Abstract
Endometrial receptivity array (ERA)—an objective tool used in assisted reproductive technology—is used for personalized embryo transfer in in vitro fertilization. Hydrosalpinx affects implantation through various mechanisms. However, its effects on ERA are not well established. In this case report, we present the diagnosis and treatment of a 34-year-old nulligravida woman with infertility for two years, obesity, double uterus with unilateral hydrosalpinx and right kidney deficiency. Based on ERA results, endometrial microbiome metagenomic analysis (EMMA), analysis of infectious chronic endometritis (ALICE), and CD138 immunostaining, the patient was treated with hormonal replacement cycle and amoxicillin/clavulanic acid. After one week of amoxicillin/clavulanic acid administration, the vitirified-warmed 4AA blastocyst was transferred to the left uterus—which was absent of hydrosalpinx and easily accessible to transfer and pregnancy was achieved. To the best of our knowledge, this case study is the first one in which we found that there were no differences between the left and right uterus in ERA, EMMA, ALICE, and CD138 immunostainings. Hence, we suggest that hydrosalpinx does not necessarily cause endometrial changes in all cases. Further research to evaluate the effects of hydrosalpinx on implantation with ERA and EMMA/ALICE is warranted.
1. Introduction
Endometrium—a highly dynamic tissue–undergoes physiological changes in response to steroid hormones to create a receptive environment for blastocyst implantation (endometrial receptivity). The optimal time period for endometrial receptivity is called the window of implantation (WOI) and is found to occur between day 19 and 21 of the menstrual cycle [1]. Endometrial receptivity array (ERA)–used in assisted reproductive technology (ART)–is an objective molecular genetics tool that uses the transcriptomic signature of 236 genes related to human endometrial receptivity to identify the WOI in in vitro fertilization (IVF) patients [1,2]. Physicians use the recommendations provided by ERA for personalized embryo transfers (ETs), synchronizing embryo transfer with the WOI of each patient for a successful implantation.
The uterine cavity was previously considered sterile. However, recent scientific evidence has demonstrated that the reproductive tract is colonized by a microbiota continuum that gradually changes from the vagina to the uterus [3]. The presence of different microorganisms in female and male reproductive tracts can impact reproductive clinical outcomes [4,5].
Hydrosalpinx causes poor ART outcomes. Hence, laparoscopic salpingectomy to exclude proximal tubal obstruction is considered as a treatment option prior to embryo transfer (ET). Although clinical approaches to treat ART patients with hydrosalpinx are established, the mechanisms by which hydrosalpinx affects the implantation environment in the uterine cavity are not clearly understood.
In this case report, we present a 34-year-old woman with infertility, double uterus and unilateral hydrosalpinx, who approached the clinic for infertility treatment. Owing to the presence of double uterus in the patient (one normal uterus and the other with hydrosalpinx), we evaluated the endometrial condition of two uteri in the same patient using ERA and next generation techniques such as 16S ribosomal RNA (16S rRNA) gene sequencing, which enables us to identify the optimal WOI and endometrial cavity microbiota profile, respectively, in a more objective method than did the classical histologic findings. We speculated that the results would differ depending on the presence or absence of hydrosalpinx, just as reported by Carranza [6]. However, to the best of our knowledge, this case study is the first one in which we found that there were no differences, in the results obtained, between the left and the right uteri. This indicates that hydrosalpinx does not necessarily cause endometrial damages that reduce implantation rates.
2. Case Presentation
A 34-year-old nulligravida woman with two years of infertility presented to our clinic seeking conception. She married to a 37-year-old man at the age of 32 and was 15 at her first menstruation; her menstrual cycle was regular with a 27–28-day cycle; the menses lasted 5–8 days. The patient had a height of 160 cm, weight of 73.1 kg, and 28.3 kg/m2 body mass index. Her blood pressure was normal (129/70 mmHg). She was diagnosed with right kidney deficiency at the age of 26. A double uterus and right hemivaginal obstruction were found at the age of 27 and she underwent vaginal septostomy (Figure 1). Chlamydia trachomatis antibody IgG and IgA were negative.
