inversely proportional to particle size.
Finally, there is no significant difference between the morphologies of the pristine and sulfonated silica particles. Almost equal and rounded (spherical) particles were obtained from both sets of particles, proving that the sulfonation process does not interfere with the overall particle shape.
3.11. Total Pore Volume
The total pore volume (TPV) is a critical parameter of particle performance, since it informs of the size of the empty spaces surrounding the silica particles. Generally, the TPV is a function of particle size, and it is also used to determine the level of agglomeration of the particles, since agglomerated particles have a compromised TVP. The higher the TVP, the more suitable the particles are for chemical reactions, including membrane reactions in fuel cells. This is based on the fact that larger pore volumes allow better mass flow to occur. Both the reactants and products percolate freely to and from the active sites of the particles at higher pore volumes, thereby improving the activity of the particles.
From Figure 10, the results in this study show that the TPV varies with the calcination duration, especially for the unsulfonated (pristine) silica particles synthesised using the sol–gel method. This is due to the connection between the particle size and pore volume. The same particles had improved surface area upon increased calcination time, an indication of the particle size reduction effect. As explained earlier, this reduction in particle size is attributed to the complete removal of pollutants surrounding the particles. Because the TPV is inversely proportional to pore volume, this occurrence is accompanied by an increased pore volume, which will subsequently result in improved performance of the silica particles. However, no improved TPV was recorded for the sulfonated particles with increased calcination time. This may be explained in terms of limited to no organic pollutants in sulfonated silica particles. As a result, no significant loss of pollutants is observed upon increased sulfonation, to create more empty volumes (pore volumes) upon increased calcination. Also, unlike the sol–gel-synthesised silica particles, there is no significant change in the TPV of the Stober-synthesised silica particles upon increased calcination time. Fundamentally, this is caused by the higher ability of the Stober method to produce highly controlled particle sizes (smaller sizes) with high surface area and pore volumes. Since these parameters are intrinsically connected to the applied method and less dependent on the calcination time, it follows naturally that increasing the calcination time does not alter the TPV by any significant amount.
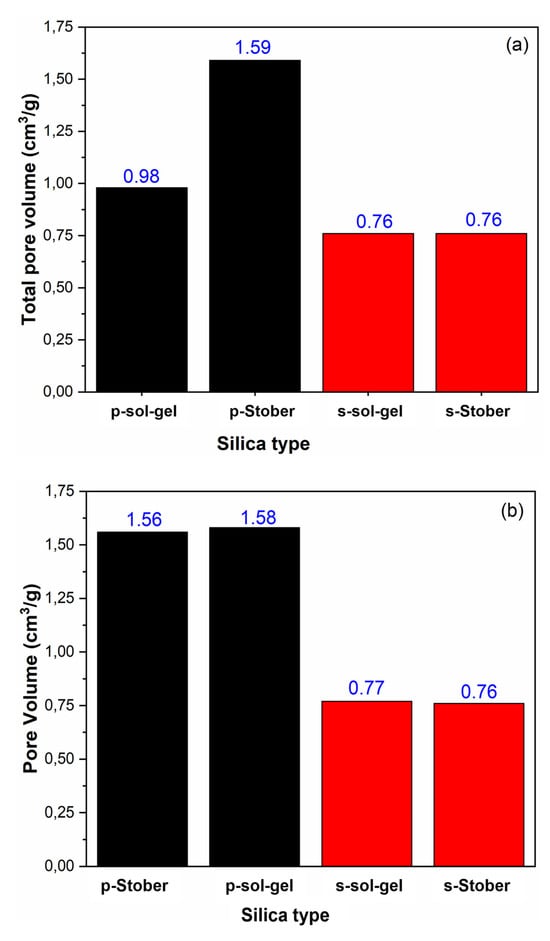
Figure 10.
Effects of the silica synthesis method and sulfonation on the total pore volume for different calcination times: (a) 2 h and (b) 24 h.
3.12. Stober Versus Sol–Gel Synthesis Method
With a 2 h calcination time, the Stober method produces silica particles with higher pore volumes than those produced by the sol–gel method. There is a general increase in the TPV with increasing calcination time from 2 h to 24 h for both methods. As the calcination time is increased to 24 h, the silica pore volumes become independent of the synthesis method. This could possibly be because the Stober method produces more pure silica particles (with less pollutants) compared to the sol–gel method. There is a marked difference in the surface areas of the pristine silica particles between the Stober method and the sol–gel method, especially with shorter (2 h) calcination times. There is, however, no significant difference in the silica particles synthesised by the Stober method versus the sol–gel method. The total pore volume of p-SiO2 synthesised by the Stober method is slightly higher than that of the sol–gel-synthesised counterpart. As stated earlier in this work, this is attributed to the precise superiority of the Stober method in controlling particle parameters during synthesis. However, no notable difference in the total pore volume is observed for s-SiO2 due to the differences in the synthesis method for both shorter and longer calcination times. This suggest that the sulfonation process uniformly introduces different structural changes for both the Stober and sol–gel processes.
