Abstract
Cardiac contractility modulation is an innovative therapy conceived for the treatment of heart failure. It is a device-based therapy, employing multiple electrodes to deliver relatively high-voltage (~7.5 V) biphasic signals to the endocardium of the right ventricular septum, in order to improve heart failure symptoms, exercise capacity and quality of life. Multiple clinical and mechanistic studies have been conducted to investigate the potential usefulness of this technology and, as of now, they suggest that it could have a place in therapy and meet a relevant medical need for a specific sub-category of underserved heart failure patients with reduced left ventricular ejection fraction. More studies are needed to further investigate its effect on outcomes such as mortality and rate of hospitalizations.
1. Introduction
Heart failure is one of the major causes of morbidity and mortality in the general population; it has a heavy impact on healthcare-related costs and its prevalence has been steadily increasing in the last 40 years [1,2,3,4]. This increase has many hypothesized causes, the most notable ones being the progressive ageing of the population, the prolonged survival of heart failure patients associated with new therapies and improved care and (somehow paradoxically) the constant improvements in the management of acute cardiovascular conditions like myocardial infarction, whose mortality keeps decreasing at the cost of higher post-acute morbidity [5,6,7]; the latter hypothesis, though, has been much debated [8].
Heart failure is a complex clinical syndrome enveloping different clinical phenotypes carrying diverse prognosis and requiring different treatments; various disease sub-categories have been identified over the years, and the latest guidelines differentiate clinical entities according to left ventricular ejection fraction (heart failure with reduced, mid-range or preserved ejection fraction), time-course of the disease (acute or chronic) and symptomatic severity (New York Heart Association (NYHA) class) [9]. This manuscript will focus on chronic heart failure with reduced ejection fraction (HFrEF), with only sparse reference to other sub-categories of heart failure.
A plethora of evidence-based therapeutic strategies are nowadays available for the treatment of HFrEF, and medical devices are part of the guideline-directed therapy advised by scientific societies. Implantable cardioverters-defibrillators (ICDs) and cardiac resynchronization therapy (CRT) have a clear role in therapy with very specific indications: ICDs are recommended for prevention of sudden death in patients with ischemic or dilated cardiomyopathy (with some limitations), while CRT is indicated for patients with an intraventricular conduction delay (QRS > 130 ms) and the recommendation is stronger in case of a left bundle branch block (LBBB) morphology [9,10,11]. CRT’s indications, though, leave many patients ineligible: patients with medically refractory disease without intraventricular conduction delay are not eligible for CRT therapy and, apart from optimal medical therapy, no strategy has until recently been available for relieving symptoms or improving quality of life in this subset of patients. The recommendation against CRT in patients with normal QRS morphology dates back to the EchoCRT trial, which not only revealed how these patients had no clear benefit from CRT, but it also found a statistically significant association between the CRT arm and an excess of deaths. This was later confirmed by subsequent subgroup analysis [12,13]. One more element that should be taken into account is that, apart from patients not eligible for CRT per guideline recommendations, there is a proportion of subjects that, after CRT implantation, show no benefit from this therapy and are considered non-responders (ranging from 20 to 40% of implanted patients according to different studies) [14,15,16,17].
In this therapeutic gap, Cardiac Contractility Modulation (CCM) could potentially play a relevant role. CCM is an emergent therapy, it employs standard pacing electrodes to deliver non-excitatory high-voltage biphasic impulses (~7.5 V/20 ms) duration during the absolute refractory period of the action potential of cardiac myocytes (Figure 1, see “Technical aspects” for more detailed information) [18,19,20].
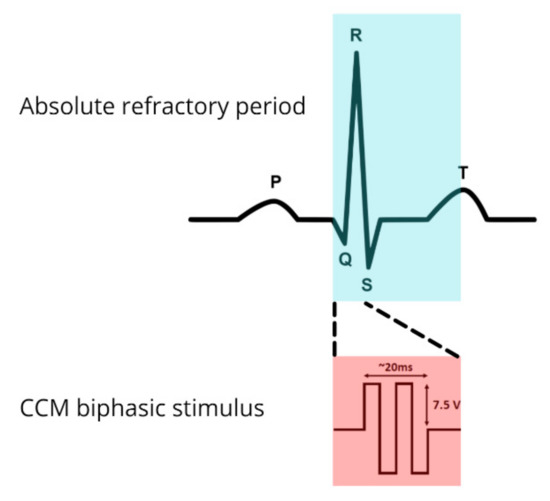
Figure 1.