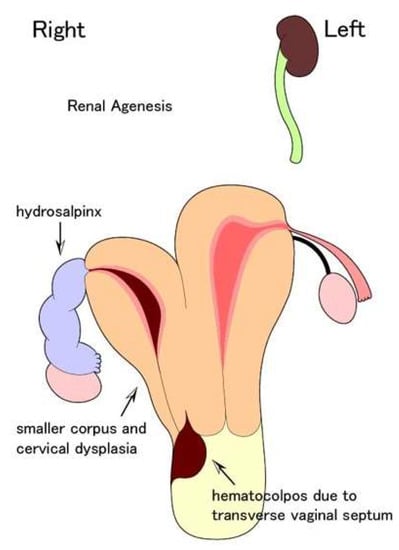
Figure 1.
Schema of the double uterus, right hemivaginal obstruction, and right renal agenesis before vaginal septostomy at the age of 27.
No vaginal septum was detected; left portio vaginalis was visible while the right was indistinct on speculum examination. Using transvaginal ultrasonography, we identified a double uterus, with the left uterus delineated continuously from the uterine body to the cervix; the right uterus was smaller than the left. Bilateral ovaries were normal in size and a multilocular mass suspected to be hydrosalpinx was found in the right adnexal region. Using magnetic resonance imaging (MRI), we confirmed a double uterus with a right hydrosalpinx and a 16 mm uterine fibroid in the left uterine body (Figure 2). Her genital tract malformation was categorized as U3bC3V3 according to the ESHRE/ESGE classification system [7].
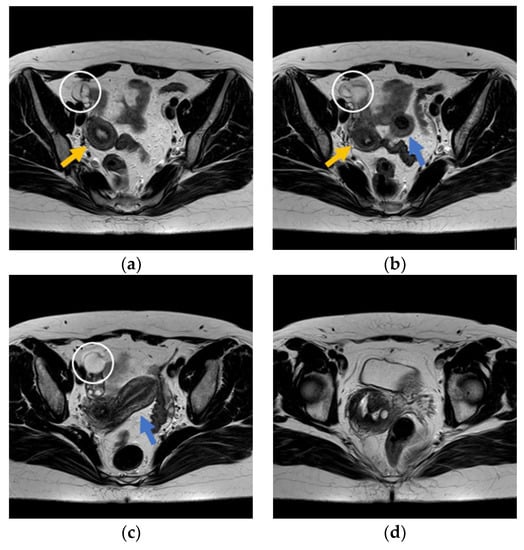
Figure 2.
Axial T2-weighted magnetic resonance images (MRI): (a) Right hydrosalpinx (white ring) mimicking a multilocular cyst, and right corpus uteri (orange arrow). (b) Double corpus uteri, formed separately (orange and blue arrows), and small myoma in left uterus fundus (blue arrow). (c) Right hydrosalpinx (white ring) and left corpus uteri (blue arrow). (d) Double uterine cervical canal, formed separately.
Hysteroscopic examination showed multiple endometrial polyps and intact uterotubal ostium in both uterine cavities (Figure 3).
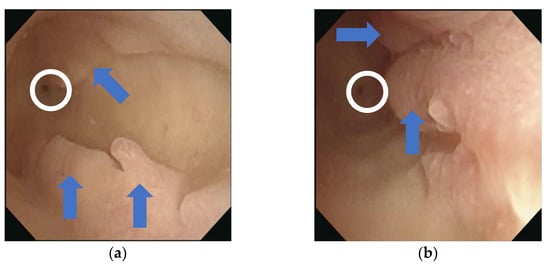
Figure 3.
Hysteroscopy images: Intact ostium of the fallopian tube (white ring) and multiple endometrial polyps (blue arrows) are visible in the bilateral (right (a) and left (b)) uterine cavities.
Given that the endometrial biopsy results of left uterus showed CD138 positive cells on immune-histological examination (Figure 4), and a chronic endometritis (CE) diagnosis score of 1 according to McQueen’s classification [8], she was administered doxycycline for 14 days.
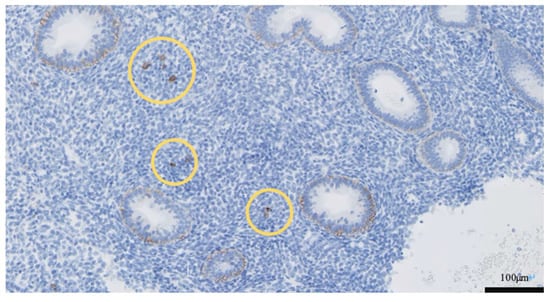
Figure 4.