It is therefore of paramount importance to highlight that neither method is entirely advantageous over the other in all areas. Considerations therefore are to be made based on the intended application of the silica produced. For instance, some of the literature highlights some of the advantages of using the sol–gel method over the Stober method, whilst some highlights the advantages of the Stober over the sol–gel method. For example, the sol–gel method is more flexible, since it accommodates more than one precursor alternative. In the same vein, the sol–gel method can be adjusted to influence the resulting silica particles’ morphology. Since each method has its own merits and demerits, it follows that the choice of the method employed is usually at the discretion of the user, based on the exact intended application of the silica particles.
3.13. Electrochemical Characterisation
The electrochemical properties of the prepared SiO2 particles were characterised by cyclic voltammetry in 0.1 M KCl in the presence of 5 mM ferri/ferricyanide at 50 cm.s−1 using FTO as the working electrode. It is well known in the electrocatalysis field that the electrocatalyst preparation method has a great effect on the properties of the resulting electrocatalyst. This effect is well observed in Figure 11a, using the Stober and sol–gel methods in the preparation of SiO2 particles. The particles prepared by the sol–gel method resulted in a low current density compared to the particles prepared by the Stober method for both the 2 h and 24 h calcination times, with the 2 h Stober-prepared electrocatalysts having the highest current density. The low current response of the sol–gel-prepared SiO2 is an indication of its low electrocatalytic activity. This proves that the Stober method is a suitable method for SiO2 preparation compared to the sol–gel method. Furthermore, these results prove that, indeed, the electrocatalyst preparation method plays a major role in its properties. Similarly, these results also demonstrate the strong effects of the calcination time on the performance of electrocatalysts. As observed in Figure 11, the voltammogram of the bare FTO electrode shows a quasi-reversible redox reaction, with high current density and oxidation–reduction potentials at 0.45 V and 0.01 V, respectively. However, upon electrode surface modification with SiO2 particles, a decrease in the current density and a peak position shift is observed as a result of the blocking effect of SiO2 particles. This effect is attributed to the electrostatic repulsion between the silanol group and the negatively charged redox probe []. Upon sulfonation (Figure 11b), the peak current increases due to the high pore diameter of the sulfonated SiO2 compared to the pristine SiO2 particles, confirmed by the BET results. Large pores reduce mass limitations, enhancing electrocatalytic activity. The high peak current response is also attributed to the favourable structural modification emanating from the electrostatic interactions between the negatively charged sulfur and the neutral SiO2, which enhances the charge transfer process within the particles compared to the pristine silica particles. Such changes promote diversity in the possible applications of the silica particles in various redox processes. Additionally, the increase in the current response is an indication of an enhanced electrochemical surface area (ECSA), which subsequently leads to improved reaction kinetics. It is of importance also to mention that the shift in peak position potentials is an indication of improved electrocatalytic activity. It is important to note that the sulfonated SiO2 particles calcined for 2 h showed a lower current response compared to the pristine SiO2. The low current response can be explained in terms of a small ECSA, since there is a linear relationship between the ECSA and the peak current response (the two are directly proportional).
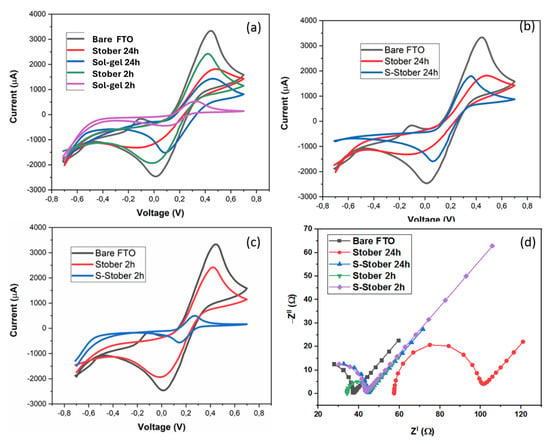
Figure 11.
