Abstract
The global energy sector is aiming to substantially reduce CO2 emissions to meet the UN climate goals. Among the proposed strategies, underground storage solutions such as radioactive disposal, CO2, NH3, and underground H2 storage (UHS) have emerged as promising options for mitigating anthropogenic emissions. These approaches require rigorous research and development (R&D), often involving laboratory-scale experiments to establish their feasibility before being scaled up to pilot plant operations. Microorganisms, which are ubiquitous in laboratory environments, can significantly influence geochemical reactions under variable experimental conditions of porous media and a salt cavern. We have selected a consortium composed of Bacillus sp., Enterobacter sp., and Cronobacter sp. bacteria, which are typically present in the laboratory environment. These microorganisms can contaminate the rock sample and develop experimental artifacts in UHS experiments. Hence, it is pivotal to sterilize the rock prior to conduct experimental research related to effects of microorganisms in the porous media and the salt cavern for the investigation of UHS. This study investigated the efficacy of various disinfection and sterilization methods, including ultraviolet irradiation, autoclaving, oven heating, ethanol treatments, and gamma irradiation, in removing the microorganisms from silica sand. Additionally, the consideration of their effects on mineral properties are reviewed. A total of 567 vials, each filled with 9 mL of acid-producing bacteria (APB) media were used to test killing efficacy of the cleaning methods. We conducted serial dilutions up to 10−8 and repeated them three times to determine whether any deviation occurred. Our findings revealed that gamma irradiation and autoclaving were the most effective techniques for eradicating microbial contaminants, achieving sterilization without significantly altering the mineral characteristics. These findings underscore the necessity of robust cleaning protocols in hydrogeochemical research to ensure reliable, reproducible data, particularly in future studies where microbial contamination could induce artifacts in laboratory research.
1. Introduction
The introduction of geo-solutions is crucial in the energy transition phase to achieve carbon neutrality targets via compressed air, anthropogenic CO2, helium, and UHS [1,2,3]. Microorganisms, which are widely distributed in both surfaces and subsurface environments, can introduce uncertainties and risks to long-term implementations of UHS [4]. To assess the potential performance of reservoir rocks for UHS, various reservoir engineering experiments have been conducted, including measurement of porosity [5], permeability [5], capillary pressure [2], interfacial tension [2], H2-brine core flooding, wettability [6], and in situ loss of H2 and H2S generation [7,8]. However, microbial contamination from laboratory surfaces, such as benches and equipment surfaces or airborne bacteria, can compromise these experiments, leading to artifacts and unreliable data [9,10].
The effective sterilization of rock samples is crucial to mitigate microbial contamination without compromising the integrity of geological material. Importantly, little information is available regarding the microbial populations residing within the underground reservoir rock samples. Sterilized samples must be stored in controlled environments, such as isolation rooms or microbial-free zones, to prevent recontamination during further testing. There have been a variety of studies published which might not adopt the right cleaning technique for the rock for UHS research. Ali et al. conducted multiple studies and examined the wettability of rock. Nevertheless, the rock was cleaned with deionized water and surface residues were removed using the ultrapure nitrogen [11,12]. Thus, inadequate cleaning of the rock may fail to sterilize it and can introduce artifacts during wettability measurements. The presence of microorganisms could alter the wettability and other reservoir properties, such as permeability and porosity [13,14]. Therefore, the proper sterilization of the rock prior to the wettability, interfacial tension, and coreflooding tests is important to understand the UHS system.
Studies of Martian UV flux provide insight into sterilization challenges. On Mars, perchlorates exhibit bactericidal properties when exposed to UV radiation, including UVC (<280 nm) and UVB (280 to 315 nm) [15]. Furthermore, two components of the Red Planet, including iron oxides and hydrogen peroxide, induce a synergetic effect with irradiated perchlorates, causing a 10.8-fold increase in cell death for Bacillus subtilis compared to cells only exposed to UV rays for 60 s [16]. Despite these findings, the complete sterilization of rock samples using UV radiation remains a challenge as the irregular shapes and rough surfaces of rocks hinder UV penetration, leaving microorganisms within cracks and crevices unaffected [17].