Histology of endometrium stained with CD138 immunohistochemistry at initial biopsy: CD138 positive cells are indicated by yellow rings. Scale bar = 100 μm.
On day three of menstruation, we found that luteinizing hormone was 9.0 mIU/mL, follicle stimulating hormone was 8.9 mIU/mL, estradiol was 19 pg/mL, and anti-müllerian hormone was 2.11 ng/mL.
We decided to treat her with ART owing to the tubal factor. The gonadotropin-releasing hormone agonist (Suprecur®, Clinigen K.K., Tokyo, Japan) long protocol was used for controlled ovarian stimulation. After 36 h of triggering with 5000 IU urinary hCG (hCG Mochida®, Mochida Pharmaceutical Co., Ltd., Tokyo, Japan), 20 matured oocytes were retrieved under transvaginal ultrasound and inseminated with the sperm of the patient’s husband. Fertilized eggs were cultured to the blastocyst stage, and finally, 12 blastocysts were vitrified (Kitazato Vitrification Media®, Kitazato, Fuji, Japan). A warmed day-5 4BB blastocyst (according to Gardner’s classification [9]) was transferred to the left uterine cavity during a hormonal replacement cycle; however, no implantation occurred. ERA, endometrial microbiome metagenomic analysis (EMMA), and analysis of infectious chronic endometritis (ALICE) of bilateral uterus were employed to evaluate endometrial condition. They were performed in a hormone replacement cycle with vaginal progesterone (Lutinus®, Ferring Pharmaceuticals Co., Ltd., Tokyo, Japan) 300 mg and oral dydrogesterone (Duphaston®, Mylan EPD G.K., Tokyo, Japan) 30 mg administration for three times a day. In both uteri, ERA was receptive; EMMA/ALICE did not detect Lactobacillus sp., whereas Escherichia sp. was the main bacterial group accounting for more than 45% of all bacterial groups. An endometrial histological study was again performed bilaterally, and CD138 immunostaining showed no CE findings in both uterine cavities (Table 1). After one week of amoxicillin/clavulanic acid administration, which was based on EMMA/ALICE results, a warmed day-5 4AA blastocyst was transferred to the left uterus, with the same hormonal replacement protocol as the ERA cycle; the patient became pregnant as a result. The course of her pregnancy was uneventful and resulted in vaginal birth with a baby weighing 3070 g after a gestation period of 39 weeks.

Table 1.
Summary of results for evaluation of endometrial receptivity, microbiota, and chronic endometritis (CE) in both uterine cavities.
3. Discussion
To the best of our knowledge, this case report is the first in which ERA, EMMA and ALICE results were the same in a patient with double uterus—one with hydrosalpinx and another one without hydrosalpinx, confirmed using ultrasonography.
Müllerian duct anomaly (MDA) represents various patterns of uterus and renal anomaly. Congenital anomalies include Herlyn-Werner syndrome, Wunderlich syndrome, or obstructed hemivagina and ipsilateral renal anomaly (OHVIRA) syndrome. However, these diseases have a common root in etiology. This physical aberration is considered to be caused by the fusion of both left and right Müllerian duct failure, where the Müllerian duct partially develops into cervix and uterus. The duct may partially develop into a urogenital sinus during embryogenesis. The extent of development disorder determines the phenotype. Furthermore, approximately 14% of this group exhibits complications with endometriosis, most of which is fortunately determined during surgery [10]. Its prevalence is probably underestimated and no evidence to treat endometriosis in early adolescence is established. It is well known that this complication causes infertility regardless of uterine anomaly. Uterus didelphys is commonly observed as a type of MDA. Nevertheless, its prevalence is controversial as most reports about this anomaly are compelled to be case series owing to its rarity. MDA is often incidentally diagnosed because it does not always show any clinical findings. However, this case was already diagnosed as not only double uterus but hemivaginal obstruction before our consultation.
The double uterus with right hydrosalpinx, which was visible in ultrasonography, was evaluated for implantation condition prior to ET. Owing to this uterine anomaly, we investigated the endometrial health of each uterine cavity with and without hydrosalpinx, individually. Although it was speculated that they might differ between the two uteri depending on the presence or absence of hydrosalpinx, there were no differences between the left and right uterus in ERA, EMMA, or ALICE results. A blastocyst was transferred to the left uterus, which was absent of hydrosalpinx and easily accessible to transfer, and pregnancy was achieved.