Cyclic voltammograms of (a) bare FTO and SiO2 particles prepared by the Stober and sol–gel methods (b,c) and p-SiO2 and s-SiO2 particles prepared by the Stober method over 2 and 24 h. (d) Nyquist plot of p-SiO2 and s-SiO2 particles prepared by the Stober method.
Figure 11d shows a Nyquist plot of the Stober-prepared electrocatalysts over the 100 kHz to 0.01 Hz frequency range. Its peak shift shows improved electrochemical properties. It can be observed that the EIS spectra have two distinct regions, the semicircular and linear segments representing electron transfer and diffusion processes, respectively. The diameter of the semicircle is an indication of the electron transfer resistance (Rct) of the electrocatalysts. These values were determined using the equivalent circuit shown in Figure 11d (insert). The bare FTO electrode has a low Rct of 30.12 Ω. After electrode coating, the Rct increased slightly to 44.13 Ω, 30.57 Ω, and 32.35 Ω for Stober 24 h, s-Stober 24 h, and s-Stober 2 h, respectively, suggesting slight repulsive interactions between the redox probe and silanol groups and slow electron transfer. However, Stober 2 h had a small Rct (10.83 Ω), clear proof that the same materials prepared with different variables possess different properties. The small electron transfer resistance of Stober 2 h explains its high activity observed on the CV voltammograms. The high conductivity of the bare FTO electrode shows that FTO is a good electrode for electrocatalysis studies, as it will not greatly influence the electrochemical analysis results of the materials, enhancing optimal characterisation.
4. Conclusions
The synthesis of pristine and sulfonated silica particles using the sol–gel and Stober methods was successfully conducted. The obtained particles were subjected to calcination at 600 °C for 2 h and 24 h. The effects of the synthesis method, calcination time, and sulfonation of the silica particles on the particle morphology in terms of the surface area, particle size, total pore volume, and shape of the silica particles produced were evaluated. The particle sizes following calcination for 2 h were found to be in the range of 1.0 µm to 2.3 µm, whilst those of particles calcinated for 24 h ranged in size from 0.3 µm to 1.2 µm. On the other hand, the pore diameters for the particles calcinated for 2 h and 24 h ranged from 10.67 nm to 21.00 nm and 12.10 nm to 21.52 nm, respectively. Silica particles synthesised using the Stober and sol–gel methods calcinated for 2 h and 24 h have short-range ordering, resulting in them having amorphous structures. Synthesised silica particles tend to agglomerate, and this was more visible in silica particles calcinated for 2 h compared to the ones calcinated for 24 h using the same method. For both shorter and longer calcination times, sulfonated silica shows a larger total pore volume compared to pristine silica. However, no significant change in the particle surface area was observed between the sulfonated and pristine silica particles synthesised by both the sol–gel and Stober methods. On the other hand, the pristine silica particles have a higher surface area than the sulfonated silica particles. No significant difference was observed between the surface area of the sol–gel- and Stober-synthesised silica. The Stober method produced silica particles with slightly larger pore volumes than the sol–gel method.
The synthesis of pristine and sulfonated silica nanoparticles using the sol–gel and Stober methods was successfully conducted. The obtained nanoparticles were subjected to calcination at 600 °C for 2 h and 24 h. The effects of the synthesis method, calcination time, and sulfonation of the silica nanoparticles on the nanoparticle morphology in terms of the surface area (nanoparticle size), total pore volume, and shape of the silica nanoparticles produced were evaluated. The particles calcinated for 2 h and 24 h were found to be within the range of 12 nm to 21 nm, with the highest pore diameters recorded for silica calcinated for 24 h. Silica particles synthesised using the Stober and sol–gel methods calcinated for 2 h and 24 h have short-range ordering, resulting in them having amorphous structures. Synthesised silica particles tend to agglomerate, and this was more visible in silica particles calcinated for 2 h compared to the ones calcinated for 24 h using the same method, and no visible agglomeration was discovered after the surface modification of silica. For both shorter and longer calcination times, sulfonated silica shows a lower total pore volume compared to pristine silica. The same trend is true for the surface areas of the sulfonated versus pristine silica nanoparticles. On the other hand, the Stober method produces silica nanoparticles with a larger surface area than those produced by the sol–gel method. With a shorter calcination time, the Stober method produces silica nanoparticles with a larger total pore volume than those produced by the sol–gel method. However, the total pore volume becomes independent of the synthesis route with longer calcination times.