Chemical disinfectants capable of penetrating microcapillaries may be required. However, chemical treatments, such as 70% and 96% ethanol, have shown limited effectiveness in creating aseptic conditions within deep cracks in limestone [18]. Furthermore, interactions between environmental microbes and rock surfaces can induce calcite dissolution and precipitation, potentially altering the rock’s mineralogy. In an overlooked phenomenon, it was illustrated that the entombment of microorganisms in a Si-rich precipitate in a nutrient-depleted environment indicates Si mobilization at ambient conditions [18]. These interactions underscore the need for sterilization methods that preserve the mineralogical integrity of geological samples.
Previous studies have employed autoclaving (~121 °C, 15 psi, and 30 min) as a sterilization method for geological samples [14,19,20]. However, it is reported that microorganisms can penetrate Berea sandstone rock quicker when sterilized by autoclaving. Additionally, the autoclaved sample showed an increased chloride content compared to dry heating, leading to aggregation and an uneven morphology in clay minerals [21]. For Mars analog rocks and minerals, gamma irradiation has been used as an alternative sterilization method. While high doses of gamma rays did not significantly affect the structural integrity of the rocks, some minerals exhibited darkening due to gamma radiation exposure. Despite this, gamma irradiation is considered a viable option for sterilizing the Mars-returned rock samples [22].
There is a need to develop a scientific approach for the sterilization of rocks intended for UHS research applications. Overall, oven heating, autoclaving, ethanol treatment, and gamma irradiation are promising candidates for microbial-focused laboratory environments. Their application can minimize microbial artifacts and ensure the reliability of experimental results in H2 and CO2 geological storage studies. In this study, we primarily focused on the effects of rock sterilization for UHS investigations. Nevertheless, given the wide range of existing literature on rock sterilization techniques for astrobiology applications, we have drawn upon these data to provide a comparative analysis of different sterilization techniques, including their merits and demerits (Table 1).

Table 1.
Effects of different sterilization techniques on the minerology of rocks related to geoscience and astrobiology applications.
2. Materials and Methods
Figure 1 illustrates the research methodology used in this study to investigate the effects of different sterilization techniques on pre-inoculated silica sand. Silica sand (super fine) was purchased from Cook Industrial Minerals, WA, Australia. Table 2 lists the techniques and factors used to sterilize the silica sand mineral.
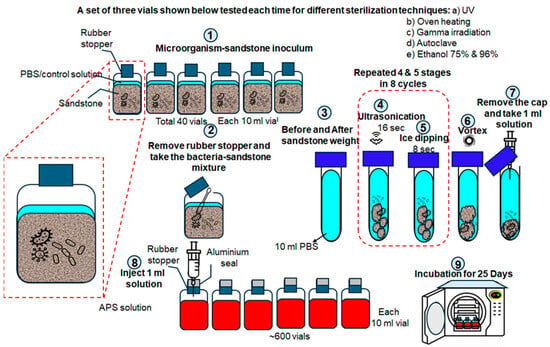
Figure 1.
Research methodology: 1. preparation of pre-inoculated silica sand mineral and exposure to different sterilization methods; 2. sampling of treated silica sand; 3. resuspension of silica sand in PBS solution; 4–5. cell detachment by ultrasonication; 6. rapid mixing of samples using the vortex machine; 7–8. aliquot for the most probable number quantification; and finally, 9. incubation of the culture media to grow surviving microorganisms.

Table 2.
Sterilization methods and parameters used in this study.
2.1. Sand Sample Preparation
To simulate core rock samples, 10 mL sterile glass vials were filled with 15.7 g of sand. Sand was aseptically transferred into each vial using sterile spatulas. A defined bacterial consortium composed of Bacillus sp., Enterobacter sp., and Cronobacter sp. was used in this study to recreate microbial contamination. Each strain was cultivated independently in separate vials under optimal growth conditions to obtain active cultures. From each culture, 1 mL was transferred into a 50 mL falcon tube containing PBS solution, resulting in a final bacterial concentration of ~107 cells/mL. 3 mL of this cell suspension were then used to inoculate the sand filled vials. Table S1 presents chemical composition of PBS.