Cardiac contractility modulation biphasic stimulus located on an electrocardiogram strip.
The first in-human experience with CCM dates back to 2001: CCM was investigated in candidates for an electrophysiological study (EP) study and its efficacy was evaluated by measuring aortic and intracardiac pressures and by evaluating echocardiographic changes before, during and after the application of CCM. This study showed promising results, in that it demonstrated how this therapy could improve the contractile capabilities of the failing left ventricle [21].
After this first experience, much research work has been conducted in the CCM domain and many improvements have been introduced. The most notable studies belong to the FIX-HF series, with the last study (FIX-HF-5C2) published in 2020 [22].
In 2019, after a considerable amount of evidence had been collected, the first device capable of delivering CCM was approved by the Food and Drug Administration (FDA): the OPTIMIZER Smart System (Impulse Dynamics Inc., Orangeburg, NY, USA) [23].
2. Underlying Mechanisms
A wealth of in vitro and in vivo experimental evidence has been collected concerning the effects of CCM during the last twenty years; this technique has demonstrated its efficacy at a cellular and macroscopic level and these studies have given many insights into the mechanisms of action underlying the observed effects.
The effects of CCM observed in vitro and in animal model are not completely understood. Various theories have been advanced, and it appears that CCM improves calcium handling in the cardiac myocyte (this theory is further supported by the observation of the abovementioned effect of ryanodine administration) and that interferes in the phosphorylation of cardiac phospholamban [24,25,26]. Pathological studies have revealed how in specimens of myocardium that underwent 3 months of CCM, there was an increased expression of SERCA2a, phospholamban, and RyR2. It appears then that CCM is capable of reverting the cardiac fetal gene program associated with heart failure [27,28].
Among the numerous in vivo experimental observations, we hereby review the most significant ones: CCM improved isometric contraction strength in rabbit papillary muscle and in myocytes obtained from the failing human myocardium; it also ameliorated isovolumic pressure generation in Langendorff-perfused ferret hearts and LV function improvements were elicited in dogs where HF was induced by coronary microembolization [29,30,31]. In Langendorff-perfused ferret hearts, the improvements evoked by CCM persisted after inotrope administration (epinephrine and digitalis, with additive effects of drugs and CCM) and were markedly blunted after exposure to ryanodine [32].
The effects of CCM appear to be pleiotropic in that it also appears to modify the expression of cytoskeletal proteins and myofilaments, possibly reducing fibrosis and further improving contractility [33]. The reverse remodeling observed with CCM looks very much like the one induced by CRT in patients with a mildly prolonged QRS (while it is much more pronounced in CRT patients with a marked QRS prolongation) [34].
At a macroscopic level, several features appear striking and potentially very favorable. CCM improves myocardial contractility without increasing myocardial oxygen consumption unlike, for example, inotrope administration [35,36]. Moreover, the improvements in myocardial contraction are not confined to the site where CCM is delivered, they are in fact global, including regions remote to the impulse delivery; this appears to be of the utmost importance considering how every single myocardial segment contributes to the pump function [37].
3. Technical Aspects
The implantation procedure of a device capable of delivering CCM is very much like the implantation of a dual-chamber pacemaker, the only difference being the placement of the two right ventricular leads that are positioned in such a way that impact on LV function is ensured [20]. The necessity of two leads in the right ventricular chamber has been questioned in 2016 by Röger et al. [38], who showed how a CCM system employing a single right ventricular lead had similar efficacy and safety compared to the traditional two-lead system, paving the way for future modifications of the stimulation protocol, and to a combined device for patients with ICD indication, which could benefit by both therapies with two endovascular leads only.
Different devices and different CCM protocols have been used in the previous years. From the “Scepter” employed by Pappone et al. in 2001 [21] to newer and more sophisticated devices like the “Optimizer” system by Impulse Dynamics, many improvements have been introduced.
A device capable of delivering CCM usually consists of an implantable pulse generator (IPG) with a rechargeable battery. Signal delivery happens through a variable number of leads and this feature has been the object of much research and many improvements since CCM was first experimented.