It is well known that patients with hydrosalpinx have reduced implantation and pregnancy rates and high miscarriage rates after ART [11]. In addition, ultrasound-confirmed hydrosalpinx is remarkably associated with poor ART outcomes [12,13]. Laparoscopic salpingectomy or proximal tubal obstruction can overcome the adverse effects of hydrosalpinx and should be considered prior to IVF [11]. Patients with unilateral hydrosalpinx also show reduced pregnancy outcome with IVF [14]; unilateral salpingectomy can significantly improve IVF pregnancy rates in these patients [15]. Although the benefits of salpingectomy prior to IVF are well known, the mechanism by which hydrosalpinx adversely affects implantation and pregnancy rates is not clearly understood. Several hypotheses have been proposed as mechanisms for impaired ART outcomes caused by hydrosalpinx [16].
First, women with hydrosalpinx might have decreased endometrial receptivity [16]. Endometrial receptivity was previously defined by histological findings with the temporal expression of αvβ3 integrin during the WOI [17]. αvβ3 integrin is less expressed in women with hydrosalpinx than that of controls [17]. HOXA10, a gene involved in embryo implantation, is also completely suppressed in the presence of hydrosalpinx [18]. Laparoscopic salpingectomy prior to ET improves the expression of αvβ3 integrin and HOXA10 in endometrium [18]. In addition, hydrosalpinx fluid contains increased level of cytokines such as interleukin (IL)-12, IL-1α, and tumor necrosis factor α than in follicular fluid and serum. These inflammatory cytokines may cause impaired endometrial receptivity owing to the resultant inflammatory milieu and can also be a cause of CE [19]. In this case, CE is defined by hysteroscopic findings and the presence of CD138-positive plasma cells. However, the number of plasma cells in the fallopian tubes of patients with hydrosalpinx is predominantly higher than those of patients without hydrosalpinx, while the number of plasma cells in the endometrium is unchanged between patients with and without hydrosalpinx [19]. Therefore, the presence of plasma cells alone might not be sufficient for CE diagnosis. Recently, with the development of ERA, endometrial receptivity has been evaluated to identify the optimal WOI more objectively than did the classical histologic findings. Using ERA, one can perform ET at an appropriate time [2]. The influence of hydrosalpinx on ERA results, however, has not been reported.
Second, hydrosalpingeal fluid may have a direct toxic effect on embryos [16]. The uterus and fallopian tubes were previously considered to be sterile, and in fact, no findings of bacterial infection were obtained in hydrosalpinx contents [20]. However, this idea was demolished by 16S rRNA gene amplicon sequencing studies using next generation sequencing, which showed the widespread presence of microbiota from uterus to the pouch of Douglas in healthy women [3]. This technology revealed that an endometrial microbiota dominated by non-lactobacillus species is associated with decreased implantation, ongoing pregnancy, and live birth rates [21]. Thus, nowadays, the use of endometrial microbiota assays prior to ET has attracted attention. However, the endometrial microbiota pattern in the presence of hydrosalpinx is not clearly understood and further investigations are warranted.
Finally, a mechanical flushing with the leakage of hydrosalpingeal fluid from the uterine cavity may result in the loss of embryo apposition to the endometrium for implantation [15,21]. The clinical feature of hydrorrhoea has been shown to be a sign of poor prognosis among patients with hydrosalpinx undergoing ART [22].
In a previous case report of double uterus with untreated unilateral hydrosalpinx, similar to the present case, the results of ERA differed between the two uteri–one was receptive and the other was not receptive [6]. However, this report lacked a detailed description or an explanation for the difference in ERA results [6]. On the contrary, in the present case, the results of ERA in both uterine cavities were consistent: “receptive.” The reason for the difference in ERA results between the previous paper and this study might be due to the different types of genital tract malformation. Furthermore, in our patient, the results of not only ERA, but also EMMA and ALICE, which evaluated endometrium microbiota, were the same in the left and right uterus. The right hydrosalpinx was diagnosed using ultrasonography and MRI but not with hysterosalpingography, which has a high risk of causing pelvic inflammatory disease. Therefore, we cannot confirm connection between hydrosalpinx and uterine cavity. After thorough evaluation of endometrial health for WOI, uterine microbiota, and CE, we decided to implant in the left uterus, which had no hydrosalpinx and was easy to access.