2.2. Sterilization Methods
UV irradiation: Three vials of sandstone–bacteria–PBS inoculum were placed inside the biosafety cabinet. The UV light was turned on, and the cabinet windows were covered to prevent external light exposure. UV irradiation was conducted for 30 min, each vial was rotated ~180° clockwise every 15 min. The rubber seal were removed during this process to allow maximum UV penetration.
Ethanol washing: ethanol was used at two different concentrations to sterilize the rock sample. The 95% and 75% ethanol solutions were prepared using ethanol pure 99.9%. A total of 95 mL of ethanol and 5 mL of water were added to a 200 mL regent bottle to make a solution of Ethanol 95 wt%. Similarly, 75 wt% ethanol was prepared using 75 mL of pure ethanol and 35 mL of water. Three sandstone–bacteria–PBS inoculum vials were washed three times with 95% ethanol, and another 3 vials were washed with 75% ethanol. The ethanol was not completely removed from the vials, so that the sandstone may remain soaked for at least 15 min.
Oven heating: A Venticell 111-Eco line oven was used to sterilize the sandstone–bacteria–PBS inoculum vials. The oven was switched on and preheated until the set temperature reached 200 °C. Three sandstone–bacteria–PBS inoculum vials were placed in the oven for approximately 2 h at a constant 200 °C.
Autoclave: the benchtop autoclave model 3870EL-D was used. Three sandstone–bacteria–PBS inoculum vials were autoclaved using the liquid cycle at 121 °C, 15 psia pressure for 30 min.
Gamma irradiation: three vials of sand–bacteria–PBS inoculum samples were provided to ChemCenter Government of Western Australia at Curtin University Campus for the irradiation of the gamma ray. The samples were irradiated for at least 32 h. The center is equipped with a gamma irradiation unit named Gammacell 220.
2.3. Microbial Quantification
The most probable number (MPN) method was employed to evaluate the efficacy of various sterilization techniques in eliminating microorganisms from rock samples. This method involves a series of dilutions and inoculation into a suitable culture medium to estimate the concentration of viable microorganisms in the sample. The culture medium used for microbial recovery was red phenol broth which supports the growth of a broad spectrum of microorganisms. The media was prepared according to standard protocols, sterilized, and poured into sterile vials (Figure 2A–C). Table S2 provides the chemical composition of culture media APB.
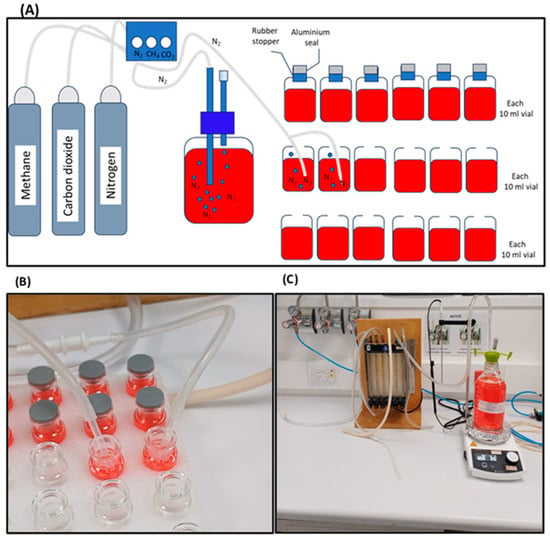
Figure 2.
(A) Schematic of the preparation of anaerobic culture medium. (B) Images show deoxygenation of 10 mL vials and (C) APB stock solution deoxygenating with N2 gas.
An amount of 2 g of the sterilized samples were inoculated into falcon tubes containing 20 mL of PBS solution. Tubes were sonicated in cycles of 15 s on and 15 s off to detach cells from the silica sand. Tubes were vortexed and 1 mL aliquots were used to inoculate vials containing the red phenol medium. Samples were processed in triplicate with a dilution factor of 10−8. After inoculation, the vials were incubated at 40 °C for 3 weeks and microbial growth was recorded using colorimetric change in the media.