The first CCM devices employed a 3-lead system, with one lead in the right atrium (sensing lead) and two into the right portion of the ventricular septum that delivered the actual electrical impulses. The most recent device to be introduced in the research setting (FIX-HF-5C2 study) is the new-generation 2-lead Optimizer system, which, at once, reduced the likelihood of lead-related adverse events (such as systemic infection and superior vena cava thrombosis), known to be higher in dual-chamber lead systems [39,40] and made it possible for patients affected by atrial fibrillation and atrial flutter to receive CCM therapy (because the atrial sensing is not necessary for this device to function) [22].
The energy delivered with CCM is about a hundred times the amount of that delivered during a standard pacemaker impulse, yet these signals do not start a depolarization because the stimulus is delivered during the absolute refractory period of ventricular cardiomyocytes, about 30–40 ms after detection of local electrical activity (during phase 2 of the action potential of cardiac myocytes) [41,42].
The arrhythmogenic potential of CCM has been demonstrated as very low since the very first studies and CCM delivering devices have safety features meant to avoid the induction of malignant arrhythmias [21,41,42].
CCM impulses have an immediate effect on myocardial contractility and the efficacy and feasibility of this technique are established by measuring peak + dP/dtmax with a Millar micromanometer right after lead implantation (within ~10 min) [20,43]. Generally, investigators have employed a cut-off of a ≥5% increase in peak + dP/dtmax to classify the patient as responder and further proceed with the implantation. If this increase could not be documented, either lead repositioning or suspension of the procedure are considered viable options [26,44].
In 2017, a detailed guide for device implantation was produced by Kuschyk et al., covering all technical aspects ranging from pocket preparation and lead positioning to device programming and postoperative care [20]. We strongly recommend referring to this work for an extensive procedural guide.
4. Clinical Significance
Due to the impressive heterogeneity of the available clinical studies involving CCM, we hereby present two synoptic tables synthesizing the different studies investigating CCM in various clinical scenarios and in different patients with the aim of giving an overview on the large CCM research landscape. We used the MEDLINE database to search for studies investigating cardiac contractility modulation; search term was “cardiac contractility modulation” and studies that were either not interventional or did not investigate mortality or cardiovascular outcomes were excluded. Nineteen studies were selected and are represented in Table 1. As observable, these studies have many different features such as criteria of inclusion and exclusion, duration of follow-up, type of device, type of recruitment, CCM stimulation protocol, blinding or unblinding, presence of control group, type of treatment in the control group, outcomes measured and sample size. It is also notable that most of these studies (except four) were conducted on less than a hundred patients and still showed in most cases significant changes in quality of life and in cardiopulmonary performance.

Table 1.
Major findings and study design of clinical trials investigating CCM.
According to the latest studies, and specifically to the FIX-HF-5 study that first introduced this concept, the subgroup of patients that seem to benefit the most from this technique are NYHA II–III patients with an EF between 35 and 45% [19,45,46]. It is on these findings that the FDA formulated its approval in 2019 and patients with an EF between 35 and 45% are specifically mentioned in the FDA label for use in the USA.
A recent individual patient metanalysis by Giallauria et al. examined all the published randomized clinical trials comparing CCM to either sham or OMT (i.e., FIX-HF 5 pilot, FIX-HF 4, FIX-HF 5 and FIX-HF 5C) [41,46,47,48] and included the recent non-randomized FIX-HF-5C2 study. This work analyzed the effects of CCM on an aggregate of 861 patients (801 without those of FIX-HF-5C2) and pooled analysis showed that CCM significantly improved peak VO2 (mean difference +0.93, 95% CI 0.56 to 1.30 mL/kg/min), 6-min walk test distance (mean difference +17.97, 95% CI 5.48 to 30.46 m), and quality of life measured by Minnesota Living With Hearth Failure Questionnaire (mean difference 7.85, 95% CI 10.76 to 4.94) [49]. These results confirmed and further extended the findings of a previous meta-analysis by the same group, which included three trials (with the same inclusion criteria) and showed similar results [50].
The impact of CCM on cardiovascular outcomes such as mortality and hospitalizations need further investigation. In Table 1, the relevant clinical studies on CCM were reported and only six of the included works investigated the effect of CCM therapy on mortality and/or other cardiovascular outcomes [46,51,52,53,54,55]. The overall effect seems favorable, but specifically designed studies are strongly encouraged in order to confirm the potential role of CCM in reducing adverse outcomes in heart failure.