Author Contributions
L.M.: methodology, formal analysis, investigation, and original draft writing. C.M.: Formal analysis, investigation, R.S.: writing (review and editing). T.M. (Tebogo Mashola): Methodology, T.M. (Touhami Mokrani): Writing, review, and F.N.: editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the internal funding of the University of South Africa and the National Research Council of South Africa (NRF) Grant number: 150341.
Data Availability Statement
All data are share in this article.
Acknowledgments
The University of South Africa contributed financially. The TEM results (CSIR) UNISA for SEM are also acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ellerbrock, R.; Stein, M.; Schaller, J. Comparing amorphous silica, short-range-ordered silicates and silicic acid species by FTIR. Sci. Rep. 2022, 12, 11708. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, Z.; Ni, S.; Ren, B.; Hu, J. Origin, formation, and transformation of different forms of silica in Xuanwei Formation coal, China, and its’ emerging environmental problem. Environ. Sci. Pollut. Res. Int. 2023, 30, 120735–120748. [Google Scholar] [CrossRef]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of silicon in plant stress tolerance: Opportunities to achieve a sustainable cropping system. 3 Biotech 2019, 9, 73. [Google Scholar] [CrossRef]
- Ying, Y.P.; Kamarudin, S.K.; Masdar, M.S. Silica-related membranes in fuel cell applications: An overview. Int. J. Hydrogen Energy 2018, 43, 16068–16084. [Google Scholar] [CrossRef]
- Li, J.; Xu, G.; Luo, X.; Xiong, J.; Liu, Z.; Cai, W. Effect of nano-size of functionalized silica on overall performance of swelling-filling modified Nafion membrane for direct methanol fuel cell application. Appl. Energy 2018, 213, 408–414. [Google Scholar] [CrossRef]
- Kusumastuti, E.; Siniwi, W.T.; Mahatmanti, F.W.; Jumaeri, J.; Atmaja, L.; Widiastuti, N. Modification of chitosan membranes with nanosilica particles as polymer electrolyte membranes. In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2016. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Vlasova, N.N.; Golovkova, L.P.; Markitan, O.; Baryshnikov, G.; Ågren, H.; Slabon, A. Nucleotide Interaction with a Chitosan Layer on a Silica Surface: Establishing the Mechanism at the Molecular Level. Langmuir 2021, 37, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Modau, L.; Sigwadi, R.; Mokrani, T.; Nemavhola, F. Chitosan Membranes for Direct Methanol Fuel Cell Applications. Membranes 2023, 13, 838. [Google Scholar] [CrossRef]
- Blachnio, M.; Zienkiewicz-Strzalka, M.; Derylo-Marczewska, A.; Nosach, L.V.; Voronin, E.F. Chitosan–Silica Composites for Adsorption Application in the Treatment of Water and Wastewater from Anionic Dyes. Int. J. Mol. Sci. 2023, 24, 818. [Google Scholar] [CrossRef]
- Da Silva, A.D.S.; Santos, J.H.Z.D. Silica particle size and polydispersity control from fundamental practical aspects of the Stöber method. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 135190. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Raimundo, I.M.; Pimentel, M.F. Revising the synthesis of Stöber silica nanoparticles: A multivariate assessment study on the effects of reaction parameters on the particle size. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 1–7. [Google Scholar] [CrossRef]
- Gonçalves, M.C. Sol-gel silica nanoparticles in medicine: A natural choice. design, synthesis and products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef]
- St, E.; Nanoparticles, S.; Mechanism, G.; Nanostructures, S.; Imaging, M. Engineering Stöber Silica Nanoparticles: Insights into the Growth Mechanism and Development of Silica-Based Nanostructures for Multimodal Imaging Oscar Hernando Moriones Botero. Doctoral Dissertation, Universitat Autònoma de Barcelona, Barcelona, Spain, 2022. [Google Scholar]