3. Results and Discussions
Figure 3A illustrates the concentration of surviving cells in the silica sand after sterilization process. The control (not subjected to sterilization) indicated that the concentration of cells in the test bottles was in the order of average 107 cell/g before sterilization treatment. Most probable number (MPN) results indicate that autoclave, oven heating, and gamma irradiation were able to eliminate all cells in the sand. These findings were well matched with those of previous studies [35,36]. However, oven heating, autoclave, and gamma rays could induce mineralogical changes. For instance, oven heating is reported for microcracking in quartz mineralogy of sand [32]. Gamma rays caused the darkening of quartz mineral (Figure 4) in silica sand, which is in line with previous findings [22].
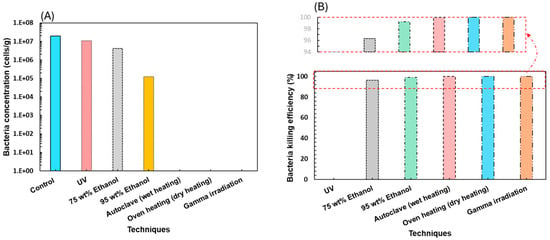
Figure 3.
Effect of different techniques on the sterilization of the sand: (A) bacteria concentration in cells/g, and (B) bacteria-killing efficiency in percentage.
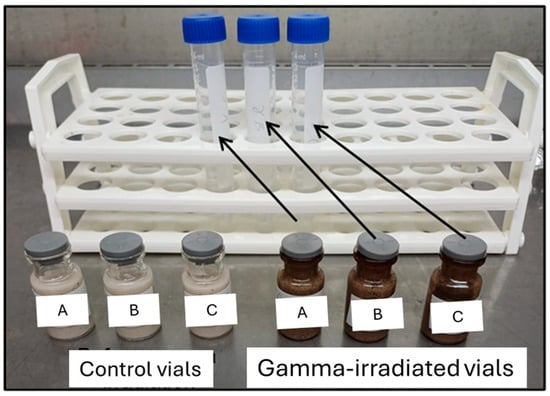
Figure 4.
Illustrated difference between color of control vials (replicates A,B,C) (left), and gamma rays treated vials (replicates A,B,C) (right).
Figure 4 illustrates that the glass of serum vials and sand changed to a blackish color after treating with gamma irradiation. These changes could affect the in-situ reservoir properties, in particular porosity, permeability, interfacial tension, capillary pressure, and wettability at the micron scale, resulting in adverse effects on the laboratory research for geological H2 storage.
Ethanol and UV radiation were not able to inactivate all the cells and a concentration between 105 to 107 cells/g surviving bacteria was still detected. Our results provide a clear comparison of the performance of cell sterilization with that of the different techniques. UV sterilization shows no effect on the sterilization of sand. We found that ethanol concentrations, including 75 wt% and 95 wt%, illustrate the lowest killing efficiency; thus, more living cells were detected compared to the other sterilization methods evaluated. (Figure 3B). This figure shows the killing efficiency of each sterilization method. UV irradiation was an inefficient technique, with a killing efficiency of 0%. This finding may be attributed to less penetration of UV from a glass of the vial into the rock. Notably, 75% ethanol achieved a killing efficiency of 96.3%, whereas 95% ethanol reached a killing efficiency of 99.2% (Figure 3B). Although killing efficiency values are high, it is important to consider that the surviving population of cells was also high, in the order of 106 and 105, respectively. This survival percentage could restore microbial activity in long-time core flooding and salt cavern bioreactor experimental setups under the influence of anaerobic conditions. Additionally, the effects of precipitation, changes in minerology, and brittle behavior of the formation were reported in calcite, clay, and sand, respectively, after the use of ethanol as a sterilizing substance. Figure 5A,B illustrates the total number of vials incubated after inoculation with cell suspension. Table S3 provides data of different sterilization methods, MPN, and dilution series.