Defining potential responders and non-responders to CCM therapy will be crucial for optimal decision-making and more data are needed in order to establish which patients are most likely to benefit from device implantation [56]. Management algorithms have been proposed by several authors based on available evidence, but many grey zones still exist and the effect of CCM in some patients, like those with a right bundle branch block or patients that stay symptomatic after CRT implantation, still remains to be elucidated. The decision-making pathway proposed in 2020 by Campbell et al. provides a good overview of the actual and potential place in therapy of CCM according to the state-of-the art research [57].
5. Future Perspectives
Scientific societies are leaning towards an evidence-driven consensus that CCM could fill a gap in HF therapy [58] and the recent approval of CCM by the FDA paves the way for future opportunity of studying long-term outcomes in patients with CCM.
Although data on mortality outcomes is still poor, research on heart failure suggests that peak VO2 is a significant prognostic determinant in heart failure [59,60]; thus, based on the demonstrated positive effects on exercise capacity, it could be speculated that by increasing peak VO2, CCM is likely to improve survival in eligible patients. Its role in HF with preserved ejection fraction, which is becoming more and more prevalent, remains to be elucidated [4,61].
As of now, evidence suggests that the subgroup that benefits the most from CCM is made up of patients with a left ventricular ejection fraction between 25 and 45% [62,63], with recent real-world studies showing even more impressive effect in a subgroup of patients with an ejection fraction between 35 and 45% [55]. Still, most studies analyzed very small cohorts; therefore, adequately powered long-term studies are eagerly awaited in order to confirm and extend previous findings, by clearly depicting the clinical efficacy and the risk/benefit ratio associated with the procedure and identifying possible non-responders. In addition, the additive beneficial effect of exercise-based Cardiac Rehabilitation on symptoms relief and outcome remains to be elucidated.
6. Conclusions
Additional evidence is needed to find CCM’s place in therapy; adequately powered studies are required to fully understand the role of this novel therapy. Several safe and effective therapies have been investigated and approved for HF treatment. The recent approval of Sacubitril-Valsartan made it clear that HFrEF treatment can in fact be improved in order to transform the ominous prognosis that this syndrome carries into a more favorable one. Still, much remains to be done, in that some patients find themselves in a state of limbo, without access to further therapies or procedures. CCM could find its place in meeting a relevant medical need, by providing an effective therapy to patients that would otherwise have no therapeutic option left.
Author Contributions
Conceptualization, F.G. and A.P.; methodology, A.D.L. and C.T.; data curation, A.D.L., C.T., C.V., A.D., G.S., M.B., and A.J.S.C.; writing—original draft preparation, F.G., A.P, C.V., A.D., G.S., M.B., and A.J.S.C.; writing—review and editing, F.G., A.P., A.D., G.S., M.B., C.V., and A.J.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fang, J.; Mensah, G.A.; Croft, J.B.; Keenan, N.L. Heart failure-related hospitalization in the U.S., 1979 to 2004. J. Am. Coll. Cardiol. 2008, 52, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, A.P. Epidemiology of heart failure in Europe. Heart Fail. Clin. 2015, 11, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Anker, S.D.; Maggioni, A.P.; Coats, A.J.; Filippatos, G.; Ruschitzka, F.; Ferrari, R.; Piepoli, M.F.; Jimenez, J.F.D.; Metra, M.; et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 2016, 18, 613–625. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics—2020 update: A report from the American heart association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. Cardiovascular medicine at the turn of the millennium: Triumphs, concerns, and opportunities. N. Engl. J. Med. 1997, 337, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Albert, N.M.; AlleHearn, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef]