In this study, although CD138 immunostaining did not show CE findings, microbial 16S rRNA gene biomarker analysis detected the presence of CE-related bacteria (Escherichia) in a substantial amount (46.78% of the total bacterial DNA). Escherichia is a facultative anaerobe commonly found in the human gastrointestinal tract. It can spread to the uterus and fallopian tubes by hematogenous seeding and/or by direct translocation from the bowel owing to the firm contact between rectum and uterine tubes [23]. Indeed, coliform bacteria are the predominant facultative pathogen found in tuboperitoneal fluid from women with salpingitis [24]. Escherichia is a genus associated with CE [25], and several mechanisms are proposed as causes of reproductive failure in CE pathophysiological models, such as endometrial inflammation, abnormal cytokine and leukocyte expression, abnormal uterine contractility, impaired immune tolerance to the embryo, altered vascular permeability, and defective decidualization and trophoblast invasion [26]. Although we cannot definitively affirm that the patient had CE at the treatment time, the presence of opportunistic pathogen Escherichia indicates that the patient was susceptible to CE in the short term. In fact, the patient in question was diagnosed with infertility and experienced one previous unsuccessful embryo transfer before antibiotic treatment. After treatment with amoxicillin/clavulanic acid, the patient underwent an additional embryo transfer that led to a successful pregnancy. Patients with CE findings show improved pregnancy outcomes after antibiotic administration [27], and the antibiotics recommended for CE are diverse, with the most common being doxycycline, ciprofloxacin, amoxicillin/clavulanic acid, and others [28]. In the present case, amoxicillin/clavulanic acid was recommended and selected based on EMMA/ALICE results before the second embryo transfer.
EMMA also detects the presence of a diverse range of other microorganisms, which accounted for an abnormal microbiome result in this case, a result which is commonly associated with poor reproductive outcomes in IVF patients [21,29]. A ratio of 0% Lactobacillus was reported in the patient’s uteri, and despite no additional treatment by vaginal probiotics, the patient achieved a successful pregnancy after antibiotic treatment. There are several studies that have reported the beneficial functions of Lactobacillus in human uterus, including: (1) production of IgA and IgG immunoglobulins for prevention of contamination from external bacteria; (2) production of hydrogen peroxide, which acts as bacteriocins and biosurfactants; and (3) promotion of Th17 and dendritic cell growth for the prevention of exogenous bacteria invasion and support of implantation environment by immune system. However, the present case indicates that endometrial Lactobacillus is not essential for a successful pregnancy.
Another interesting point about this case is that, even though the patient was positive for a CE-related bacterial strain, the ERA report showed “receptive” results for both uteri, showing that the endometrial WOI did not shift in the presence of the detected strain. As ERA analyzes endometrial genes that are related to apoptosis and inflammation, patients who present an advanced stage of CE are more prone to receiving ERA results indicating a “non-receptive” endometrium [30]. Since the patient in question had a successful implantation and pregnancy, it is reasonable to say that the WOI also did not shift after antibiotics administration and the putative elimination of facultative pathogen.
A growing body of scientific evidence indicates that abnormal vaginal and endometrial microbiomes can contribute to, or even be the cause of, hydrosalpinx, which is often related to acute or chronic salpingitis [31]. Although sexually transmitted organisms such as Chlamydia trachomatis and Neisseria gonorrhoeae are extensively described as the main causative agents of salpingitis, the majority of cases have no known etiology [32,33,34], and rDNA PCR and sequencing have identified novel bacterial phylotypes associated with this inflammatory condition [35]. Microorganisms ascending from the vagina and endocervix can cause inflammation and tissue damage to the endometrium and fallopian tubes [32], and an abnormal vaginal flora is epidemiologically linked with upper reproductive-tract infection, including salpingitis [32,33,36]. Moreover, the serous liquid that accumulates in the hydrosalpinx can serve as a medium that fosters further bacterial growth [37].