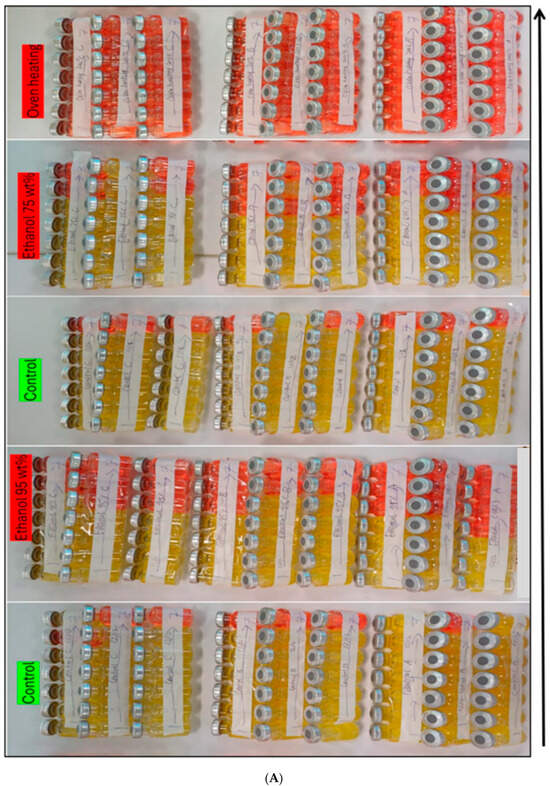
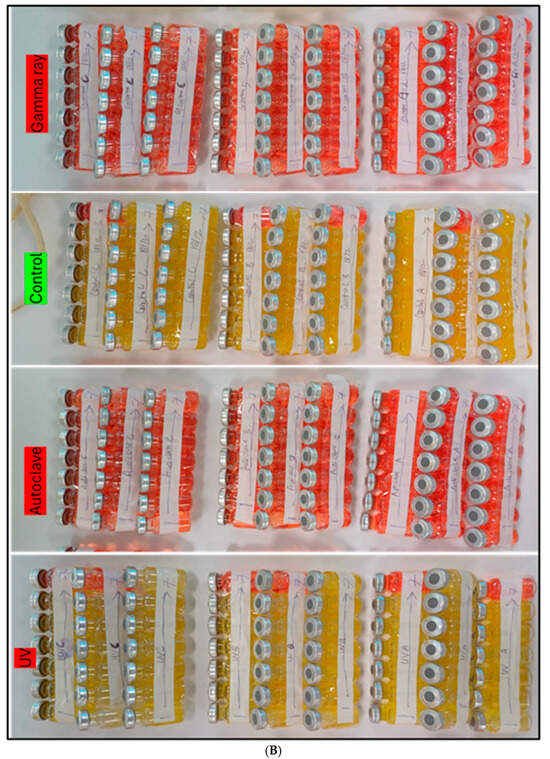
Figure 5.
(A) Control from left ran parallel with 95 wt% ethanol sterilization. (A,B) Another control ran for 75 wt% ethanol, oven heating experiments, UV, and autoclave sterilization techniques. (B) The second last control was run parallel to the Gamma ray. There are 567 vials, each 10 mL, as illustrated in the figure. We conducted serial dilutions up to 10−7, and repeated it three times to determine if any deviation occurred in the values. Note: Red color vials show no growth. Yellow color vials illustrate positive growth of microorganisms in the APB media solution.
Overall, physical methods, such as UV irradiation, oven heating, and autoclaving, are widely used for different kinds of sterilization. UV irradiation revealed a not considerable level of sterilization. UV can be useful for the sterilization of surface and air. Nevertheless, the limited penetration depth restricts its effectiveness for bulk sterilization. Gamma irradiation was very effective and a very high sterilization. Gamma irradiation damages cellular components, such as DNA, which results in microbial killing. This is a very effective technique with strong penetration capability. Similarly, autoclave revealed very strong sterilization. Autoclave shows a variety of merits, such as faster cycle time, in-house control and accessibility, lower cost, and less impact on the properties of materials in comparison to gamma irradiation. Autoclave disrupts bacterial proteins and cellular structure, leading to better and rapid sterilization.