- Hellermann, J.P.; Goraya, T.Y.; Jacobsen, S.J.; Weston, S.A.; Reeder, G.S.; Gersh, B.J.; Redfield, M.M.; Rodeheffer, R.J.; Yawn, B.P.; Roger, V.L. Incidence of Heart failure after myocardial infarction: Is It changing over time? Am. J. Epidemiol. 2003, 157, 1101–1107. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.B.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Circulation 2017, 136, 776–803. [Google Scholar] [CrossRef]
- Ruschitzka, F.; Abraham, W.T.; Singh, J.P.; Bax, J.J.; Borer, J.S.; Brugada, J.; Dickstein, K.; Ford, I.; Gorcsan, J.; Gras, D.; et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N. Engl. J. Med. 2013, 369, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Robertson, M.; Singh, J.P.; Abraham, W.T.; Bax, J.J.; Borer, J.S.; Dickstein, K.; Ford, I.; Gorcsan, J.; Gras, D.; et al. The effect of QRS duration on cardiac resynchronization therapy in patients with a narrow QRS complex: A subgroup analysis of the EchoCRT trial. Eur. Heart J. 2015, 36, 1983–1989. [Google Scholar] [CrossRef]
- Daubert, C.; Behar, N.; Martins, R.P.; Mabo, P.; Leclercq, C. Avoiding non-responders to cardiac resynchronization therapy: A practical guide. Eur. Heart J. 2016, 38, 1463–1472. [Google Scholar] [CrossRef]
- Daubert, J.C.; Saxon, L.; Adamson, P.B.; Auicchio, A.; Berger, D.R.; Beshai, J.F.; Breithard, O.; Brignole, M.; Cleland, J.; DeLurgio, D.B.; et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: Implant and follow-up recommendations and management. Europace 2012, 14, 1236–1286. [Google Scholar] [CrossRef] [PubMed]
- Aranda, J.M.; Woo, G.W.; Schofield, R.S.; Handberg, E.M.; Hill, J.A.; Curtis, A.B.; Sears, S.F.; Goff, J.S.; Pauly, D.F.; Conti, J.B. Management of heart failure after cardiac resynchronization therapy. J. Am. Coll. Cardiol. 2005, 46, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Prinzen, F.W.; Vernooy, K.; Auricchio, A. Cardiac resynchronization therapy. Circulation 2013, 128, 2407–2418. [Google Scholar] [CrossRef]
- Tschöpe, C.; Kherad, B.; Klein, O.; Lipp, A.; Blaschke, F.; Gutterman, D.; Burkhoff, D.; Hamdani, N.; Spillmann, F.; Van Linthout, S. Cardiac contractility modulation: Mechanisms of action in heart failure with reduced ejection fraction and beyond. Eur. J. Heart Fail. 2019, 21, 14–22. [Google Scholar] [CrossRef]
- Borggrefe, M.; Burkhoff, D. Clinical effects of cardiac contractility modulation (CCM) as a treatment for chronic heart failure. Eur. J. Heart Fail. 2012, 14, 703–712. [Google Scholar] [CrossRef]
- Kuschyk, J.; Kloppe, A.; Schmidt-Schweda, S.; Bonnemeier, H.; Rousso, B.; Röger, S. Cardiac contractility modulation: A technical guide for device implantation. Rev. Cardiovasc. Med. 2017, 18, 1–13. [Google Scholar]
- Pappone, C.; Vicedomini, G.; Salvati, A.; Meloni, C.; Haddad, W.; Aviv, D.R.; Mika, D.Y.; Darvish, N.; Kimchy, D.Y.; Shemer, I.; et al. Electrical modulation of cardiac contractility: Clinical aspects in congestive heart failure. Heart Fail. Rev. 2001, 6, 55–60. [Google Scholar] [CrossRef]
- Wiegn, P.; Chan, R.; Jost, C.; Saville, B.R.; Parise, H.; Prutchi, D.; Carson, P.E.; Stagg, A.; Goldsmith, R.L.; Burkhoff, D. Safety, performance, and efficacy of cardiac contractility modulation delivered by the 2-lead optimizer smart system. Circ. Heart Fail. 2020, 13, e006512. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. OPTIMIZER Smart System—P180036. 2019. Available online: https://www.fda.gov/medical-devices/recently-approved-devices/optimizer-smart-system-p180036 (accessed on 3 November 2020).