- Mujiyanti, D.R.; Surianthy, M.D.; Junaidi, A.B. The Initial Characterization of Nanosilica from Tetraethylorthosilicate (TEOS) with the Addition Polivynil Alcohol by Fourier Transform Infra Red. IOP Conf. Ser. Earth Environ. Sci. 2018, 187, 012056. [Google Scholar] [CrossRef]
- Spitzmüller, L.; Nitschke, F.; Rudolph, B.; Berson, J.; Schimmel, T.; Kohl, T. Dissolution control and stability improvement of silica nanoparticles in aqueous media. J. Nanopart. Res. 2023, 25, 40. [Google Scholar] [CrossRef]
- Muhammud, A.M.; Gupta, N.K. Nanostructured SiO2 material: Synthesis advances and applications in rubber reinforcement. RSC Adv. 2022, 12, 18524–18546. [Google Scholar] [CrossRef]
- Therese, J.B.A.J.H.; Gayathri, R.; Selvakumar, K.; Prabhu, M.R.; Sivakumar, P. Incorporation of sulfonated silica nano particles into polymer blend membrane for PEM fuel cell applications. Mater. Res. Express 2019, 6, 115336. [Google Scholar] [CrossRef]
- Su, Y.H.; Liu, Y.L.; Sun, Y.M.; Lai, J.Y.; Wang, D.M.; Gao, Y.; Liu, B.; Guiver, M.D. Proton exchange membranes modified with sulfonated silica nanoparticles for direct methanol fuel cells. J. Memb. Sci. 2007, 296, 21–28. [Google Scholar] [CrossRef]
- Petreanu, I.; Niculescu, V.C.; Enache, S.; Iacob, C.; Teodorescu, M. Structural Characterization of Silica and Amino-Silica Nanoparticles by Fourier Transform Infrared (FTIR) and Raman Spectroscopy. Anal. Lett. 2023, 56, 390–403. [Google Scholar] [CrossRef]
- Martina, P.; Gayathri, R.; Pugalenthi, M.R.; Cao, G.; Liu, C.; Prabhu, M.R. Nanosulfonated silica incorporated SPEEK/SPVdF-HFP polymer blend membrane for PEM fuel cell application. Ionics 2020, 26, 3447–3458. [Google Scholar] [CrossRef]
- Kalaiselvimary, J.; Sundararajan, M.; Prabhu, M.R. Preparation and characterization of chitosan-based nanocomposite hybrid polymer electrolyte membranes for fuel cell application. Ionics 2018, 24, 3555–3571. [Google Scholar] [CrossRef]
- Rangasamy, V.S.; Thayumanasundaram, S.; Seo, J.W.; Locquet, J.P. Vibrational spectroscopic study of pure and silica-doped sulfonated poly(ether ether ketone) membranes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 138, 693–699. [Google Scholar] [CrossRef]
- Sompech, S.; Dasri, T.; Thaomola, S. Preparation and Characterization of Amorphous Silica and Calcium Oxide from Agricultural Wastes. Orient. J. Chem 2016, 32, 1923–1928. [Google Scholar] [CrossRef]
- Trisunaryanti, W.; Larasati, S.; Triyono, T.; Santoso, N.R.; Paramesti, C. Selective production of green hydrocarbons from the hydrotreatment of waste coconut oil over Ni- And NiMo-supported on amine-functionalized mesoporous silica. Bull. Chem. React. Eng. Catal. 2020, 15, 415–431. [Google Scholar] [CrossRef]
- Ren, G.; Su, H.; Wang, S. The combined method to synthesis silica nanoparticle by Stöber process. J. Solgel Sci. Technol. 2020, 96, 108–120. [Google Scholar] [CrossRef]
- Sivolapov, P.; Myronyuk, O.; Baklan, D. Synthesis of Stober silica nanoparticles in solvent environments with different Hansen solubility parameters. Inorg. Chem. Commun. 2022, 143, 109769. [Google Scholar] [CrossRef]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle Characterization: What to Measure? Adv. Mater. 2019, 31, 1901556. [Google Scholar] [CrossRef] [PubMed]
- Schmid, O.; Stoeger, T. Surface area is the biologically most effective dose metric for acute nanoparticle toxicity in the lung. J. Aerosol Sci. 2016, 99, 133–143. [Google Scholar] [CrossRef]
- Dolai, J.; Mandal, K.; Jana, N.R. Nanoparticle Size Effects in Biomedical Applications. ACS Appl. Nano Mater. 2021, 4, 6471–6496. [Google Scholar] [CrossRef]
- Hickey, J.W.; Santos, J.L.; Williford, J.M.; Mao, H.Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control Release 2015, 219, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control Release 2016, 238, 176–185. [Google Scholar] [CrossRef]
- Javdani, H.; Khosravi, R.; Etemad, L.; Moshiri, M.; Zarban, A.; Hanafi-bojd, M.Y. Microporous and Mesoporous Materials Tannic acid-templated mesoporous silica nanoparticles as an effective treatment in acute ferrous sulfate poisoning. Microporous Mesoporous Mater. 2020, 307, 110486. [Google Scholar] [CrossRef]