4. Conclusions
Currently, underground hydrogen storage (UHS) experiments are being carried out, often impacting the integrity of rock samples by introducing a variety of anthropogenic microorganisms. In response, we have developed a laboratory protocol and procedure for rock sterilization. Our experiments demonstrated that the eradication of microorganisms can be achieved in UHS experiments when differentiating between biological activity and abiotic processes is critical. This research provides valuable information to safeguard the accuracy and reliability of future hydrogen geological laboratory-scale experiments. UV irradiation was not an efficient technique, with a killing efficiency of 0%. This finding may be attributed to lower penetration of UV into the rock. Notably, 75% ethanol achieved a killing efficiency of 96.3%, whereas 95% ethanol reached a killing efficiency of 99.2%. We conclude that autoclave process, oven heating, and gamma irradiation methods achieved 100% killing efficiency and absolutely eliminate microbial life from the rock. However, gamma irradiation caused the discoloration of sand, and oven heating may induce micro-cracks, potentially compromising the integrity of the sand. Therefore, we recommend the autoclave process as the most suitable sterilization method due to its relatively low operating temperature and pressure, which are well suited for sand sterilization. Further research is needed to assess the impact of these sterilization methods on the mineralogy of rocks, including petrophysical properties and surface behavior for large-scale geological hydrogen storage experiments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fuels6030052/s1, Table S1: Chemical composition of PBS 1000 mL; Table S2: Chemical composition of culture media APB 1000 mL; Table S3: provides data of different sterilization methods, MPN, and dilution series.
Author Contributions
Conceptualization, A.A. and M.S.; methodology, S.J.S.-C.; software, A.A.; validation, S.J.S.-C.; formal analysis, S.J.S.-C. and M.S.; investigation, A.A.; resources, M.S. and A.S.; data curation, A.A.; writing—original draft preparation, A.A.; writing—review and editing, A.A. and S.J.S.-C.; visualization, A.A. and M.S.; supervision, M.S., S.J.S.-C. and Q.X.; project administration, M.S. and A.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank Curtin University, Australia and Government of Australia for funding the first author’s PhD scholarship through the Research Training Program.
Data Availability Statement
All the data included in this study is presented in the manuscript.
Acknowledgments
We would like to extend our sincere thanks to Adeeba Hussain for her invaluable assistance with the experimental work conducted in the B601 MIC Laboratory at the Curtin Corrosion Centre, Australia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Truche, L.; Donzé, F.-V.; Goskolli, E.; Muceku, B.; Loisy, C.; Monnin, C.; Dutoit, H.; Cerepi, A. A deep reservoir for hydrogen drives intense degassing in the Bulqizë ophiolite. Science 2024, 383, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Aftab, A.; Al-Yaseri, A.; Nzila, A.; Hamad, J.A.; Sarmadivaleh, M. Microbial impact on basalt-water-hydrogen system: Insights into wettability, capillary pressure, and interfacial tension for subsurface hydrogen storage. Greenh. Gases Sci. Technol. 2024, 14, 546–560. [Google Scholar] [CrossRef]
- Aftab, A.; Hassanpouryouzband, A.; Xie, Q.; Machuca, L.L.; Sarmadivaleh, M. Toward a fundamental understanding of geological hydrogen storage. Ind. Eng. Chem. Res. 2022, 61, 3233–3253. [Google Scholar] [CrossRef]
- Thaysen, E.M.; Butler, I.B.; Hassanpouryouzband, A.; Freitas, D.; Alvarez-Borges, F.; Krevor, S.; Heinemann, N.; Atwood, R.; Edlmann, K. Pore-scale imaging of hydrogen displacement and trapping in porous media. Int. J. Hydrog Energy 2023, 48, 3091–3106. [Google Scholar] [CrossRef]