- Gupta, R.C.; Mishra, S.; Wang, M.; Jiang, A.; Rastogi, S.; Rousso, B.; Mika, Y.; Sabbah, H.N. Cardiac contractility modulation electrical signals normalize activity, expression, and phosphorylation of the Na+-Ca2+ exchanger in heart failure. J. Card. Fail. 2009, 15, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Mishra, S.; Rastogi, S.; Wang, M.; Rousso, B.; Mika, Y.; Remppis, A.; Sabbah, H.N. Ca2+-Binding proteins in dogs with heart failure: Effects of cardiac contractility modulation electrical signals. Clin. Transl. Sci. 2009, 2, 211–215. [Google Scholar] [CrossRef]
- Abraham, W.T.; Burkhoff, D.; Nademanee, K.; Carson, P.; Bourge, R.; Ellenbogen, K.A.; Parides, M.; Kadish, A. A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation in patients with systolic heart failure: Rationale, design, and baseline patient characteristics. Am. Heart J. 2008, 156, 641–648. [Google Scholar] [CrossRef]
- Butter, C.; Rastogi, S.; Minden, H.-H.; Meyhöfer, J.; Burkhoff, D.; Sabbah, H.N. Cardiac contractility modulation electrical signals improve myocardial gene expression in patients with heart failure. J. Am. Coll. Cardiol. 2008, 51, 1784–1789. [Google Scholar] [CrossRef]
- Imai, M.; Rastogi, S.; Gupta, R.C.; Mishra, S.; Sharov, V.G.; Stanley, W.C.; Mika, Y.; Rousso, B.; Burkhoff, D.; Ben-Haim, S.; et al. Therapy with cardiac contractility modulation electrical signals improves left ventricular function and remodeling in dogs with chronic heart failure. J. Am. Coll. Cardiol. 2007, 49, 2120–2128. [Google Scholar] [CrossRef]
- Morita, H.; Suzuki, G.; Haddad, W.; Mika, Y.; Tanhehco, E.J.; Sharov, V.G.; Goldstein, S.; Ben-Haim, S.; Sabbah, H.N. Cardiac contractility modulation with nonexcitatory electric signals improves left ventricular function in dogs with chronic heart failure. J. Card. Fail. 2003, 9, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Mohri, S.; Shimizu, J.; Mika, Y.; Shemer, I.; Wang, J.; Ben-Haim, S.; Burkhoff, D. Electric currents applied during refractory period enhance contractility and systolic calcium in the ferret heart. Am. J. Physiol. Circ. Physiol. 2003, 284, H1119–H1123. [Google Scholar] [CrossRef] [PubMed]
- Brunckhorst, C.B.; Shemer, I.; Mika, Y.; Ben-Haim, S.A.; Burkhoff, D. Cardiac contractility modulation by non-excitatory currents: Studies in isolated cardiac muscle. Eur. J. Heart Fail. 2005, 8, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Burkhoff, D.; Shemer, I.; Felzen, B.; Shimizu, J.; Mika, Y.; Dickstein, M.; Prutchi, D.; Darvish, N.; Ben-Haim, S.A. Electric currents applied during the refractory period can modulate cardiac contractility in vitro and in vivo. Heart Fail. Rev. 2001, 6, 27–34. [Google Scholar] [CrossRef]
- Rastogi, S.; Mishra, S.; Zacà, V.; Mika, Y.; Rousso, B.; Sabbah, H.N. Effects of chronic therapy with cardiac contractility modulation electrical signals on cytoskeletal proteins and matrix metalloproteinases in dogs with heart failure. Cardiology 2007, 110, 230–237. [Google Scholar] [CrossRef]
- Zhang, Q.; Chan, Y.-S.; Liang, Y.-J.; Fang, F.; Lam, Y.-Y.; Chan, C.-P.; Lee, A.P.-W.; Chan, K.C.-Y.; Wu, E.B.; Yu, C.-M. Comparison of left ventricular reverse remodeling induced by cardiac contractility modulation and cardiac resynchronization therapy in heart failure patients with different QRS durations. Int. J. Cardiol. 2013, 167, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Butter, C.; Wellnhofer, E.; Schlegl, M.; Winbeck, G.; Fleck, E.; Sabbah, H.N. Enhanced inotropic state of the failing left ventricle by cardiac contractility modulation electrical signals is not associated with increased myocardial oxygen consumption. J. Card. Fail. 2007, 13, 137–142. [Google Scholar] [CrossRef]