Removal of hydrosalpinx prior to ART clearly improves its outcome. Although concerns that salpingectomy or tubal ligation may decrease ovarian reserve have been raised [38], no remarkable differences in response to ovarian stimulation, dose or duration of gonadotropin administration before and after tubal resection have been observed [39]. In addition, patients with hydrosalpinx often have a history of pelvic infection or adnexal adhesions, which may place them at a high risk for surgeries such as salpingectomy. To avoid these risks, minimally invasive approaches, such as ultrasound-guided aspiration of hydrosalpingeal fluid at the time of oocyte retrieval, or sclerotherapy with instillation of ethanol into fallopian tube, are being explored [11]. The effects of a simple aspiration are questioned owing to the high likelihood of fluid reaccumulation. Sclerotherapy, which causes contraction, sclerosis, and decreased secretory function of fallopian tubes, is speculated to aid the prevention of hydrosalpingeal fluid recurrence [40]. Aspiration of a hydrosalpinx with or without sclerotherapy may be superior to no treatment at all [11]. However, further studies are essential to investigate ERA, EMMA, and ALICE results of this less invasive procedure for hydrosalpinx.
Thus, it remains undetermined how treatment of hydrosalpinx influences on endometrial receptivity and microbiota. In infertile women complicated with hydrosalpinx, clinicians may need to consider fertility treatment plans in respects of endometrial environment.
4. Conclusions
The results of ERA, EMMA/ALICE, and endometrial histology for CE were not different between the uterine cavities with and without hydrosalpinx. Hydrosalpinx does not necessarily cause endometrial changes that reduce implantation rates in all cases. Further research to evaluate the effects of hydrosalpinx on implantation with ERA and EMMA/ALICE is essential.
Author Contributions
Conceptualization, K.K. and T.I.; methodology, K.K.; data collection, M.T., K.H. and Y.F.; writing—original draft preparation, J.M. and T.I.; writing—review and editing, N.M., Y.S. and V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approval was waived for this report due to case report by Kameda IVF Clinic Makuhari.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
V.C. and Y.S. are employees of Igenomix Japan, company has no role in this study. Other authors declare no conflict of interest.
References
- Díaz-Gimeno, P.; Horcajadas, J.A.; Martinez-Conejero, J.A.; Esteban, F.J.; Alama, P.; Pellicer, A.; Simon, C. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil. Steril. 2011, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gimeno, P.; Ruiz-Alonso, M.; Blesa, D.; Bosch, N.; Martínez-Conejero, J.A.; Alamá, P.; Garrido, N.; Pellicer, A.; Simón, C. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil. Steril. 2013, 99, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Scott, R.T., Jr. Reproductive tract microbiome in assisted reproductive technologies. Fertil. Steril. 2015, 104, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Koedooder, R.; Singer, M.; Schoenmakers, S.; Savelkoul, P.H.M.; Morré, S.A.; De Jonge, J.D.; Poort, L.; Cuypers, W.J.S.S.; Beckers, N.G.M.; Broekmans, F.J.M.; et al. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: A prospective study. Hum. Reprod. 2019, 34, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Carranza, F.; González-Ravina, A.; Blasco, V.; Fernández-Sánchez, M. Different endometrial receptivity in each hemiuterus of a woman with uterus didelphys and previous failed embryo transfers. J. Hum. Reprod. Sci. 2018, 11, 297–299. [Google Scholar] [CrossRef]
- Grimbizis, G.F.; Gordts, S.; Sardo, A.D.S.; Brucker, S.; De Angelis, C.; Gergolet, M.; Li, T.-C.; Tanos, V.; Brölmann, H.; Gianaroli, L.; et al. The ESHRE/ESGE consensus on the classification of female genital tract congenital anomalies. Hum. Reprod. 2013, 28, 2032–2044. [Google Scholar] [CrossRef]