- Aftab, A.; Hassanpouryouzband, A.; Martin, A.; Kendrick, J.E.; Thaysen, E.M.; Heinemann, N.; Utley, J.; Wilkinson, M.; Haszeldine, R.S.; Edlmann, K. Geochemical integrity of wellbore cements during geological hydrogen storage. Environ. Sci. Technol. Lett. 2023, 10, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Al-Yaseri, A.; Fatah, A.; Al-Abdrabalnabi, R.; Alafnan, S.; Salmachi, A. Investigation of gas residuals in sandstone formations via X-ray core-flooding experiments: Implication for subsurface hydrogen storage. Int. J. Hydrog Energy 2024, 78, 268–278. [Google Scholar] [CrossRef]
- Gholami, R. Hydrogen storage in geological porous media: Solubility, mineral trapping, H2S generation and salt precipitation. J. Energy Storage 2023, 59, 106576. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Joonaki, E.; Edlmann, K.; Haszeldine, R.S. Offshore geological storage of hydrogen: Is this our best option to achieve net-zero? ACS Energy Lett. 2021, 6, 2181–2186. [Google Scholar] [CrossRef]
- Harvey, R.W.; Harms, H.; Landkamer, L. Transport of microorganisms in the terrestrial subsurface: In situ and laboratory methods. Man. Environ. Microbiol. 2007, 872–897. [Google Scholar] [CrossRef]
- van der Schoor, A.S.; Severin, J.A.; Klaassen, C.H.; Gommers, D.; Bruno, M.J.; Hendriks, J.M.; Voor, A.F.; Vos, M.C. Environmental contamination with highly resistant microorganisms after relocating to a new hospital building with 100% single-occupancy rooms: A prospective observational before-and-after study with a three-year follow-up. Int. J. Hyg. Environ. Health 2023, 248, 114106. [Google Scholar] [CrossRef]
- Ali, M.; Kumar, N.; Alsubhi, M.; Alissa, F.; Ghamdi, A.; Hoteit, H. Effects of Hydrogenated and De-Hydrogenated Organic Hydrogen Carriers on Carbonate Wettability for Hydrogen Geological Storage. Energy Fuels 2025, 39, 5550–5561. [Google Scholar] [CrossRef]
- Ali, M.; Yekeen, N.; Pal, N.; Keshavarz, A.; Iglauer, S.; Hoteit, H. Influence of pressure, temperature and organic surface concentration on hydrogen wettability of caprock; implications for hydrogen geo-storage. Energy Rep. 2021, 7, 5988–5996. [Google Scholar] [CrossRef]
- Boon, M.; Buntic, I.; Ahmed, K.; Dopffel, N.; Peters, C.; Hajibeygi, H. Microbial induced wettability alteration with implications for Underground Hydrogen Storage. Sci. Rep. 2024, 14, 8248. [Google Scholar] [CrossRef] [PubMed]
- Aftab, A.; Al-Yaseri, A.; Nzila, A.; Al Hamad, J.; Amao, A.O.; Sarmadivaleh, M. Quartz–H2–brine bacterium wettability under realistic geo-conditions: Towards geological hydrogen storage. Energy Fuels 2023, 37, 5623–5631. [Google Scholar] [CrossRef]
- Andrady, A.L. The Martian and extraterrestrial UV radiation environment—1. Biological and closed-loop ecosystem considerations. Acta Astronaut. 1999, 44, 53–62. [Google Scholar]
- Wadsworth, J.; Cockell, C.S. Perchlorates on Mars enhance the bacteriocidal effects of UV light. Sci. Rep. 2017, 7, 4662. [Google Scholar] [CrossRef]
- Volpon, A.; Elias, C.; Drozdowicz, A. An efficient device for sterilizing irregular surfaces. J. Microbiol. Methods 1990, 11, 51–58. [Google Scholar] [CrossRef]
- Johnston, V.E.; Martín-Pérez, A.; Skok, S.; Mulec, J. Microbially-mediated carbonate dissolution and precipitation; towards a protocol for ex-situ, cave-analogue cultivation experiments. Int. J. Speleol. 2021, 50, 3. [Google Scholar] [CrossRef]
- Abdelhamid, G.M.; Abdelkader, R.S.; Weesa, S.E. Streptomyces rock phosphate ore biomining evaluation in vitro. J. Environ. Radioact. 2024, 272, 107361. [Google Scholar] [CrossRef]
- Rolland, C.; Burzan, N.; Leupin, O.X.; Boylan, A.A.; Frutschi, M.; Wang, S.; Jacquemin, N.; Bernier-Latmani, R. Microbial hydrogen sinks in the sand-bentonite backfill material for the deep geological disposal of radioactive waste. Front. Microbiol. 2024, 15, 1359677. [Google Scholar] [CrossRef]