- Patel, P.A.; Nadarajah, R.; Ali, N.; Gierula, J.; Witte, K.K. Cardiac contractility modulation for the treatment of heart failure with reduced ejection fraction. Heart Fail. Rev. 2021, 26, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-M.; Chan, J.Y.-S.; Zhang, Q.; Yip, G.W.; Lam, Y.-Y.; Chan, A.; Burkhoff, D.; Lee, P.-W.; Fung, J.W.-H. Impact of cardiac contractility modulation on left ventricular global and regional function and remodeling. JACC Cardiovasc. Imaging 2009, 2, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Röger, S.; Said, S.; Kloppe, A.; Lawo, T.; Emig, U.; Rousso, B.; Gutterman, D.; Borggrefe, M.; Kuschyk, J. Cardiac contractility modulation in heart failure patients: Randomized comparison of signal delivery through one vs. two ventricular leads. J. Cardiol. 2017, 69, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Kirkfeldt, R.E.; Johansen, J.B.; Nohr, E.A.; Moller, M.; Arnsbo, P.; Nielsen, J.C. Risk factors for lead complications in cardiac pacing: A population-based cohort study of 28,860 Danish patients. Heart Rhythm. 2011, 8, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Van Eck, J.; Van Hemel, N.; Zuithof, P.; Van Asseldonk, J.; Voskuil, T.; Grobbee, D.; Moons, K. Incidence and predictors of in-hospital events after first implantation of pacemakers. Eurospace 2007, 9, 884–889. [Google Scholar] [CrossRef]
- Borggrefe, M.M.; Lawo, T.; Butter, C.; Schmidinger, H.; Lunati, M.; Pieske, B.; Misier, A.R.; Curnis, A.; Böcker, D.; Remppis, A.; et al. Randomized, double blind study of non-excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur. Heart J. 2008, 29, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Cappannoli, L.; Scacciavillani, R.; Rocco, E.; Perna, F.; Narducci, M.L.; Vaccarella, M.; D’Amario, D.; Pelargonio, G.; Massetti, M.; Crea, F.; et al. Cardiac contractility modulation for patient with refractory heart failure: An updated evidence-based review. Heart Fail. Rev. 2021, 26, 227–235. [Google Scholar] [CrossRef]
- Lyon, A.R.; Samara, M.A.; Feldman, D.S. Cardiac contractility modulation therapy in advanced systolic heart failure. Nat. Rev. Cardiol. 2013, 10, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Lawo, T.; Borggrefe, M.; Butter, C.; Hindricks, G.; Schmidinger, H.; Mika, Y.; Burkhoff, D.; Pappone, C.; Sabbah, H.N. Electrical signals applied during the absolute refractory period. J. Am. Coll. Cardiol. 2005, 46, 2229–2236. [Google Scholar] [CrossRef] [PubMed]
- Burkhoff, D. Does contractility modulation have a role in the treatment of heart failure? Curr. Heart Fail. Rep. 2011, 8, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Kuck, K.-H.; Goldsmith, R.L.; Lindenfeld, J.; Reddy, V.Y.; Carson, P.E.; Mann, D.L.; Saville, B.; Parise, H.; Chan, R.; et al. A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. JACC Heart Fail. 2018, 6, 874–883. [Google Scholar] [CrossRef]
- Neelagaru, S.B.; Sanchez, J.E.; Lau, S.K.; Greenberg, S.M.; Raval, N.Y.; Worley, S.; Kalman, J.; Merliss, A.D.; Krueger, S.; Wood, M. Nonexcitatory, cardiac contractility modulation electrical impulses: Feasibility study for advanced heart failure in patients with normal QRS duration. Heart Rhythm. 2006, 3, 1140–1147. [Google Scholar] [CrossRef]
- Kadish, A.; Nademanee, K.; Volosin, K.; Krueger, S.; Neelagaru, S.; Raval, N.; Obel, O.; Weiner, S.; Wish, M.; Carson, P.; et al. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. Am. Heart J. 2011, 161, 329–337. [Google Scholar] [CrossRef]
- Giallauria, F.; Cuomo, G.; Parlato, A.; Raval, N.Y.; Kuschyk, J.; Coats, A.J.S. A comprehensive individual patient data meta-analysis of the effects of cardiac contractility modulation on functional capacity and heart failure-related quality of life. ESC Heart Fail. 2020, 7, 2922–2932. [Google Scholar] [CrossRef]
- Giallauria, F.; Vigorito, C.; Piepoli, M.F.; Coats, A.J.S. Effects of cardiac contractility modulation by non-excitatory electrical stimulation on exercise capacity and quality of life: An individual patient’s data meta-analysis of randomized controlled trials. Int. J. Cardiol. 2014, 175, 352–357. [Google Scholar] [CrossRef]