- McQueen, D.B.; Perfetto, C.O.; Hazard, F.K.; Lathi, R.B. Pregnancy outcomes in women with chronic endometritis and recurrent pregnancy loss. Fertil. Steril. 2015, 104, 927–931. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M.; Stevens, J.; Schlenker, T.; Schoolcraft, W.B. Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil. Steril. 2000, 73, 1155–1158. [Google Scholar] [CrossRef]
- Tong, J.; Zhu, L.; Chen, N.; Lang, J. Endometriosis in association with Herlyn-Werner-Wunderlich syndrome. Fertil. Steril. 2014, 102, 790–794. [Google Scholar] [CrossRef]
- American Society for Reproductive Medicine. The Practice Committee. Role of tubal surgery in the era of assisted reproductive technology: A committee opinion. Fertil. Steril. 2021, 115, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Strandell, A.; Lindhard, A.; Waldenström, U.; Thorburn, J.; Janson, P.O.; Hamberger, L. Hydrosalpinx and IVF outcome: A prospective, randomized multicentre trial in Scandinavia on salpingectomy prior to IVF *. Hum. Reprod. 1999, 14, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- de Wit, W.; Gowrising, C.J.; Kuik, D.J.; Lens, J.W.; Schats, R. Only hydrosalpinges visible on ultrasound are associated with reduced implantation and pregnancy rates after in-vitro fertilization. Hum. Reprod. 1998, 13, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Kassabji, M.; Sims, J.A.; Butler, L.; Muasher, S.J. Reduced pregnancy outcome in patients with unilateral or bilateral hy-drosalpinx after in vitro fertilization. Eur. J. Obstet. Gynecol. Reprod. Biol. 1994, 56, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Shelton, K.E.; Butler, L.; Toner, J.P.; Oehninger, S.; Muasher, S.J. Salpingectomy improves the pregnancy rate in in-vitro fertilization patients with hydrosalpinx. Hum. Reprod. 1996, 11, 523–525. [Google Scholar] [CrossRef] [PubMed]
- American Society for Reproductive Medicine. Practice Committee of the American Society for Reproductive Medicine in collaboration with The Society of Reproductive Surgeons. Salpingectomy for hydrosalpinx prior to in vitro fertilization. Fertil. Steril. 2008, 90, S66–S68. [Google Scholar] [CrossRef]
- Meyer, W.R.; Castelbaum, A.J.; Somkuti, S.; Sagoskin, A.W.; Doyle, M.; Harris, J.E.; Lessey, B.A. Hydrosalpinges adversely affect markers of endometrial receptivity. Hum. Reprod. 1997, 12, 1393–1398. [Google Scholar] [CrossRef]
- Daftary, G.S.; Kayisli, U.; Seli, E.; Bukulmez, O.; Arici, A.; Taylor, H.S. Salpingectomy increases peri-implantation endometrial HOXA10 expression in women with hydrosalpinx. Fertil. Steril. 2007, 87, 367–372. [Google Scholar] [CrossRef]
- Copperman, A.B.; Wells, V.; Luna, M.; Kalir, T.; Sandler, B.; Mukherjee, T. Presence of hydrosalpinx correlated to endometrial inflammatory response in vivo. Fertil. Steril. 2006, 86, 972–976. [Google Scholar] [CrossRef]
- Strandell, A.; Sjögren, A.; Bentin-Ley, U.; Thorburn, J.; Hamberger, L.; Brännström, M. Hydrosalpinx fluid does not adversely affect the normal development of human embryos and implantation in vitro. Hum. Reprod. 1998, 13, 2921–2925. [Google Scholar] [CrossRef]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Strandell, A.; Lindhard, A.; Fernandez-Sanchez, M. Why does hydrosalpinx reduce fertility? The importance of hydrosalpinx fluid. Hum. Reprod. 2002, 17, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Hurni, Y.; Bonollo, M.; Ferrero, L.; Taraschi, G.; Canonica, C.; Lozano, S.V.R. Pyosalpinx complicating chronic hydrosalpinx in a 50-year old virgo woman: A case report. BMC Women’s Health 2018, 18, 90. [Google Scholar] [CrossRef]