- Jenneman, G.E.; McINERNEY, M.J.; Crocker, M.E.; Knapp, R.M. Effect of sterilization by dry heat or autoclaving on bacterial penetration through Berea sandstone. Appl. Environ. Microbiol. 1986, 51, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.C.; Albert, F.G.; Combie, J.; Banin, A.; Yablekovitch, Y.; Kan, I.; Bodnar, R.J.; Hamilton, V.E.; Jolliff, B.L.; Kuebler, K. Effects of sterilizing doses of gamma radiation on Mars analog rocks and minerals. J. Geophys. Res. Planets 1999, 104, 27043–27066. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, F.; Farfan, G.A.; Xu, H. Low-temperature synthesis of disordered dolomite and high-magnesium calcite in ethanol–water solutions: The solvation effect and implications. ACS Omega 2021, 7, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.-S.; Han, C.; Wee, J.-H.; Park, J.-K.; Ahn, J.-W. Synthesis of calcium carbonate in a pure ethanol and aqueous ethanol solution as the solvent. J. Cryst. Growth 2005, 276, 680–687. [Google Scholar] [CrossRef]
- Sand, K.; Rodriguez-Blanco, J.; Makovicky, E.; Benning, L.G.; Stipp, S. Crystallization of CaCO3 in water–alcohol mixtures: Spherulitic growth, polymorph stabilization, and morphology change. Cryst. Growth Des. 2012, 12, 842–853. [Google Scholar] [CrossRef]
- Sharma, S.; Slater, L.; Ntarlagiannis, D.; Werkema, D.; Szabo, Z. Specific polarizability of sand–clay mixtures with varying ethanol concentration. Near Surf. Geophys. 2017, 15, 615–624. [Google Scholar] [CrossRef]
- Dawson, G.; Pearce, J.; Biddle, D.; Golding, S. Experimental mineral dissolution in Berea Sandstone reacted with CO2 or SO2–CO2 in NaCl brine under CO2 sequestration conditions. Chem. Geol. 2015, 399, 87–97. [Google Scholar] [CrossRef]
- Zárraga, R.; Alvarez-Gasca, D.E.; Cervantes, J. Solvent effect on TEOS film formation in the sandstone consolidation process. Silicon Chem. 2002, 1, 397–402. [Google Scholar] [CrossRef]
- Momma, N.; Kobara, Y.; Uematsu, S.; Kita, N.; Shinmura, A. Development of biological soil disinfestations in Japan. Appl. Microbiol. Biotechnol. 2013, 97, 3801–3809. [Google Scholar] [CrossRef]
- Jabarov, S.; Huseynov, R.; Ayyubova, G.S.; Trukhanov, S.; Trukhanov, A.; Aliyev, Y.; Thabethe, T.; Mauyey, B.; Kuterbekov, K.; Kaminski, G. Evaluation of structural characteristics BaFe(12-x)InxO19 hexaferrite compounds at high temperatures. Solid State Commun. 2024, 386, 115529. [Google Scholar] [CrossRef]
- Jabarov, S.; Nabiyeva, A.K.; Samadov, S.; Abiyev, A.; Sidorin, A.; Trung, N.; Orlov, O.; Mauyey, B.; Trukhanov, S.; Trukhanov, A. Study defects formation mechanism in La1-xBaxMnO3 perovskite manganite by positron annihilation lifetime and Doppler broadening spectroscopy. Solid State Ion. 2024, 414, 116640. [Google Scholar] [CrossRef]
- Hajpál, M.; Török, Á. Mineralogical and colour changes of quartz sandstones by heat. Environ. Geol. 2004, 46, 311–322. [Google Scholar] [CrossRef]
- Gamliel, A.; Austerweil, M.; Kritzman, G. Non-chemical approach to soilborne pest management–organic amendments. Crop Prot. 2000, 19, 847–853. [Google Scholar] [CrossRef]
- Bank, T.L.; Kukkadapu, R.K.; Madden, A.S.; Ginder-Vogel, M.; Baldwin, M.; Jardine, P. Effects of gamma-sterilization on the physico-chemical properties of natural sediments. Chem. Geol. 2008, 251, 1–7. [Google Scholar] [CrossRef]
- Querejeta, G.A. Sterilize methods comparison for soils: Cost, time, and efficiency. Int. J. Methodol. 2023, 2, 34–40. [Google Scholar] [CrossRef]
- King, W.L.; Grandinette, E.M.; Trase, O.; Rolon, M.L.; Salis, H.M.; Wood, H.; Bell, T.H. Autoclaving is at least as effective as gamma irradiation for biotic clearing and intentional microbial recolonization of soil. Msphere 2024, 9, e0047624. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).