- Schau, T.; Seifert, M.; Meyhöfer, J.; Neuss, M.; Butter, C. Long-term outcome of cardiac contractility modulation in patients with severe congestive heart failure. Eurospace 2011, 13, 1436–1444. [Google Scholar] [CrossRef]
- Röger, S.; Michels, J.; Heggemann, F.; Stach, K.; Rousso, B.; Borggrefe, M.; Kuschyk, J. Long term impact of cardiac contractility modulation on QRS duration. J. Electrocardiol. 2014, 47, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Kuschyk, J.; Roeger, S.; Schneider, R.; Streitner, F.; Stach, K.; Rudic, B.; Weiß, C.; Schimpf, R.; Papavasilliu, T.; Rousso, B.; et al. Efficacy and survival in patients with cardiac contractility modulation: Long-term single center experience in 81 patients. Int. J. Cardiol. 2015, 183, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Kloppe, A.; Lawo, T.; Mijic, D.; Schiedat, F.; Muegge, A.; Lemke, B. Long-term survival with cardiac contractility modulation in patients with NYHA II or III symptoms and normal QRS duration. Int. J. Cardiol. 2016, 209, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Borggrefe, M.; Neuser, H.; Ohlow, M.; Röger, S.; Goette, A.; Remppis, B.A.; Kuck, K.; Najarian, K.B.; Gutterman, D.D.; et al. Cardiac contractility modulation improves long-term survival and hospitalizations in heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2019, 21, 1103–1113. [Google Scholar] [CrossRef]
- Biffi, M.; Aspromonte, N.; Bongiorni, M.G.; Clemenza, F.; D’Onofrio, A.; De Ferrari, G.M.; Grimaldi, M.; Oliva, F.; Senni, M.; Tondo, C.; et al. Modulazione della contrattilità cardiaca nello scompenso cardiaco a frazione di eiezione ridotta: Revisione critica delle evidenze ed aspetti decisionali pratici. G. Ital. Cardiol. 2021. Available online: https://www.giornaledicardiologia.it/anticipazioni/articoli/35134/ (accessed on 3 November 2020).
- Campbell, C.M.; Kahwash, R.; Abraham, W.T. Optimizer Smart in the treatment of moderate-to-severe chronic heart failure. Futur. Cardiol. 2020, 16, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, P.M.; Ponikowski, P.; Anker, S.D.; Bauersachs, J.; Chioncel, O.; Cleland, J.G.; De Boer, R.A.; Drexel, H.; Ben Gal, T.; Hill, L.; et al. Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 1169–1186. [Google Scholar] [CrossRef]
- Francis, D.P.; Shamim, W.; Davies, L.C.; Piepoli, M.F.; Ponikowski, P.; Anker, S.D.; Coats, A.J.S. Cardiopulmonary exercise testing for prognosis in chronic heart failure: Continuous and independent prognostic value from VE/VCO2slope and peak VO2. Eur. Heart J. 2000, 21, 154–161. [Google Scholar] [CrossRef]
- Swank, A.M.; Horton, J.; Fleg, J.L.; Fonarow, G.C.; Keteyian, S.; Goldberg, L.; Wolfel, G.; Handberg, E.M.; Bensimhon, D.; Illiou, M.-C.; et al. Modest Increase in Peak VO2 is related to better clinical outcomes in chronic heart failure patients. Circ. Heart Fail. 2012, 5, 579–585. [Google Scholar] [CrossRef]
- Vasan, R.S.; Xanthakis, V.; Lyass, A.; Andersson, C.; Tsao, C.; Cheng, S.; Aragam, J.; Benjamin, E.J.; Larson, M.G. Epidemiology of left ventricular systolic dysfunction and heart failure in the framingham study. JACC Cardiovasc. Imaging 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Liu, M.; Fang, F.; Luo, X.X.; Shlomo, B.-H.; Burkhoff, D.; Chan, J.Y.; Chan, C.-P.; Cheung, L.; Rousso, B.; Gutterman, D.; et al. Improvement of long-term survival by cardiac contractility modulation in heart failure patients: A case–control study. Int. J. Cardiol. 2016, 206, 122–126. [Google Scholar] [CrossRef]
- Abraham, W.T.; Nademanee, K.; Volosin, K.; Krueger, S.; Neelagaru, S.; Raval, N.; Obel, O.; Weiner, S.; Wish, M.; Carson, P.; et al. Subgroup analysis of a randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. J. Card. Fail. 2011, 17, 710–717. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).