- Laufer, N.; Simon, A.; Schenker, J.G.; Sekeles, E.; Cohen, R. Fallopian Tubal Mucosal Damage Induced Experimentally by Escherichia coli in the Rabbit: A Scanning Electron Microscopic Study. Pathol.-Res. Pract. 1984, 178, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; Matteo, M.; Tinelli, R.; Pinto, V.; Marinaccio, M.; Indraccolo, U.; De Ziegler, D.; Resta, L. Chronic Endometritis due to Common Bacteria Is Prevalent in Women with Recurrent Miscarriage as Confirmed by Improved Pregnancy Outcome after Antibiotic Treatment. Reprod. Sci. 2014, 21, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Benner, M.; Ferwerda, G.; Joosten, I.; Van Der Molen, R.G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Updat. 2018, 24, 393–415. [Google Scholar] [CrossRef]
- Cicinelli, E.; Matteo, M.; Tinelli, R.; Lepera, A.; Alfonso, R.; Indraccolo, U.; Marrocchella, S.; Greco, P.; Resta, L. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum. Reprod. 2015, 30, 323–330. [Google Scholar] [CrossRef]
- Kitaya, K.; Takeuchi, T.; Mizuta, S.; Matsubayashi, H.; Ishikawa, T. Endometritis: New time, new concepts. Fertil. Steril. 2018, 110, 344–350. [Google Scholar] [CrossRef]
- Moreno, I.; Garcia-Grau, I.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Bahçeci, M.; Barrionuevo, M.J.; Taguchi, S.; Puente, E.; Dimattina, M.; Lim, M.W.; et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 2022, 10, 1. [Google Scholar] [CrossRef]
- Kuroda, K.; Horikawa, T.; Moriyama, A.; Nakao, K.; Juen, H.; Takamizawa, S.; Ojiro, Y.; Nakagawa, K.; Sugiyama, R. Impact of chronic endometritis on endometrial receptivity analysis results and pregnancy outcomes. Immun. Inflamm. Dis. 2020, 8, 650–658. [Google Scholar] [CrossRef]
- Puttemans, P.J.; Brosens, I.A. Preventive salpingectomy of hydrosalpinx prior to IVF: Salpingectomy improves in-vitro fertilization outcome in patients with a hydrosalpinx: Blind victimization of the Fallopian tube? Hum. Reprod. 1996, 11, 2079–2081. [Google Scholar] [CrossRef] [PubMed]
- Soper, D.E.; Brockwell, N.J.; Dalton, H.P.; Johnson, D. Observations concerning the microbial etiology of acute salpingitis. Am. J. Obstet. Gynecol. 1994, 170, 1008–1017. [Google Scholar] [CrossRef]
- Sweet, R.L.; Draper, D.L.; Hadley, W.K. Etiology of acute salpingitis: Influence of episode number and duration of symptoms. Obstet. Gynecol. 1981, 58, 62–68. [Google Scholar] [PubMed]
- Cohen, C.R.; Sinei, S.; Reilly, M.; Bukusi, E.; Eschenbach, D.; Holmes, K.K.; Ndinya-Achola, J.O.; Bwayo, J.; Grieco, V.; Stamm, W.; et al. Effect of Human Immunodeficiency Virus Type 1 Infection upon Acute Salpingitis: A Laparoscopic Study. J. Infect. Dis. 1998, 178, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Hebb, J.K.; Cohen, C.R.; Astete, S.G.; Bukusi, E.A.; Totten, P.A. Detection of Novel Organisms Associated with Salpingitis, by Use of 16S rDNA Polymerase Chain Reaction. J. Infect. Dis. 2004, 190, 2109–2120. [Google Scholar] [CrossRef]
- Hillier, S.L.; Kiviat, N.B.; Hawes, S.E.; Hasselquist, M.B.; Hanssen, P.W.; Eschenbach, D.A.; Holmes, K.K. Role of bacterial vaginosis–associated microorganisms in endometritis. Am. J. Obstet. Gynecol. 1996, 175, 435–441. [Google Scholar] [CrossRef]
- Nikolic, B.; Nguyen, K.; Martin, L.G.; Redd, D.C.; Best, I.; Silverstein, M.I. Pyosalpinx Developing from a Preexisting Hydrosalpinx after Uterine Artery Embolization. J. Vasc. Interv. Radiol. 2004, 15, 297–301. [Google Scholar] [CrossRef]
- Chan, C.C.; Ng, E.H.; Li, C.F.; Ho, P.C. Impaired ovarian blood flow and reduced antral follicle count following laparoscopic salpingectomy for ectopic pregnancy. Hum. Reprod. 2003, 18, 2175–2180. [Google Scholar] [CrossRef]
- Strandell, A.; Lindhard, A.; Waldenström, U.; Thorburn, J. Prophylactic salpingectomy does not impair the ovarian response in IVF treatment. Hum. Reprod. 2001, 16, 1135–1139. [Google Scholar] [CrossRef]
- Song, X.-M.; Jiang, H.; Zhang, W.-X.; Zhou, Y.; Ni, F.; Wang, X.-M. Ultrasound sclerotherapy pretreatment could obtain a similar effect to surgical intervention on improving the outcomes of in vitro fertilization for patients with hydrosalpinx. J. Obstet. Gynaecol. Res. 2017, 43, 122–127. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).