- Kumar, S.; Malik, M.M.; Purohit, R. Synthesis of high surface area mesoporous silica materials using soft templating approach. Mater. Today Proc. 2018, 5, 4128–4133. [Google Scholar] [CrossRef]
- Thahir, R.; Wahab, A.W.; La Nafie, N.; Raya, I. Synthesis of high surface area mesoporous silica SBA-15 by adjusting hydrothermal treatment time and the amount of polyvinyl alcohol. Open Chem. 2019, 17, 963–971. [Google Scholar] [CrossRef]
- Zeng, S.Z.; Zeng, X.; Huang, L.; Wu, H.; Yao, Y.; Zheng, X.; Zou, J. The formation mechanisms of porous silicon prepared from dense silicon monoxide. RSC Adv. 2017, 7, 7990–7995. [Google Scholar] [CrossRef]
- Rizzi, F.; Castaldo, R.; Latronico, T.; Lasala, P.; Gentile, G.; Lavorgna, M.; Striccoli, M.; Agostiano, A.; Comparelli, R.; Depalo, N.; et al. High surface area mesoporous silica nanoparticles with tunable size in the sub-micrometer regime: Insights on the size and porosity control mechanisms. Molecules 2021, 26, 4247. [Google Scholar] [CrossRef]
- Ge, Q.; Ding, L.; Wu, T.; Xu, G.; Yang, F.; Xiang, M. Effect of surfactant on morphology and pore size of polysulfone membrane. J. Polym. Res. 2018, 25, 21. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.K.; Park, J.O.; Choi, S.W.; Kim, K.H.; Ko, T.; Pak, C.; Lee, J.C. Highly reinforced pore-filling membranes based on sulfonated poly(arylene ether sulfone)s for high-temperature/low-humidity polymer electrolyte membrane fuel cells. J. Memb. Sci. 2017, 537, 11–21. [Google Scholar] [CrossRef]
- Huber, L. The influence of the ammonia concentration and the water content on the water sorption behavior of ambient pressure dried silica xerogels. J. Sol-Gel Sci. Technol. 2020, 96, 197–206. [Google Scholar] [CrossRef]
- Calderon, V.S.; Ribeiro, T.; Farinha, J.P.S.; Baleizão, C.; Ferreira, P.J. On the Structure of Amorphous Mesoporous Silica Nanoparticles by Aberration-Corrected STEM. Small 2018, 14, 1802180. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Subhani, T.; Husain, S.W. Synthesis and characterization of silica nanoparticles from clay. J. Asian Ceram. Soc. 2016, 4, 91–96. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Subhani, T.; Husain, S.W. Synthesis of silica nanoparticles from sodium silicate under alkaline conditions. J. Solgel Sci. Technol. 2016, 77, 753–758. [Google Scholar] [CrossRef]
- Issa, H.K.; Taherizadeh, A.; Maleki, A. Atomistic-level study of the mechanical behavior of amorphous and crystalline silica nanoparticles. Ceram. Int. 2020, 46, 21647–21656. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Y.; Bi, Y. Rapid Synthesis of SiO2 by Ultrasonic-Assisted Stober Method as Controlled and pH-Sensitive Drug Delivery. J. Nanopart. Res. 2018, 20, 304. [Google Scholar] [CrossRef]
- Hagar, M.E.; Afifi, T.H. Catalytic Activity of Sulfated and Phosphated Catalysts towards the Synthesis of Substituted Coumarin. Catalysts 2018, 8, 36. [Google Scholar] [CrossRef]
- Dhaneswara, D.; Fatriansyah, J.F.; Situmorang, F.W.; Haqoh, A.N. Synthesis of Amorphous Silica from Rice Husk Ash: Comparing HCl and CH3COOH Acidification Methods and Various Alkaline Concentrations. Int. J. Technol. 2020, 11, 200–208. [Google Scholar] [CrossRef]
- Usgodaarachchi, L.; Thambiliyagodage, C.; Wijesekera, R.; Bakker, M.G. Synthesis of mesoporous silica nanoparticles derived from rice husk and surface-controlled amine functionalization for efficient adsorption of methylene blue from aqueous solution. Curr. Res. Green. Sustain. Chem. 2021, 4, 100116. [Google Scholar] [CrossRef]
- Dhaffouli, A.; Salazar-Carballo, P.A.; Carinelli, S.; Holzinger, M.; Barhoumi, H. Improved electrochemical sensor using functionalized silica nanoparticles (SiO2-APTES) for high selectivity detection of lead ions. Mater. Chem. Phys. 2024, 318, 129253. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).